Distinct population and single-neuron selectivity for executive and episodic processing in human dorsal posterior cingulate
Figures
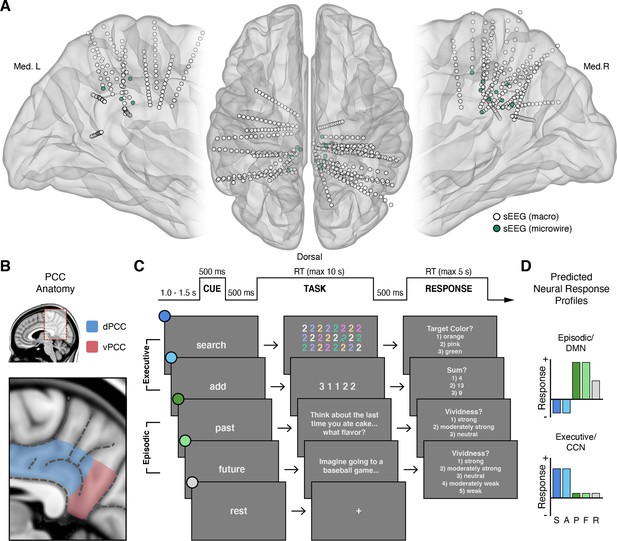
Intracranial recordings sites, PCC anatomy, task design, and predicted neural responses.
(A) Anatomical locations, normalized to MNI space, of intracranial sEEG depth electrodes targeting the posterior aspect of the cingulate cortex, with the distal contact of probes containing microwires indicated in green. (B) Anatomy of human PCC, differentiating dorsal (dPCC) and ventral (vPCC) subregions and demarcating regional sulci following the most recent definitions in PCC (Petrides, 2018; Willbrand et al., 2022). (C) Attention/Memory Switch task procedure and example stimuli. The task included two executive attention conditions: search and add, two episodic memory conditions: past and future, and a rest condition. Each trial was proceeded by a cue indicating the forthcoming task condition and was followed by a trial response period, excluding rest (see Methods). (D) Schematic of the putative neural response profiles to the task conditions for episodic/DMN (default mode network) selectivity vs. executive/CCN (cognitive control network) selectivity.
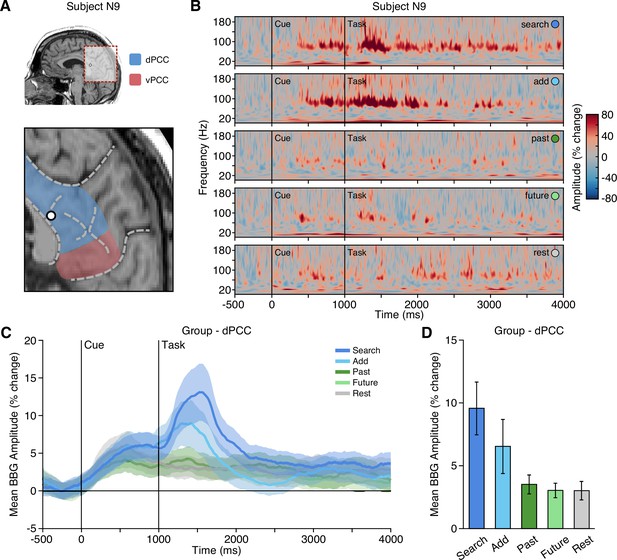
Executive condition selectivity of LFP BBG task responses in dPCC.
(A) Location of a representative dPCC electrode in subject N9. (B) Time-frequency spectrogram for electrode in (A) depicting amplitude (% change) dynamics over time for each task condition. Amplitude in the BBG frequency range is increased during the cue and early task performance of attention conditions (search/add). (C) Group mean (nprobes = 29, nsubjects = 19) BBG amplitude (with s.e.m. shading) over time, aligned to trial onset, across conditions for all dPCC electrodes. Black vertical lines indicate time of cue and task onset. (D) Group mean BBG amplitude (with s.e.m.) during early task execution (task onset to 1000 ms) across conditions for all dPCC electrodes. Together, these data show increased activity of dPCC populations during early task execution of executive attention conditions, consistent with the CCN. See Figure 2—figure supplement 1 for vPCC data.
