Structure of Geobacter OmcZ filaments suggests extracellular cytochrome polymers evolved independently multiple times
Figures
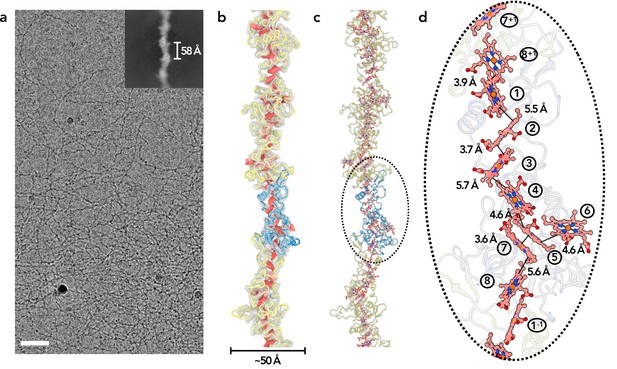
Cryo-EM of OmcZ filaments.
(a) Cryo-EM image of the purified OmcZ filaments from ΔomcS G. sulfurreducens strain grown on electrodes. The sample was treated with DNase I prior to freezing. Scale bar = 50 nm. The upper right is a two-dimensional class average of the OmcZ filament, showing the rise of 58 Å between adjacent subunits. (b) The cryo-EM reconstruction (transparent gray) with backbone trace of the OmcZ subunits. The density of heme molecules is shown in red. One OmcZ subunit is colored in blue and the rest are yellow. (c) The atomic model of OmcZ filaments. The protein backbone trace is shown and the heme molecules are shown in ball and stick representation. (d) A zoomed region to show the heme array in OmcZ, with the minimum observed edge-to-edge distances indicated between adjacent porphyrin rings. Heme molecules are labeled with numbers in circles, The superscripts ‘+1’ and ‘–1’ indicate protein subunits above and below the central subunit, respectively.
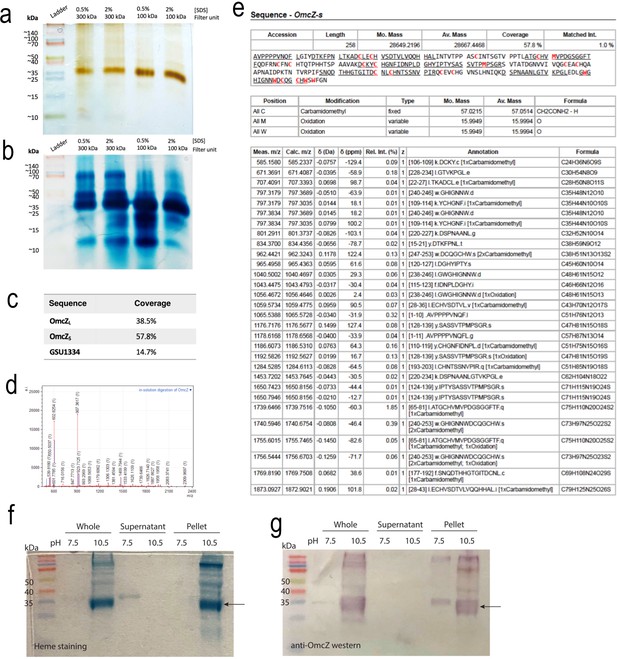
Isolation of OmcZ filaments.
(a) Silver stain and (b) heme stain of filament preparations after dialysis. (c) Coverage of OmcZ forms and related GSU1334 protein by tandem mass spectrometry(MS/MS) after dialysis with 300 kDa filter. (d) MALDI-TOF of preparation and (e) detected peptides within the heme domain (‘OmcZs’) of OmcZ. (f) Heme stain showing effect the of pH 7.5 Tris vs. pH 10.5 ethanolamine buffer on raw filament recovery from biofilms prior to further enrichment, dialysis, or purification from sheared biofilms, and (g) Western blot using anti-OmcZ antibodies of the same preparations. Arrow indicates a region consistent with OmcZs. Raw, uncropped images are provided for the gels in (a ,b, f and g) as Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Raw data for panels a, b, f and g.
- https://cdn.elifesciences.org/articles/81551/elife-81551-fig1-figsupp1-data1-v2.zip
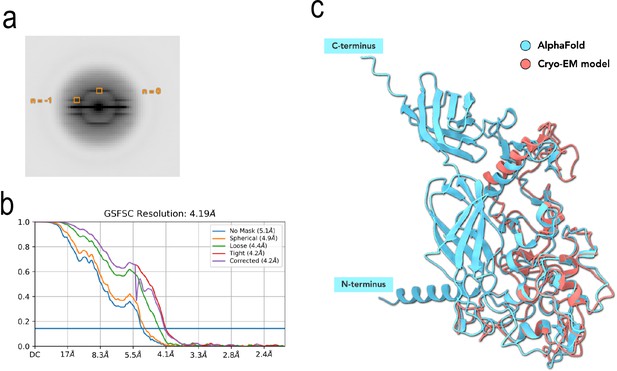
Cryo-EM analysis of the OmcZ filaments.
(a) Averaged power spectra from OmcZ filaments. (b) The map:map Fourier shell correlation (FSC) calculation of OmcZ filament (0.143 cutoff). (c) Cryo-EM model of single OmcZ subunits (red), aligned by the full-length OmcZ prediction, generated by AlphaFold (cyan). The AlphaFold predicted subunit contains tandem Ig-domains at the C-terminus that are cleaved and not present in the filament.
