Efficacy of ultra-short, response-guided sofosbuvir and daclatasvir therapy for hepatitis C in a single-arm mechanistic pilot study
Figures
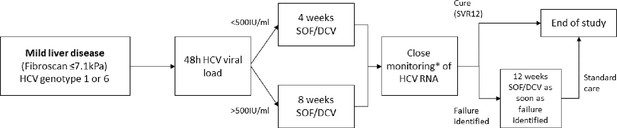
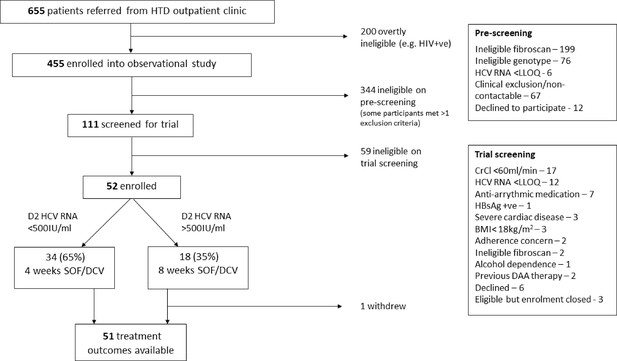
Study design.
*HCV RNA on days 0, 1, 2, 7, 10, 14, 17, 21, 24, 28, (42, 56), EOT +3, EOT +7, EOT +10, EOT +14, EOT +17, EOT +21, EOT +24, EOT +28s, EOT +56, EOT +84.
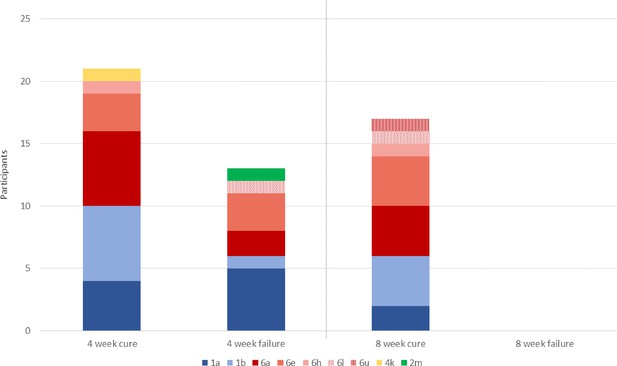
Primary outcome, with HCV subtypes (n=51).
All 13 individuals who experience treatment failure with 4-week SOF/DCV were cured with 12-week SOF/DCV retreatment.
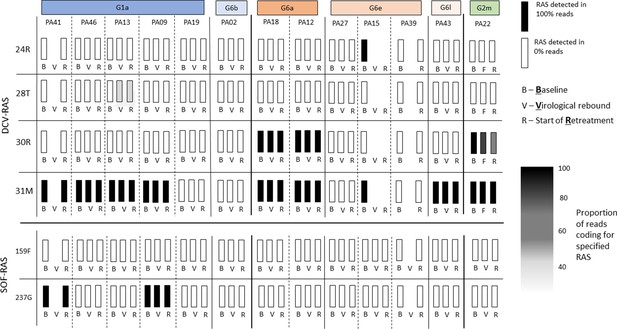
Sofosbuvir RAS and Daclatasvir RAS at baseline, treatment failure, and at start of retreatment in all participants who failed first-line treatment.