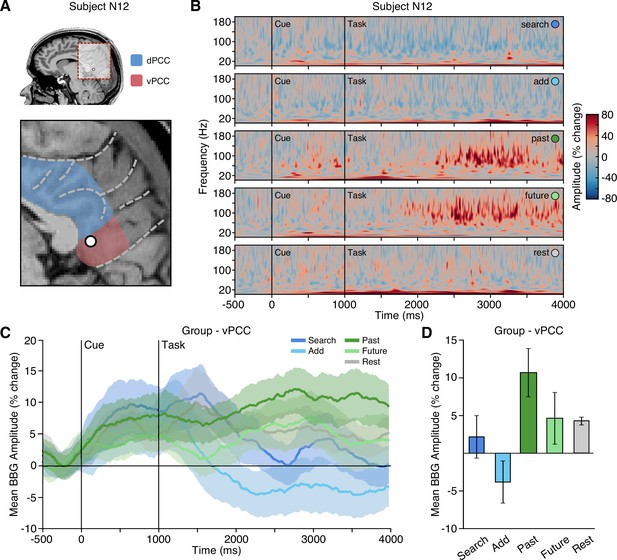
Episodic condition selectivity of LFP BBG task responses in vPCC.
(A) Location of a representative vPCC electrode in subject N12. (B) Time-frequency spectrogram from electrode in (A) depicting amplitude (% change) dynamics over time for each task condition. Amplitude in the BBG frequency range is increased during the execution of episodic conditions (past/future). (C) Group mean (nprobes = 6) BBG amplitude (with s.e.m. shading) over time, aligned to trial onset, across conditions for all vPCC electrodes. Black vertical lines indicate time of cue and task onset. (D) Group mean BBG amplitude (with s.e.m.) during late task execution (2000–3000 ms after task onset) across conditions for all vPCC electrodes. Together, these data show sustained increased activity of vPCC populations during execution of episodic conditions, consistent with the DMN.
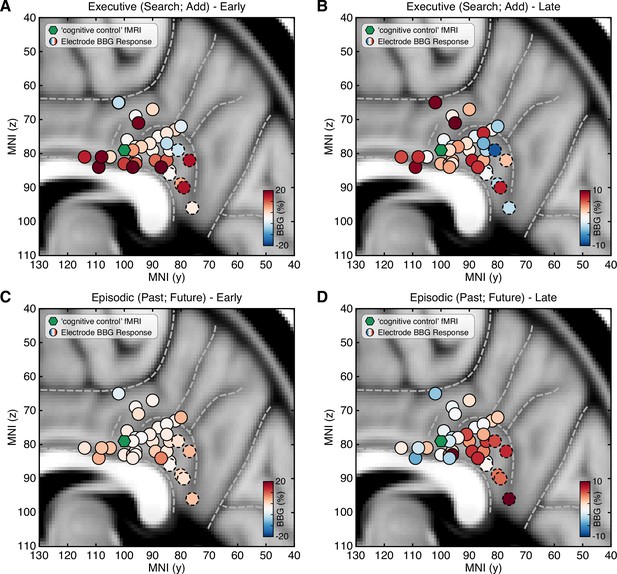
Spatial clustering of LFP BBG responses across PCC.
Mean LFP BBG response for each macro-electrode site within PCC averaged for the executive (search/add) (A/B) and episodic (past/future) (C/D) task conditions during early (task onset to 1000 ms; left) and late (2000–3000 ms into task; right) task execution. Electrode coordinates are shown in MNI voxel space, collapsed for the x-dimension (i.e. collapsed for left/right hemispheres). Green symbol indicates the centroid maxima of the association map for search term ‘cognitive control’ at z>4.0 from Neurosynth meta-analysis. BBG responses predominate for the executive task conditions during the early task window, specifically for electrodes more proximal to the callosal sulcus. However, BBG responses to the episodic task conditions emerge during the late task window, specifically for more ventral sites. Ventral PCC electrodes (n=6) are indicated with a dashed outline.
