Huntingtin recruits KIF1A to transport synaptic vesicle precursors along the mouse axon to support synaptic transmission and motor skill learning
Figures
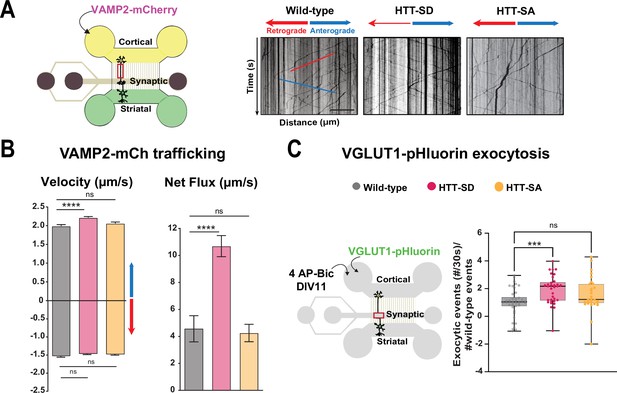
HTT phosphorylation at S421 increases synaptic vesicle precursor (SVP) anterograde axonal transport and SV exocytosis.
(A) Diagram of the microfluidic device for reconstituting a corticostriatal network compatible with live-cell imaging of axons. Cortical axons grow in the cortical chamber (yellow) and connect with the striatal dendrites in the striatal chamber (green) through synapses in the synaptic compartment (purple). On the right, representative kymographs of VAMP2-mCherry vesicle transport in axons for each genotype. Scale bar = 25 µm. (B) Segmental anterograde (**** p<0.0001, N = 1078 wild-type [WT] vesicles, 1886 HTT-SD vesicles, and 1384 HTT-SA vesicles), retrograde velocities (ns: non-significant; N=1029 WT vesicles, 1564 HTT-SD vesicles, 2019 HTT-SA vesicles) and directional net flux (****p<0.0001; N=118 WT axons, 157 HTT-SD axons, 132 HTT-SA axons) of VAMP2-mCherry vesicles. Histograms represent means ± SEM of three independent experiments. Significance was determined using one-way ANOVA followed by Dunn’s multiple comparison test. (C) Schematic of the three-compartment microfluidic device. Cortical neurons were infected with a lentivirus expressing VGLUT1 linked to a pH-sensitive variant of GFP (pHluorin); they were stimulated with 4AP-bicuculline at day in vitro (DIV) 11. The number of VGLUT-1 pHluorin exocytosis events within the synaptic chamber of the corticostriatal network, as compared to that of WT and to that of non-stimulated condition is shown here (*p<0.05; N=6712 events in WT, 4640 events in HTT-SD and 5176 events in HTT-SA neurons). The box-whisker plots show the median, the 25th and the 75th percentiles, the smallest and the largest values of three independent experiments using a total of N=WT 11, 10 HTT-SD, and 10 HTT-SA neurons seeded within microfluidic devices with at least three fields per device. Significance was determined using one-way ANOVA followed by Dunn’s multiple comparison test.
-
Figure 1—source data 1
Data analyzed for anterograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-data1-v3.xlsx
-
Figure 1—source data 2
Data analyzed for retrograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-data2-v3.xlsx
-
Figure 1—source data 3
Data analyzed for net flux.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-data3-v3.xlsx
-
Figure 1—source data 4
Data analyzed for VGLUT1 pHluorin exocytosis number of events.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-data4-v3.xlsx
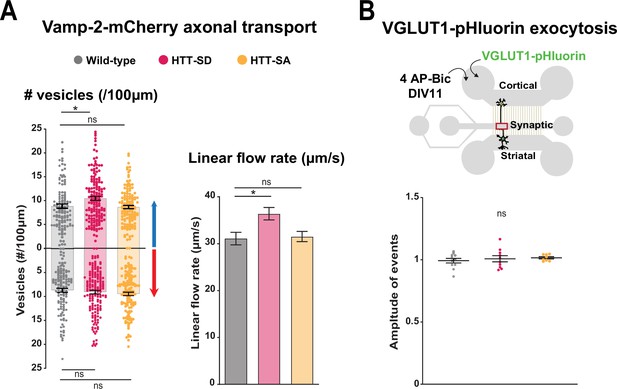
HTT phosphorylation at S421 increases synaptic vesicle precursor (SVP) anterograde axonal transport without affecting the quantity of SV released.
(A) Left: Number of anterograde (*p<0.05; N=117 WT axons, 156 HTT-SD axons, and 132 HTT-SA axons) and retrograde (*p<0.05; 118 WT axons, 159 HTT-SD axons, and 134 HTT-SA axons) VAMP2-mCherry vesicles in 100 µm of axon. Right: Their linear flow rate (*p<0.05; 118 WT axons, 158 HTT-SD axons, and 133 HTT-SA axons). Histograms represent means ± SEM of three independent experiments. Significance was determined using a one-way ANOVA followed by Dunn’s multiple comparison test. (B) Diagram of the three-compartment microfluidic device with an indication of lentiviral transduction and stimulation with 4AP-bicuculline. The amplitude of VGLUT-1 pHluorin exocytosis events within the synaptic chamber of the corticostriatal network was compared to that of non-stimulated condition (see Materials and methods). Histograms represent means ± SEM of three independent experiments. Significance was determined using one-way ANOVA followed by Dunn’s multiple comparison test (ns: non-significant).