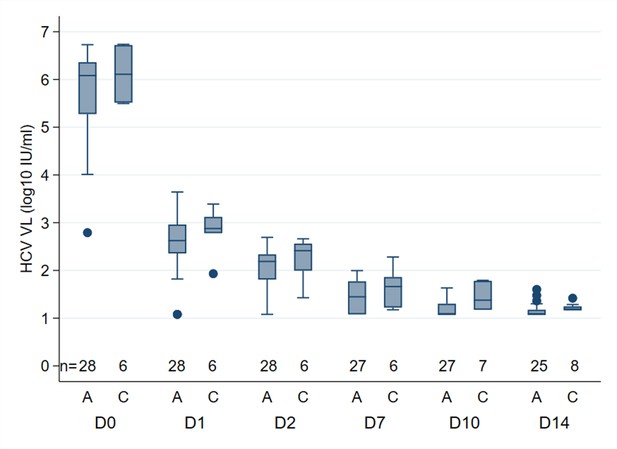
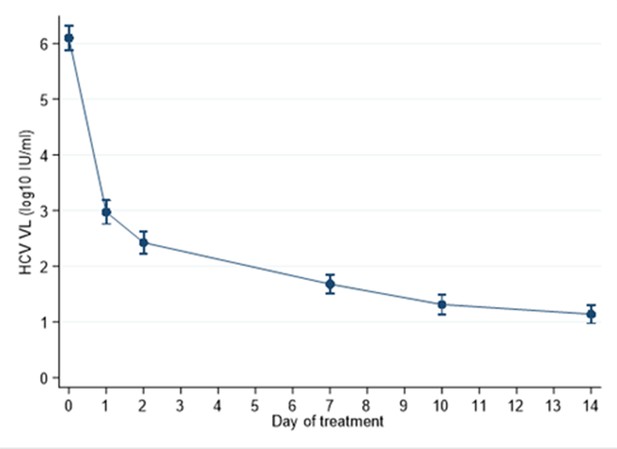
Median HCV RNA (log10), by PCR assay, at different time points in participants treated with 4 weeks SOF/DCV A = Abbott Architect (LLOQ = 12 IU/ml).
C=COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, version 2.0 (Roche Molecular Systems, LLOQ = 15 IU/ml)
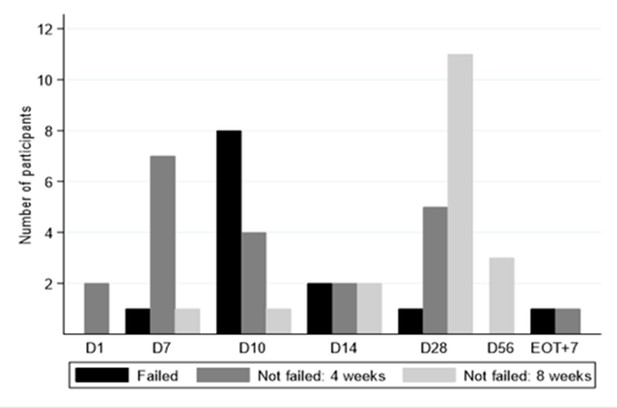
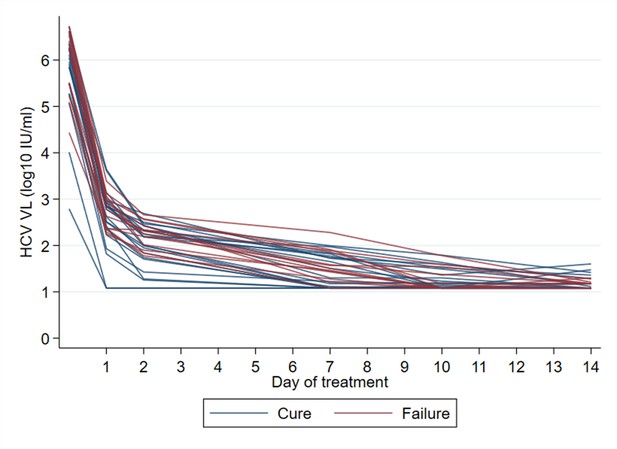
Time to viral suppression <LLOQ and eventual treatment outcome.
*No treatment failures in 8 week arm. D28 is the EOT visit for those who received 4 weeks. D56 visit is the EOT visit for those who received 8 weeks.
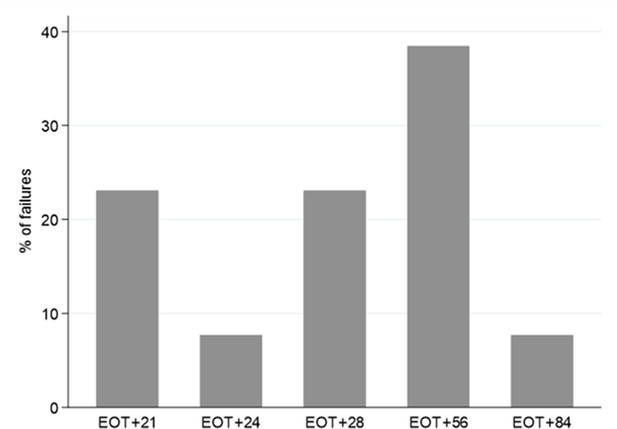
Timing of treatment failure (confirmed HCV VL >2000 IU/mL) (n=13).