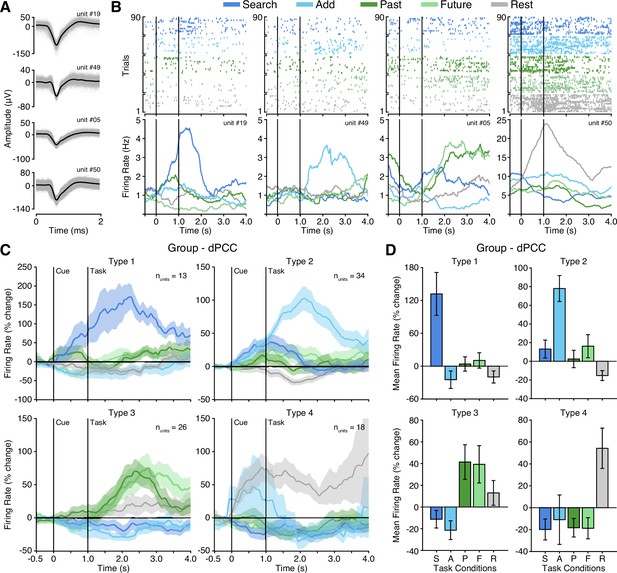
Four functional single unit types in dPCC.
(A) Example isolated single unit waveforms. (B) Raster plots and instantaneous firing rate traces for four example isolated single units shown in (A). Example units show distinct functional selectivity profiles across task conditions. (C) Mean instantaneous firing rates (with s.e.m. shading) across task conditions for the four functional single unit types identified based on unsupervised similarity clustering (See Methods & Figure 4—figure supplement 1; Type 1 nunits = 13; Type 2 nunits = 34; Type 3 nunits = 26; Type 4 nunits = 18). (D) Mean firing rate (with s.e.m.) for the four functional single unit types (averaged over task onset to 2 s into task execution). In (B) and (C), black vertical lines indicate time of cue and task onset.
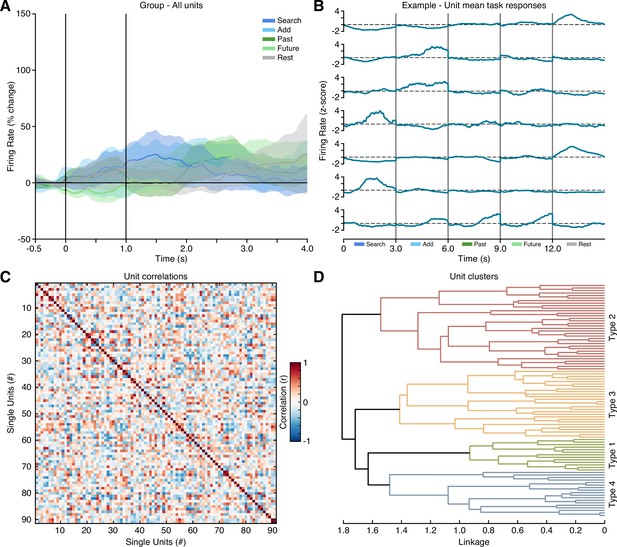
Single unit response clustering.
(A) Group averaged firing rate (with s.e.m.) for all isolated units (nunits = 91) across task conditions. Overall, group data showed modest task modulation and condition selectivity. This initial observation led to the examination of individual unit response profiles, suggesting a diversity in task selectivity. Therefore, single unit data was grouped based on an unsupervised hierarchical clustering approach. To do so, a task response feature was constructed by concatenating mean firing rates for each task condition (window: cue +3 s) to capture the putative dynamics and selectivity of each unit, with seven example units depicted here (B). All unit response features were then correlated and clustered to identify putative functional response types. (C) Unsorted correlation matrix based on all unit response features. (D) Dendrogram showing agglomerative hierarchical clustering of all units (based on correlation distance, i.e. 1-r). Clustering revealed four distinct functional response types (See Methods).
Additional files
-
Supplementary file 1
Subject demographic and electrode information.
Demographic and electrode information is reported for each subject (1-20), including sex (male/female), age at time of experiment (years), total number of PCC electrodes, number of dPCC electrodes, number of vPCC electrodes, and number of microwire probes.
- https://cdn.elifesciences.org/articles/80722/elife-80722-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/80722/elife-80722-mdarchecklist1-v1.docx