-
Figure 1—figure supplement 1—source data 1
Data analyzed for number of anterograde vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-figsupp1-data1-v3.xlsx
-
Figure 1—figure supplement 1—source data 2
Data analyzed for number of retrograde vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-figsupp1-data2-v3.xlsx
-
Figure 1—figure supplement 1—source data 3
Data analyzed for the linear flow rate.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-figsupp1-data3-v3.xlsx
-
Figure 1—figure supplement 1—source data 4
Data analyzed for VGLUT1 pHluorin exocytosis amplitude of events.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig1-figsupp1-data4-v3.xlsx
Movie showing the glutamate release (VGLUT-pHluorin) in wild-type (WT) neurons after the stimulation.
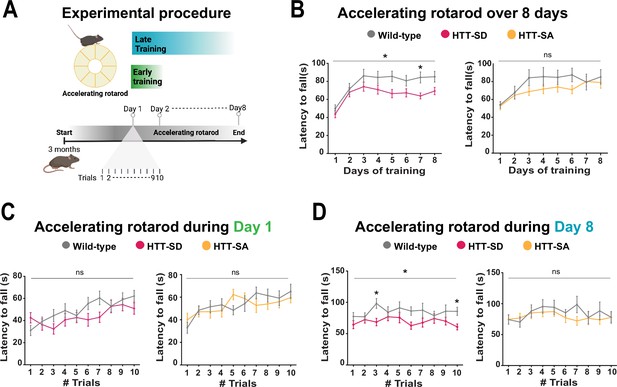
Constitutive phosphorylation of HTT at S421 impairs motor skill learning in mice.
(A) Schematic of the accelerating rotarod protocol assessing motor skill learning over 8 days with 10 sessions per day. (B) Mean latency to fall each day for 8 days, for HTT-SD mice (*p<0.05: two-way ANOVA followed by Sidak’s multiple comparisons test where p<0.01 at day 7) and HTT-SA mice (ns: non-significant: two-way ANOVA). (C) Mean time to fall off the rotarod per session, over 10 sessions, during the first or (D) the last day for HTT-SD (ns: non-significant: two-way ANOVA at day 1 and *p<0.05: two-way ANOVA at day 8 followed by Sidak’s multiple comparison test where p<0.01 at trials 3 and 10) and HTT-SA (ns: non-significant: two-way ANOVA at days 1 and 8). We compared 3-month-old male mice: 20 wild-type (WT) with 20 HTT-SD littermates, and 13 WT with 18 HTT-SA littermates.
-
Figure 2—source data 1
Data analyzed for accelerating rotarod over 8 days.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig2-data1-v3.xlsx
-
Figure 2—source data 2
Data analyzed for accelerating rotarod during day 1.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig2-data2-v3.xlsx
-
Figure 2—source data 3
Data analyzed for accelerating rotarod during day 8.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig2-data3-v3.xlsx
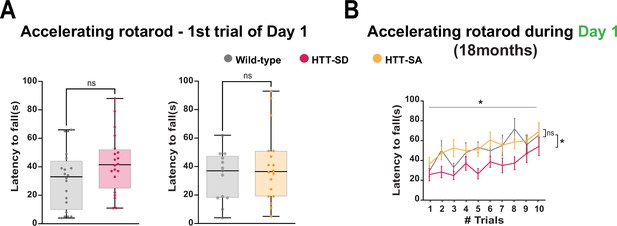
HTT phosphorylation at S421 impairs motor skill learning without affecting motor performance.
(A) Results of the first rotarod trial of the first day for HTT-SD and HTT-SA mice (ns: non-significant, Mann-Whitney test). The box-whisker plots show the median, the 25th and the 75th percentiles, the smallest and the largest values for N=20 wild-type (WT) vs. 20 HTT-SD and 13 WT vs. 18 HTT-SA 3-month-old littermate male mice. (B) Performance during the 10 sessions of the first day for 8 WT, 10 HTT-SD, and 12 HTT-SA 18-month-old mice. Significance was determined using two-way ANOVA followed by Holm-Sidak’s multiple comparison test (*p<0.05 between WT and HTT-SD).
-
Figure 2—figure supplement 1—source data 1
Data analyzed for accelerating rotarod first trial at day 1.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig2-figsupp1-data1-v3.xlsx
-
Figure 2—figure supplement 1—source data 2
Data analyzed for accelerating rotarod during day 1 at 18 months.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig2-figsupp1-data2-v3.xlsx
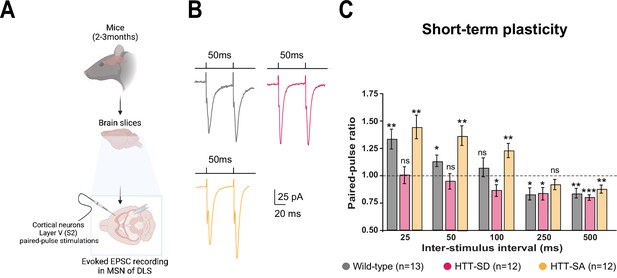
HTT phosphorylation at S421 increases short-term plasticity in the corticostriatal network ex vivo.