*Note twice weekly sampling in first 4 weeks after EOT, monthly thereafter.
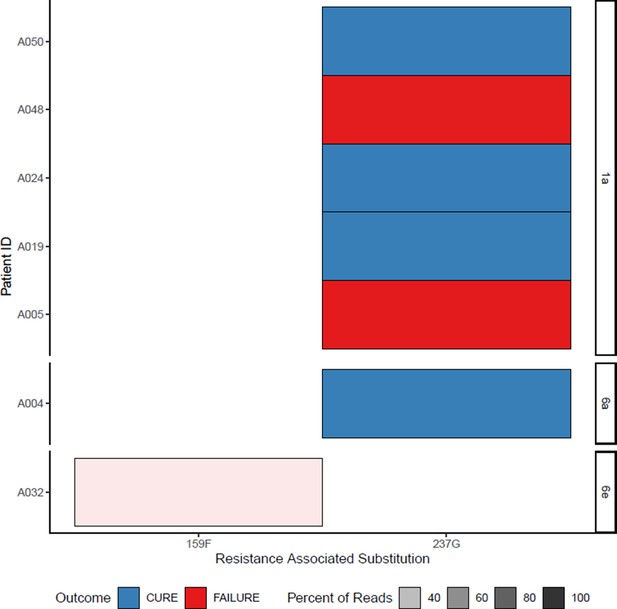
All sofosbuvir resistance-associated substitutions at baseline (with treatment outcome).
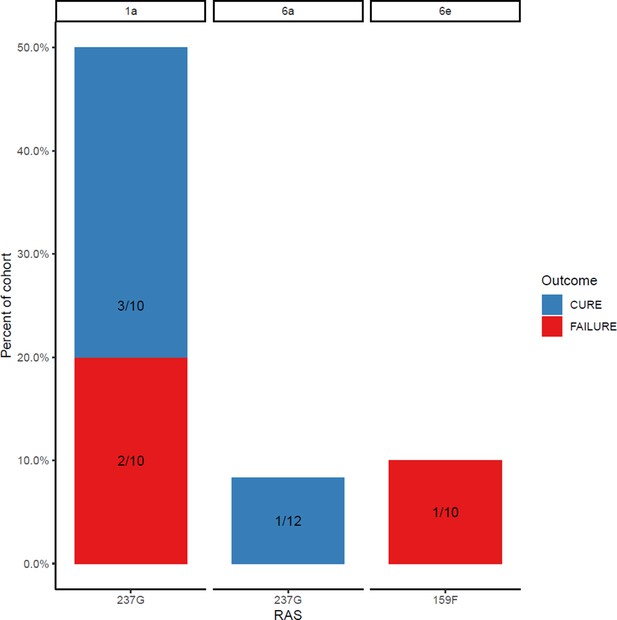
Proportion of each subtype with sofosbuvir resistance-associated substitutions at baseline (with treatment outcome).
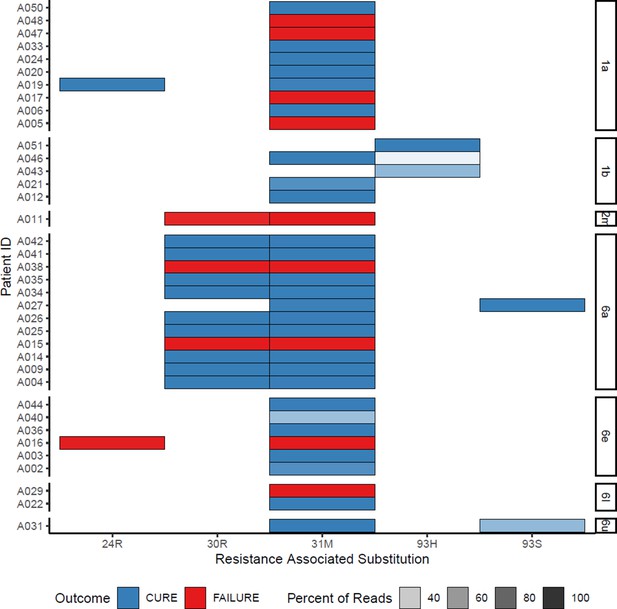
All daclatasvir resistance-associated substitutions at baseline (with treatment outcome).