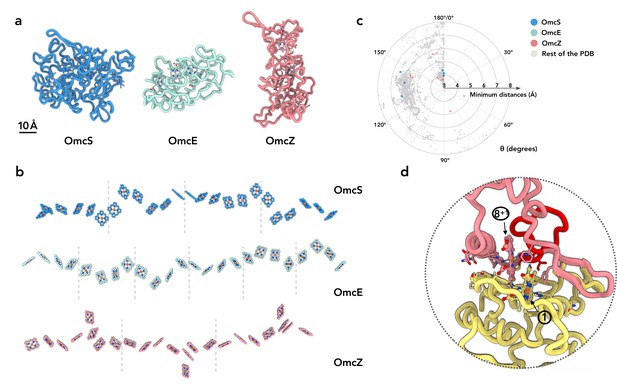
OmcZ differs from OmcS/OmcE in protein fold, heme arrangements and coordination.
(a) The protein fold of OmcS, OmcE, and OmcZ. (b) The heme arrays of OmcS, OmcE, and OmcZ. Dashed lines indicate the separation of cytochrome protein subunits. (c) The heme-heme orientation plot. One heme can be aligned to the adjacent heme by a rotation and a translation. Only 25 non-hydrogen atoms in the porphyrin ring are used in the alignment. The heme (ID: HEC or HEM) pairs in all PDB structures were analyzed. The minimum distances refer to the smallest distance between two porphyrin rings, regardless of the atom type. The angle θ was determined from the alignment rotation matrix between heme pairs. For example, θ = 0° means two porphyrin rings are perfectly parallel, while θ = 180° means two porphyrin rings are perfectly antiparallel (flipped). All porphyrin rings pairs with a minimum distance less than or equal to 6 Å are shown. The porphyrin ring pairs in the OmcS, OmcE, and OmcZ filaments are highlighted in blue, green, and red, respectively. (d) The subunit-subunit interface in the OmcZ filament. The residues between C77 and H93 are highlighted in red. Heme molecules are labeled with numbers in circles.
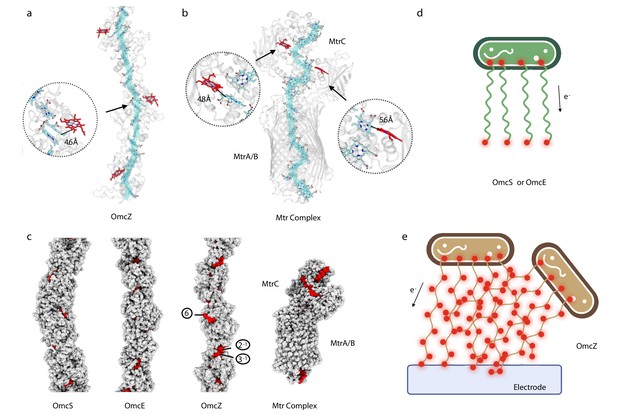
OmcZ filaments possess solvent-accessible hemes.
(a) OmcZ and (B) Mtr complex (PDB 6R2Q). The main heme chain is traced in cyan while the solvent-accessible hemes are shown in red. The protein backbones are shown in transparent cartoon representation. The branched heme in OmcZ is 4.6 Å away from a heme in the linear chain, (a) while the two branched hemes in MtrC are 4.8 Å and 5.6 Å away from hemes in the linear chain (b). (c) Atomic models of OmcS filament, OmcE filament, OmcZ filament, and outer membrane-spanning protein complex MtrABC. Models without hydrogens are shown with atoms represented by spheres having the appropriate van der Waals radii. The protein residues are colored in gray while heme molecules are in red. The (d) and (e) schematic models of Geobacter cells producing different type of nanowires (OmcS or OmcE in (d) and OmcZ in (e)). Red dots indicate solvent-accessible heme molecules.
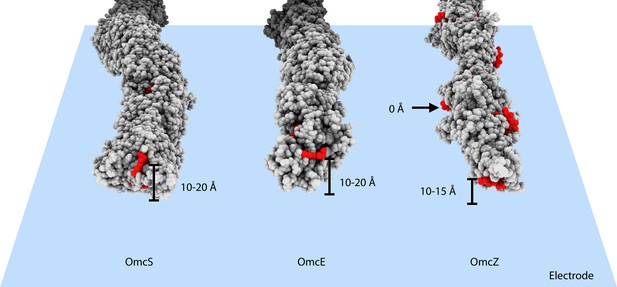
Heme arrangements may affect conductivity measurements.
The atomic structures of OmcS, OmcE, and OmcZ filaments are shown. All protein atoms are colored in gray, while heme molecules are colored in red. When those filaments touch an electrode surface, shown as a blue plane, the estimated heme-to-surface distances are labeled. The possible tip proteins on these cytochrome filaments are not considered here.
Tables
Cryo-EM and refinement statistics of OmcZ filaments.
| Parameter | OmcZ filament |
|---|---|
| Data collection and processing | |
| Voltage (kV) | 300 |
| Electron exposure (e− Å−2) | 48 |
| Pixel size (Å) | 1.08 |
| Particle images (n) | 92,170 |
| Shift (pixel) | 60 |
| Helical symmetry | |
| Point group | C1 |
| Helical rise (Å) | 58.1 |
| Helical twist (°) | –158.2 |
| Map resolution (Å) | |
| Map:map Fourier shell correlation (FSC, 0.143) | 4.2 |
| Model:map FSC (0.5) | 4.4 |
| Refinement and model validation | |
| Ramachandran favored (%) | 88.0 |
| Ramachandran outliers (%) | 0.0 |
| Real space correlation coefficient | 0.80 |
| Clashscore | 22.1 |
| Bonds RMSD, length (Å) | 0.006 |
| Bonds RMSD, angles (°) | 0.990 |
| Deposition ID | |
| Protein Data Bank (model) | 8D9M |
| Electron Microscopy Data Bank (map) | EMD-27266 |