(A) Schematic of medium-sized spiny neurons (MSNs) recording in the dorsolateral striatum (DLS) after paired-pulse stimulations in S2 cortex of mice at 2–3 months of age. (B) Representative traces of the paired-pulse ratio per interstimulus interval of electrophysiological response of MSNs in the DLS after stimulation in S2 in 2- to 3-month-old wild-type (WT) (gray), HTT-SD (pink), and HTT-SA (orange) mice (C). Quantification of (B). In contrast to WT and HTT-SA MSNs, HTT-SD MSN responses from 25 to 50 ms showed no facilitation (paired-pulse ratio~1) but only depression from 100 ms (*p<0.05, **p<0.001, and ***p<0.0001; ns means non-significant). Paired-pulse ratios were recorded from 13 WT, 12 HTT-SD, and 12 HTT-SA MSNs from at least N=3 mice.
-
Figure 3—source data 1
Data analyzed for short-term plasticity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig3-data1-v3.xlsx
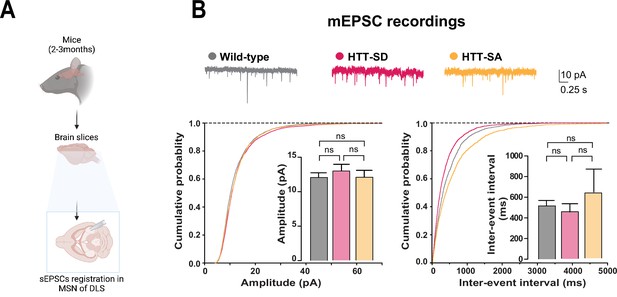
HTT phosphorylation does not regulate the spontaneous excitatory postsynaptic currents (sEPSCs) in the corticostriatal synapse.
(A) Schematic of the procedure for sEPSC recording in medium-sized spiny neurons (MSNs) within the dorsolateral striatum (DLS). (B) Representative traces, cumulative probability of the mean amplitude, and mean interevent intervals (p<0.05) of sEPSCs in MSNs within the DLS in 2- to 3-month-old wild-type (WT) and HTT-SD mice. sEPSCs were recorded from WT (10 MSN) and HTT-SD mice (10 MSN). There were no significant differences in cumulative probability between WT, HTT-SD, and HTT-SA mice (ns: non-significant).
-
Figure 3—figure supplement 1—source data 1
Data analyzed for mEPSC recordings.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig3-figsupp1-data1-v3.xlsx
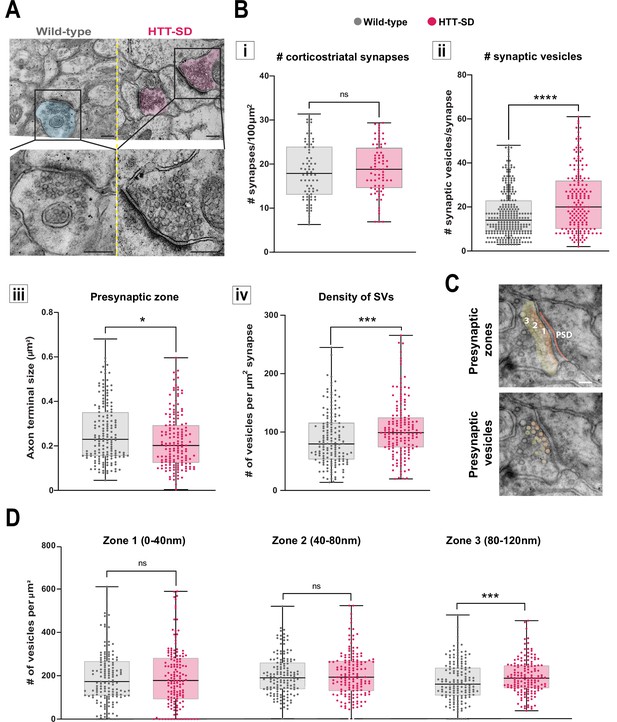
HTT phosphorylation increases the number of synaptic vesicles (SVs) distally to the presynaptic active zone.
(A) Representative images of SVs at the corticostriatal synapse, obtained by electronic microscopy, in dorsolateral striatum (DLS) slices from three wild-type (WT) and HTT-SD mice 3-month-old male. Scale = 200 nm. (B) Quantification of (i) the number of synapses at the corticostriatal synapse per 100 µm2 in DLS on n=74 WT and 74 HTT-SD striatal areas (ns: non-significant), (ii) the number of SVs per corticostriatal synapse from five WT and three HTT-SD mouse brains (N=279 WT and 171 HTT-SD axon terminals; ****p<0.0001), (iii) size of the cortical axon terminal in 158 WT and 156 HTT-SD corticostriatal synapses (*p<0.05), and (iv) the density of SVs within these axon terminals (number of vesicles per μm2) in N=157 WT and 162 HTT-SD corticostriatal synapses (***p<0.001). (C) Representative images showing the 40-nm-wide zones in the axon terminal. Zone 1 is the closest to the synaptic cleft and contains the active zone. Zone 2 (40–80 nm) is adjacent to zone 1, and zone 3 (80–120 nm) is farthest from the active zone. Dark orange denotes the PSD within the striatal postsynaptic element. Scale = 100 nm. (D) The number of SVs per zone within the distal 120 nm of the axon terminal in at least 149±2 axon terminals (ns: non-significant, *p<0.05). The box-whisker plots show the median, the 25th and the 75th percentiles, the smallest and the largest value from at least three brains for each condition. Significances were determined using the Mann-Whitney test.