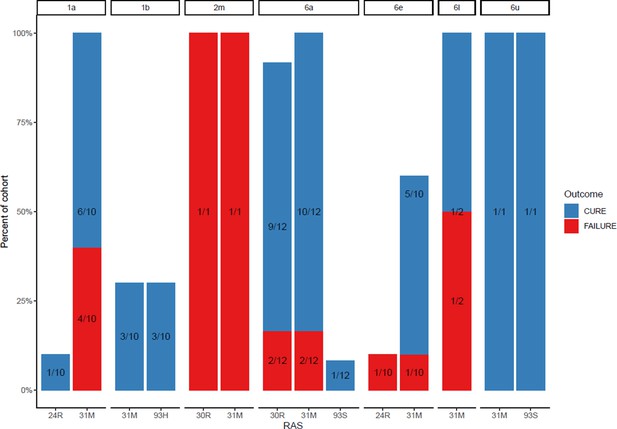
Proportion of each subtype with daclatasvir resistance-associated substitutions at baseline (with treatment outcome).
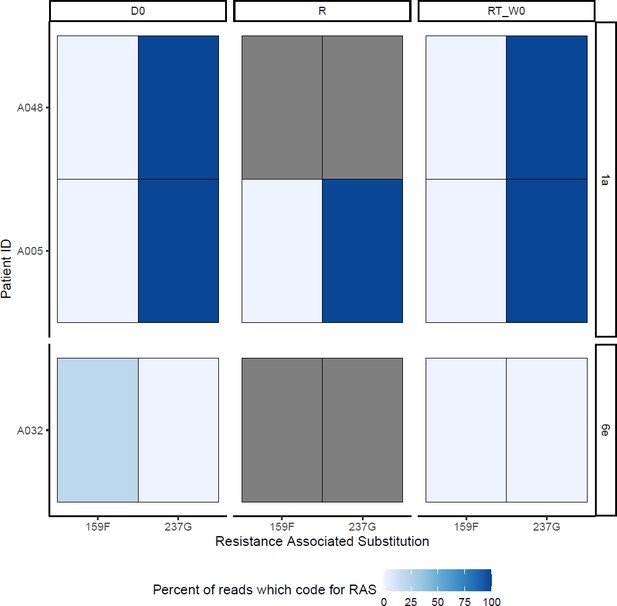
SOF-RAS at baseline (D0), time of virological rebound (R) and start of retreatment (RT_W0) Grey boxes represent missing data.
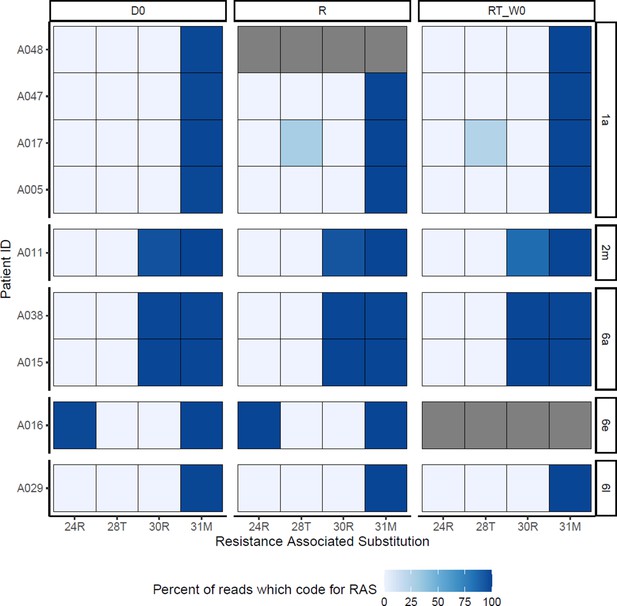
DCV-RAS at baseline (D0), time of virological rebound (R) and start of retreatment (RT_W0)Grey boxes represent missing data.