-
Figure 4—source data 1
Data analyzed for the number of the corticostriatal synapses (i).
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-data1-v3.xlsx
-
Figure 4—source data 2
Data analyzed for the number of the synaptic vesicles (ii).
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-data2-v3.xlsx
-
Figure 4—source data 3
Data analyzed for the number of the axon terminal size in the presynaptic zone.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-data3-v3.xlsx
-
Figure 4—source data 4
Data analyzed for the density of synaptic vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-data4-v3.xlsx
-
Figure 4—source data 5
Data analyzed for the number of vesicles per zone.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-data5-v3.xlsx
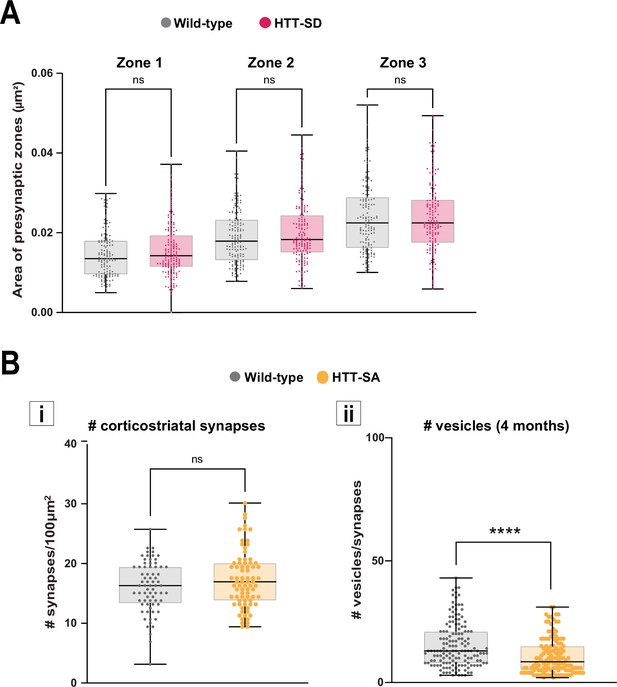
Characterization of the three presynaptic zones of HTT-SD corticostriatal axon terminals and analysis of HTT-SA corticostriatal synapses.
(A) Area of the three zones in wild-type (WT) and HTT-SD axon terminals studied in 145±9 axon terminals from three WT and HTT-SD 3-month-old mice (ns: non-significant, two-way ANOVA followed by Dunn’s multiple comparisons test). (B) Left (i). The number of corticostriatal synapses in 72 WT and 77 HTT-SA striatal areas from three WT and HTT-SA 4-month-old mice (ns: non-significant, Mann-Whitney). Right (ii). The number of vesicles at 4 months in 148 WT and 130 HTT-SA corticostriatal axon terminals (****p<0.0001, Mann-Whitney). The box-whisker plots show the median, the 25th and the 75th percentiles, using at least three brains of each genotype.
-
Figure 4—figure supplement 1—source data 1
Data analyzed for the area of the presynaptic zones.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-figsupp1-data1-v3.xlsx
-
Figure 4—figure supplement 1—source data 2
Data analyzed for the the number of the corticostriatal synapses (i).
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-figsupp1-data2-v3.xlsx
-
Figure 4—figure supplement 1—source data 3
Data analyzed for the number of the synaptic vesicles (ii).
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig4-figsupp1-data3-v3.xlsx
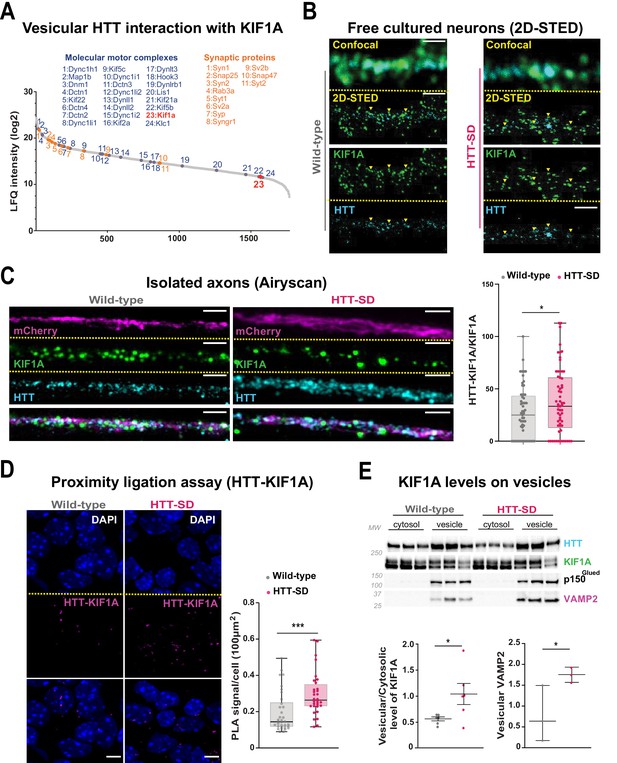
HTT phosphorylation recruits KIF1A on VAMP2-mCherry vesicles.