Tables
Baseline characteristics.
| N/ median | %/range | |
|---|---|---|
| Total participants | 52 | |
| Age in years | 49.5 | (25.0, 67.0) |
| Female | 29 | (56%) |
| Body-mass index in kg/m2 | 23.3 | (18.7, 30.6) |
| Genotype 1 | 22 | (43%) |
| 1a | 11 | |
| 1b | 12 (1 withdrew) | |
| Genotype 6 | 27 | (53%) |
| 6a | 12 | |
| 6e | 10 | |
| 6h | 2 | |
| 6l | 2 | |
| 6u | 1 | |
| Genotype 2(m) | 1 | |
| Genotype 4(k) | 1 | |
| Baseline HCV viral load in IU/ml | 1,932,775 | (618, 11,200,000) |
| HCV viral load – log10 IU/ml (range) | 6.3 | (2.8, 7.0) |
| Past medical history: | ||
| Illicit drug use | 4 | (8%) |
| Alcohol dependence (historic; current excluded) | 4 | (8%) |
| Diabetes | 2 | (4%) |
| Hypertension | 7 | (13%) |
| Ischaemic heart disease | 1 | (2%) |
| Tuberculosis | 2 | (4%) |
| Current smoker | 18 | (35%) |
| Previous spontaneous clearance of HCV with re-infection | 2 | (4%) |
Treatment outcome.
| N/median | %/range | |
|---|---|---|
| Detectable HCV viral load (HCV VL) at day 2 | 50 | 96% |
| Abbott | 39/41 | 95% |
| COBAS | 11/11 | 100% |
| Median (IQR) HCV VL at day 2 in IU/ml | 269 | (104, 690) |
| Abbott | 217 | (101, 690) |
| COBAS | 459 | (209, 832) |
| Below threshold—for 4-week therapy | 34 | (65%) |
| Abbott | 31 | (66%) |
| COBAS | 3 | (60%) |
| Above threshold—for 8-week therapy | 18 | (35%) |
| Abbott | 16 | (34%) |
| COBAS | 2 | (40%) |
| Mean (SD) duration of first-line therapy received in days | 37 | (13.7) |
| Mean (SD) duration of all therapy received in days | 58 | (34.2) |
| Median weeks from enrolment to last visit (range) | 20 | (1, 42) |
| Primary outcome | ||
| Outcome available | 51 | |
| SVR12 by intention-to-treat analysis and per protocol analysis | 38 | (75% [95% CI 63, 86]) |
| SVR12 by sensitivity analysis (i) [missing results = failure] | 38 | (73% [95% CI 61, 85]) |
| SVR12 by post hoc analysis (ii) [G1 and G6 only] | 37 | (76% [95% CI 63, 88]) |
| Secondary endpoints | ||
| Lack of initial virological response | 0 | (0% [97.5% CI 0, 7]) |
| Serious adverse events | 0 | (0% [97.5% CI 0, 7]) |
| Grade 3/4 clinical adverse events | 0 | (0% [97.5% CI 0, 7]) |
| Non-serious adverse reactions | 18 | (35% [95% CI 22, 48]) |
| Adverse events or reactions leading to change in study medication | 0 | (0% [97.5% CI 0, 7]) |
-
Where not labelled, data presented as n (%; 97.5% confidence interval).