(A) Mass spectrometry analysis of vesicles purified from mouse brains identifies KIF1A (red) among HTT-associated vesicular proteins. (B) Confocal and two-dimensional stimulated emission depletion (2D-STED) images of free-cultured neurons at day in vitro (DIV) 5 showing the colocalization of KIF1A and HTT. Scale bar: 1 μm. (C) Representative immunofluorescence labeling revealing HTT (cyan), KIF1A (green), and VAMP2-mCherry (magenta) within wild-type (WT) and HTT-SD cortical axons in the long channels of the microfluidic devices. The images were acquired in a specific region of interest and processed by an Airyscan detector (scale bar: 1 µm). Distribution analysis shows that HTT and KIF1A were more likely to colocalize on KIF1A+ vesicles in the HTT-SD condition. The graph represents means ± SEM of three independent experiments reproducing a corticostriatal network of WT or HTT-SD neurons in at least three microfluidic devices per experiment. Significance determined by the Mann-Whitney test (*p<0.05; N=61). (D) Proximity ligation assay (PLA) in WT or HTT-SD neurons, nuclei stained with DAPI. Representative images are from three independent experiments. Scale bar: 10 µm. Significance was determined by the Mann-Whitney test (***p<0.0001; N=32–34). (E) Western blot analysis for HTT, KIF1A (both bands), p150Glued, and tubulin from vesicular fractions from six WT and six HTT-SD brains. Significance was determined using the Mann-Whitney test (*p<0.05).
-
Figure 5—source data 1
Data analyzed for HTT-KIF1A colocalization in axons.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-data1-v3.xlsx
-
Figure 5—source data 2
Data analyzed for the proximity ligation assay performed between HTT and KIF1A.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-data2-v3.xlsx
-
Figure 5—source data 3
Data analyzed for the protein content of KIF1A and VAMP2 levels in vesicular fractions.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-data3-v3.xlsx
-
Figure 5—source data 4
Western blot scans for the data presented in Figure 5E (KIF1A and VAMP2 levels in brain vesicular fractions).
Shown in red are the cropped regions presented in Figure 5E. Films containing the second batch of samples (Gel 2) are shown.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-data4-v3.zip
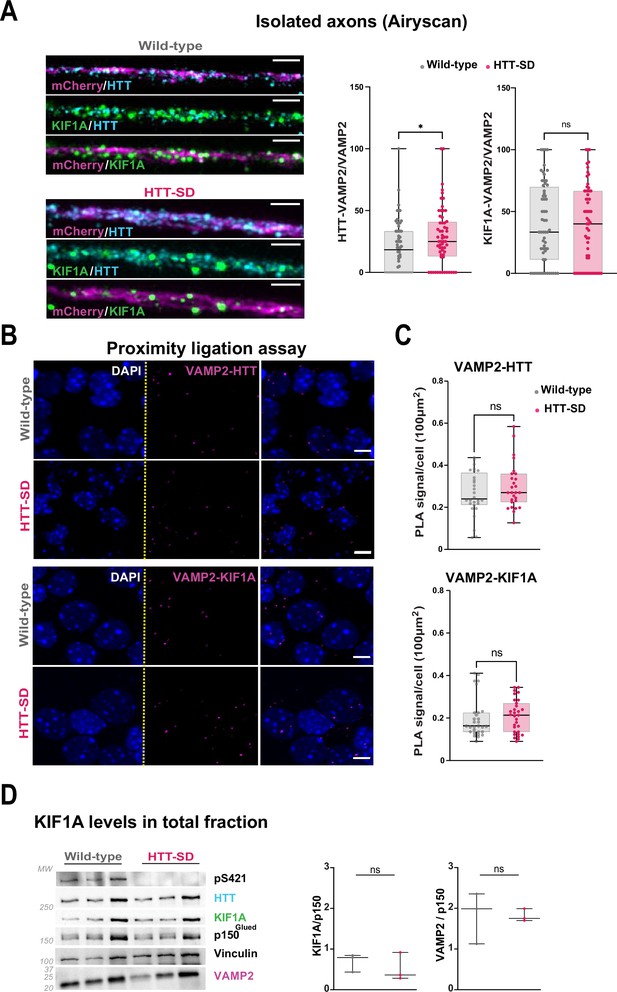
HTT phosphorylation and subcellular localization and interaction of HTT and KIF1A with VAMP2.
(A) Representative immunostainings revealing HTT (cyan), KIF1A (green), and VAMP2-mCherry (magenta) within cortical axons localized in the long channels of the microfluidic devices. The images were acquired in a specific region of interest and processed by an Airyscan detector (scale bar: 1 µm). The distribution analysis shows that there is greater colocalization of HTT and KIF1A on KIF1A+ vesicles in the HTT-SD condition compared to wild-type (WT) neurons. The graph represents means ± SEM of three independent experiments reconstituting corticostriatal networks of WT or HTT-SD neurons in at least three microfluidic devices per experiment. Significance was determined using the Mann-Whitney test (ns: non-significant; n=61). (B) Western blot analysis for HTT, HTT S421 phosphorylation, KIF1A (both bands), p150Glued, and tubulin from vesicular fractions of N=3 WT and 3 HTT-SD brains. Significance was determined using the Mann-Whitney test (ns: non-significant). As previously reported, HTT-SD is not recognized by the anti-pS421-HTT antibody. (C–D) Proximity ligation assay (PLA) in WT and HTT-SD neurons. Nuclei were stained with DAPI. Shown are representative images from three independent experiments. Scale bar: 10 µm. Significance was determined using the t-test (ns: non-significant; N=29–31).
-
Figure 5—figure supplement 1—source data 1
Data analyzed for HTT-VAMP2 colocalization in axons.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data1-v3.xlsx
-
Figure 5—figure supplement 1—source data 2
Data analyzed for KIF1A-VAMP2 colocalization in axons.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data2-v3.xlsx
-
Figure 5—figure supplement 1—source data 3
Data analyzed for the proximity ligation assay performed between VAMP2/HTT.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data3-v3.xlsx
-
Figure 5—figure supplement 1—source data 4
Data analyzed for the proximity ligation assay performed between VAMP2/KIF1A.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data4-v3.xlsx
-
Figure 5—figure supplement 1—source data 5
Data analyzed for the protein content of KIF1A and VAMP2 levels in total fractions.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data5-v3.xlsx
-
Figure 5—figure supplement 1—source data 6
Western blot scans for the data presented in Figure 5—figure supplement 1D (KIF1A levels in whole brain lysates).
Shown in red are the cropped regions presented in Figure 5—figure supplement 1D. Films containing the first batch of samples (Gel 1) are shown.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig5-figsupp1-data6-v3.zip
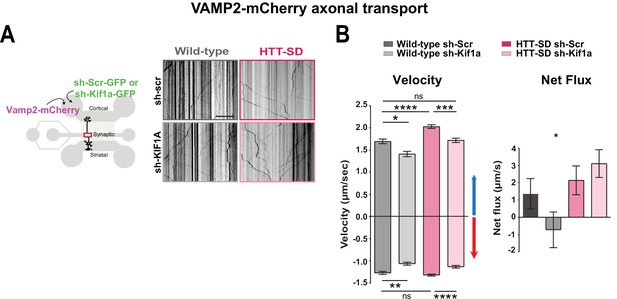
HTT-dependent axonal transport of synaptic vesicle precursors (SVPs) is mediated by KIF1A.
(A) Diagram indicating lentiviral transduction of VAMP2-mCherry and sh-scramble (sh-Scr-GFP) or sh-Kif1a (sh-Kif1a-GFP) lentiviruses at day in vitro (DIV) 8 in the microfluidic device. On the right, representative kymographs of VAMP2-mCherry vesicle transport in axons for each condition. Scale bar = 25 µm. (B) Segmental anterograde and retrograde velocities (anterograde: *p<0.05, ***p<0.001, ****p<0.0001; N=548 vesicles wild-type [WT] sh-Scr, 318 vesicles WT sh-Kif1a, 1129 vesicles HTT-SD sh-Scr, 628 vesicles HTT-SD sh-Kif1a) (retrograde: *p<0.05, **p<0.01, ****p<0.0001; N=583 vesicles WT sh-Scr, 396 vesicles WT sh-Kif1a, 1282 vesicles HTT-SD sh-Scr, 620 vesicles HTT-SD sh-Kif1a) and directional net flux (*p<0.01; N=79 axons WT sh-Scr, 59 axons WT sh-KIFA,112 axons HTT-SD sh-Scr, 89 axons HTT-SD sh-Kif1a; one-way ANOVA test) of VAMP2-mCherry vesicles in WT and HTT-SD neurons transduced with sh-Scr or sh-Kif1a lentiviruses. Histograms represent means ± SEM of three independent experiments. Significance was determined using a one-way ANOVA followed by Dunn’s multiple comparison test.
-
Figure 6—source data 1
Data analyzed for anterograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-data1-v3.xlsx
-
Figure 6—source data 2
Data analyzed for retrograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-data2-v3.xlsx
-
Figure 6—source data 3
Data analyzed for net flux.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-data3-v3.xlsx
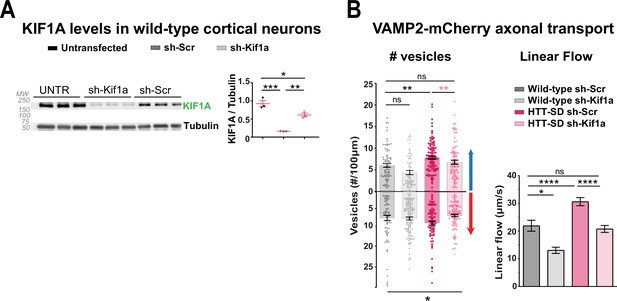
KIF1A levels in HTT-SD neurons regulate VAMP2 axonal transport.