Comparison of baseline factors, drugs levels and virological response in individuals failed to achieve SVR12 with 4-week therapy versus those who cured with 4- or 8-week therapy.
| 4-week cures (n=21) | 4-week failures (n=13) | p | 8-week cures (n=17) | |
|---|---|---|---|---|
| Host factors | ||||
| Male (%) | 62% | 38% | 0.18 | 29% |
| Mean age | 45 | 48 | 0.23 | 55 |
| Mean BMI | 23 | 23 | 0.40 | 24 |
| Median ALT | 54 | 36 | 0.10 | 31 |
| Median AST | 34 | 28 | 0.44 | 33 |
| IFNL4 delG/TT and TT/TT genotypes (rs368234815) | 71% | 58% | 0.47 | 69% |
| Virus factors | ||||
| Median D0 HCV VL | 916,000 | 2,139,258 | 0.20 | 4,982,889 |
| Abbott | 960,913 | 1,972,841 | 0.47 | 4,625,118 |
| COBAS | 916,000 | 5,260,000 | 0.40 | 4,605,000 |
| D2 VL<LLOQ | 2/21 (10%) | 0/13 (0%) | 0.51 | 0% |
| Abbott | 2/18 (11%) | 0/10 (0%) | 0.41 | 0/13 (0%) |
| COBAS | 0/3 (0%) | 0/3 (0%) | – | 0/5 (0%) |
| D7 VL<LLOQ | 9/21 (43%) | 1/12 (8%)* | 0.054 | 0% |
| Abbott | 8/18 (44%) | 1/9 (11%) | 0.09 | 0/13 (0%) |
| COBAS | 1/3 (33%) | 0/3 (0%) | 1.00 | 0/5 (0%) |
| D10 VL<LLOQ | 9/21 (43%) | 9/13 (69%) | 0.17 | 6% |
| Abbott | 8/17 (47%) | 8/10 (80%) | 0.12 | 1/10 (10%) |
| COBAS | 1/4 (25%) | 1/3 (33%) | 1.00 | 0/6 (0%) |
| D14 VL<LLOQ | 14/21 (68%) | 9/13 (69%) | 1.00 | 18% |
| Abbott | 11/16 (69%) | 6/9 (67%) | 1.00 | 1/11 (18%) |
| COBAS | 2/4 (50%) | 3/4 (75%) | 1.00 | 1/6 (17%) |
| HCV genotype 1 | 10/21 (48%) | 6/13 (46%) | 1.00 (vs Gt 6) | 6/17 (35%) |
| 1a | 4/21 (19%) | 5/13 (38%) | 0.15 (vs 1b) | 2/17 (12%) |
| 1b | 6/21 (24%) | 1/13 (8%) | 4/17 (24%) | |
| HCV genotype 6 | 10/21 (48%) | 6/13 (46%) | 11/17 (65%) | |
| 6a | 6/21 (29%) | 2/13 (15%) | 0.58 (vs. 6e) | 4/17 (24%) |
| 6e† | 3/21 (14%) | 3/13 (23%) | 4/17 (24%) | |
| Resistance-associated substitutions | ||||
| Median (range) SOF-RAS | 0 (0–1) | 0 (0–2) | 0.76 | 0 (0–1) |
| Median (range) DCV-RAS | 2 (0–2) | 1 (0–2) | 0.17 | 2 (0–4) |
| Median (range) SOF- & DCV-RAS combined | 2 (0–3) | 2 (1–2) | 0.12 | 2 (0–4) |
| Drug exposure (n=37) § | n=15 | n=8 | n=14 | |
| Median AUClast, SOF (h×ng/ml) ‡ | 2360 (1120–4550) | 2220 (937–3910) | 0.975 | 2120 (1430–2610) |
| Median AUClast GS-331007 (h×ng/ml) ‡ | 11,700 (8420–14,100) | 15,100 (9240–19,700) | 0.023 | 14,000 (10,200–17,400) |
| Median AUClast, DCV (h×ng/mL) ‡ | 13,000 (6800–22,300) | 13,200 (6630–27,000) | 0.728 | 14,200 (9210–17,000) |
-
Results presented as median (5th–95th percentile).
-
*
n=12, no HCV VL data for one participant’s day 7 visit.
-
†
h, l and u subtypes excluded from the table/analysis due to small numbers (≤2).
-
‡
AUClast is the total exposure to the last time point (8 hr for SOF and 24 hr for GS-331007 and DCV).
-
§
Complete d0 and d28 data only available for 37 participants.
Pharmacokinetic parameters from the naïve-pooled analysis.
| Sofosbuvir | GS-331007 | Daclatasvir | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| Cmax (ng/mL) | 1,320 | 1,070 | 988 | 1,230 | 1,170 | 1,110 |
| tmax (h) | 1.00 | 1.00 | 3.00 | 4.00 | 3.00 | 3.00 |
| t1/2 (h) | 0.670 | 0.650 | 9.20 | 12.4 | 7.31 | 8.18 |
| AUClast (h×ng/mL)* | 1,550 | 1,600 | 10,500 | 14,600 | 11,400 | 12,400 |
| AUCINF (h×ng/mL)* | 1,550 | 1,600 | 12,700 | 20,400 | 12,800 | 14,400 |
-
Cmax is the maximum observed concentration, tmax is the time to reach the maximum concentration, t1/2 is the terminal elimination half-life (calculated using the 3–6 last concentration measurements, depending on drug and day), AUClast is the total exposure to the last time point (8 hours for SOF and 24 hours for GS-331007 and DCV), AUCinf is the total exposure extrapolated to infinity.
-
*
Extrapolation based on the last observed concentration measurement.
Pharmacokinetic exposure from the individual analysis and pharmacodynamic parameters.
| Pharmacokinetics | |||
|---|---|---|---|
| Sofosbuvir | GS-331007 | Daclatasvir | |
| AUClast (h×ng/mL) | 1,140 (598-2,150) | 3,430 (2,200-4,720) | 9,770 (5,080-16,200) |
| Pharmacodynamics | |||
| AUC (days ×IU/mL) | 252,000 (19,200-1,370,000) | ||
| t1/2 (days) | 2.25 (0.986–5.22) | ||
| %ReductionEnrolment-Day1 | 99.9 (99.0–100) | ||
| %ReductionEnrolment-Day7 | 100 (100–100) |
-
Data is presented as median (5th –95th percentile). AUClast is the total exposure to the last time point (8 hours for SOF and 24 hours for GS-331007 and DCV). AUC14 is the area under the viral load-time curve from enrolment (day 0) to day 14, t1/2 is the terminal viral half-life (estimated using at least three measurements), %ReductionEnrolment-Day1 is the reduction in viral load from enrolment to day 1, %ReductionEnrolment-Day7 is the reduction in viral load from enrolment to day 7.
-
The half-life could not be determined for one participant due to only one sample above the lower limit of quantification.
Pharmacokinetic-pharmacodynamic linear regression analysis.
| Viral outcome measurement vs. | Sofosbuvir AUClast | GS-331007 AUClast | Daclatasvir AUClast | |||
|---|---|---|---|---|---|---|
| Slope (95% CI) | p | Slope (95% CI) | p | Slope (95% CI) | p-value | |
| Area under the viral load-time curve | –157 (−423–109) | 0.239 | 16.2 (−74.4–107) | 0.719 | –14.2 (−67.1–38.6) | 0.589 |
| Viral elimination half-life | 1.55×10–4 (–8.70×10–4 - 5.60×10–4) | 0.662 | –3.64×10–5 (–2.74×10–4 - 2.01×10–4) | 0.757 | 2.17×10–5 (–1.16×10–4 - 1.60×10–4) | 0.751 |
| Relative reduction in viral load at day 1 | 1.31×10–6 (–4.54×10–6 - 7.16×10–6) | 0.652 | 2.67×10–8 (–1.94×10–6 - 1.99×10–6) | 0.978 | 2.81×10–7 (–8.62×10–7 - 1.42×10–6) | 0.621 |
| Relative reduction in viral load at day 7 | 2.53×10–7 (–2.81×10–7 - 7.86×10–7) | 0.343 | 5.09×10–8 (–1.29×10–7 - 2.31×10–7) | 0.569 | 1.44*10–8 (–9.11×10–8 - 1.20×10–7) | 0.783 |
-
95%CI is the 95% confidence interval around the slop.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/81801/elife-81801-mdarchecklist1-v2.docx
-
Source data 1
Pseudo anonymised HCV RNA data for every participant.
- https://cdn.elifesciences.org/articles/81801/elife-81801-data1-v2.xlsx