(A) Analysis of KIF1A levels by western blot in cortical neurons either not-transduced or transduced with sh-Kif1a or sh-Scr lentiviruses (*p<0.05, **p<0.01, ***p<0.001; one-way ANOVA followed by Dunn’s multiple comparisions test). Histograms represent the means ± SEM. (B) Number of anterograde (*p<0.05, **p<0.01; n=76 axons wild-type [WT] sh-Scr, 59 axons WT sh-Kif1a, 110 axons HTT-SD sh-Scr, and 86 axons HTT-SD sh-Kif1a) and retrograde (*p<0.05; n=60 WT sh-Scr axons, 114 WT sh-Kif1a axons, 79 HTT-SD sh-Scr axons, and 95 HTT-SD sh-Kif1a axons) VAMP2-mCherry axonal vesicles along 100 µm of axon in WT and HTT-SD and their linear flow rate (*p<0.05; 75 WT sh-Scr axons, 59 WT sh-Kif1a axons, 107 HTT-SD sh-Scr axons, and 85 HTT-SD sh-Kif1a axons; one-way ANOVA followed by Dunn’s test). Histograms represent the means ± SEM of at least three independent experiments.
-
Figure 6—figure supplement 1—source data 1
Western blot scans for the data presented in Figure 6—figure supplement 1A (KIF1A levels in cortical neurons).
Shown in red are the cropped regions presented in Figure 6—figure supplement 1A. Films containing the samples are shown.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp1-data1-v3.zip
-
Figure 6—figure supplement 1—source data 2
Data analyzed for number of anterograde vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp1-data2-v3.xlsx
-
Figure 6—figure supplement 1—source data 3
Data analyzed for number of retrograde vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp1-data3-v3.xlsx
-
Figure 6—figure supplement 1—source data 4
Data analyzed for the linear flow rate.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp1-data4-v3.xlsx
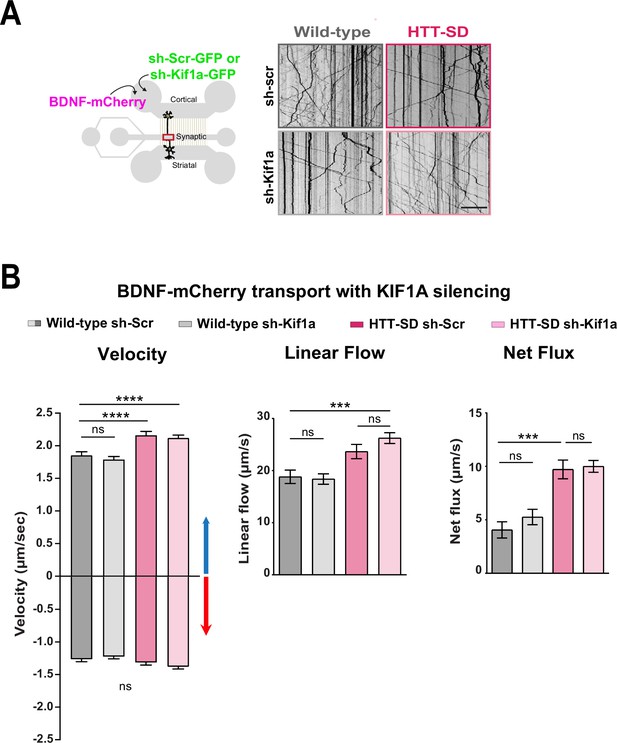
KIF1A silencing doesn’t affect BDNF-mCherry transport.
(A) Diagram indicating transduction of BDNF-mCherry and sh-scramble (sh-scr-GFP) or sh-Kif1a (sh-Kif1a-GFP) lentiviruses (left). Representative kymographs of BDNF-mCherry vesicle transport within wild-type (WT) or HTT-SD axons transduced with sh-Scr or sh-Kif1a at day in vitro (DIV) 8. Scale bar = 25 µm. (B) Segmental anterograde (****p<0.0001; n=618 WT sh-Scr vesicles, 901 WT sh-Kif1a vesicles, 1735 HTT-SD sh-Scr vesicles, and 2830 HTT-SD sh-Kif1a vesicles) and retrograde velocities. There were no significant differences between genotypes in the retrograde segmental velocities and KIF1A silencing conditions (ns = non-significant), linear flow (***p<0.001; 75 WT sh-Scr axons, 102 WT sh-Kif1a axons, 114 HTT-SD sh-Scr axons, and 191 HTT-SD sh-Kif1a axons), or net flux (***p<0.001; n=75 WT sh-Scr axons, 103 WT sh-Kif1a axons, 123 HTT-SD sh-Scr axons, and 186 HTT-SD sh-Kif1a axons) of BDNF-mCherry vesicles. Histograms represent means ± SEM of three independent experiments. Significance was determined using one-way ANOVA followed by Dunn’s multiple comparisons test.
-
Figure 6—figure supplement 2—source data 1
Data analyzed for for anterograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp2-data1-v3.xlsx
-
Figure 6—figure supplement 2—source data 2
Data analyzed for for retrograde velocity.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp2-data2-v3.xlsx
-
Figure 6—figure supplement 2—source data 3
Data analyzed for the linear flow rate.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp2-data3-v3.xlsx
-
Figure 6—figure supplement 2—source data 4
Data analyzed for the net flux.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig6-figsupp2-data4-v3.xlsx
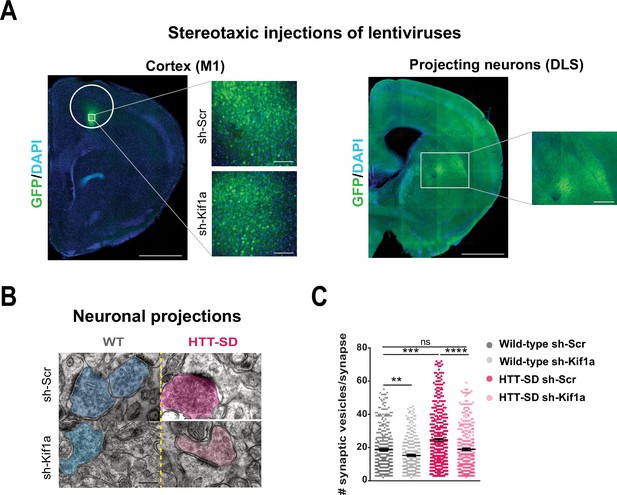
In vivo KIF1A silencing in mice restores the synaptic vesicle (SV) synaptic pool.
(A) Immunolabeling of GFP within the injection site on a slice located at 1.5 mm before the bregma (left). Scale = 1 cm (insets, 100 µm). Immunolabeling of GFP within the projection site on a slice located at –0.3 mm after the bregma (right). Scale = 1 cm (inset, 250 µm). Nuclei are labeled with DAPI. (B) Representative images from electron microscopy of corticostriatal synapses and (C) quantification of the number of SVs at the corticostriatal synapse of three wild-type (WT) male mice injected with either sh-Scr or sh-Kif1a and three HTT-SD mice injected with sh-Scr or sh-Kif1a (**p<0.01, ***p<0.001, ****p<0.0001; N=360 WT sh-Scr synapses, 324 WT sh-Kif1a synapses, 417 HTT-SD sh-Scr synapses, 337 HTT-SD sh-Kif1a synapses). Scale = 200 nm. Histograms represent means ± SEM. Significance was determined using one-way ANOVA followed by Dunn’s multiple comparison test.
-
Figure 7—source data 1
Data analyzed for the number of the synaptic vesicles.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig7-data1-v3.xlsx
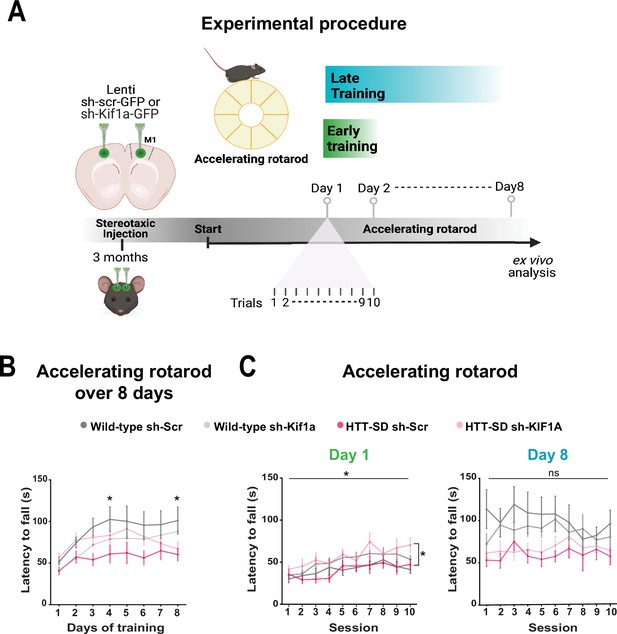
Motor skill learning defects of S421D mice are rescued by KIF1A silencing in vivo.
(A) Schematic of the experimental procedure consisting in bilateral stereotaxic injections in the mouse brain followed 3 weeks later by the accelerating rotarod protocol over 8 days. (B) Mean time to fall off the rotarod per day over 8 days. Two-way ANOVA comparing the four conditions showed significant differences between genotypes and silencing conditions (****p<0.0001). Holm-Sidak’s post hoc analysis revealed significant differences between wild-type (WT) sh-Scr and HTT-SD sh-Scr mice at day 4 and day 8 (*p<0.05). (C) Mean time to fall off the rotarod the first day (*p<0.01) (left) and the last day (ns: non-significant, right), per sessions. Holm-Sidak’s post hoc analysis revealed significant differences between HTT-SD sh-Scr and HTT-SD sh-Kif1a mice during the first day (*p<0.05). At least three cohorts containing 12 WT sh-Scr, 11 WT sh-Kif1a, 10 HTT-SD sh-Scr, and 12 HTT-SD sh-Kif1a 3-month-old littermate male mice were analyzed.
-
Figure 8—source data 1
Data analyzed for accelerating rotarod over 8 days.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig8-data1-v3.xlsx
-
Figure 8—source data 2
Data analyzed for accelerating rotarod during day 1.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig8-data2-v3.xlsx
-
Figure 8—source data 3
Data analyzed for accelerating rotarod during day 8.
- https://cdn.elifesciences.org/articles/81011/elife-81011-fig8-data3-v3.xlsx





