Fecal transplant from myostatin deletion pigs positively impacts the gut-muscle axis
Figures
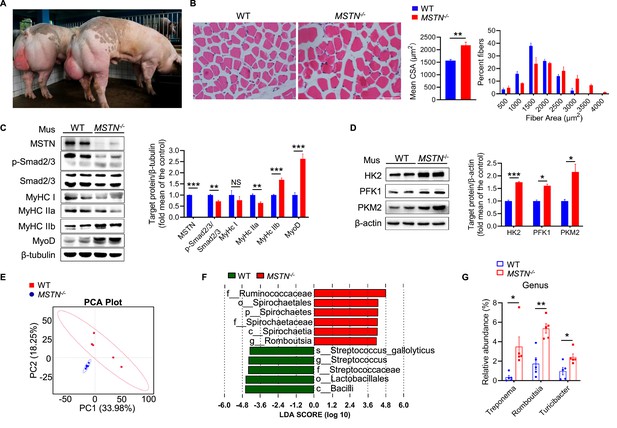
MSTN deletion stimulates muscle hypertrophy and alters composition of gut microbiota in pigs (n=5).
(A and B) Representative images of MSTN−/− pigs and hematoxylin eosin staining longissimus dorsi. Magnification is ×200. Scale bar, 100 μm. MSTN−/− pigs showed skeletal muscle hypertrophy and significantly increased the muscle fiber area. (C) Relative to WT pigs, MSTN−/− pigs showed no expression of MSTN, downregulate phosphorylation of Smad2/3 and MyHC IIa, and upregulate MyHC IIb and MyoD in longissimus dorsi (Mus). (D) MSTN−/− pigs showed increased glycolysis enzymes HK2, PFK1 and PKM2 in longissimus dorsi (Mus). (E) Plots shown were generated using the weighted version of the Unifrac-based PCA. (F) Discriminative taxa determined by LEfSe between two groups (log10 LDA >4.8). (G) Comparison proportion of genus levels in feces detected by pyrosequencing analysis showed Treponema, Romboutsia, and Turicibacter were increased in MSTN−/− pigs. Statistical analysis is performed using Student’s t-test between WT and MSTN−/− pigs. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 1—source data 1
Raw data for Figure 1.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig1-data1-v2.zip
-
Figure 1—source data 2
Raw western blot images for Figure 1C and D.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig1-data2-v2.zip
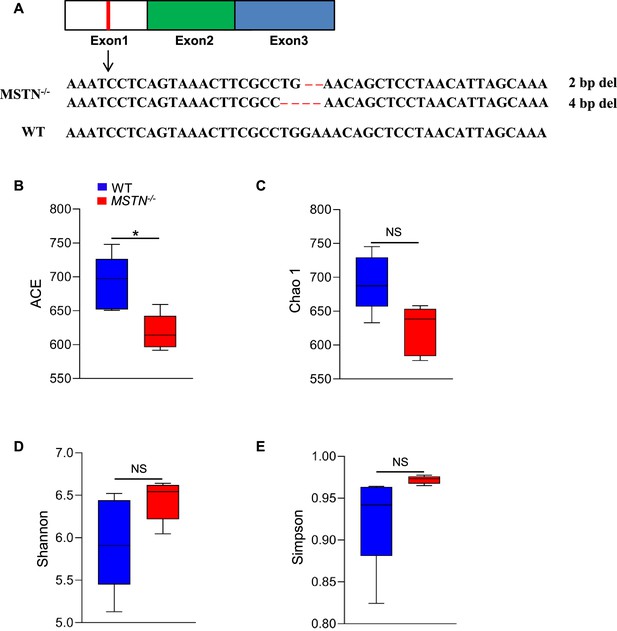
Gene sequence and alpha-diversity of microbiota of MSTN−/− pigs.
(A) Gene sequence of MSTN−/− pigs generated by genome editing. (B) Alpha-diversity analyses showed that the ACE was lower in MSTN−/− pigs and had no difference in (C) Chao 1, (D) Shannon, (E) Simpson index between MSTN−/− and WT pigs. Statistical analysis is performed using Student’s t-test between WT and MSTN−/− pigs. Data are means ± SEM. *p<0.05; NS, not statistically significant.
-
Figure 1—figure supplement 1—source data 1
Raw data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig1-figsupp1-data1-v2.zip
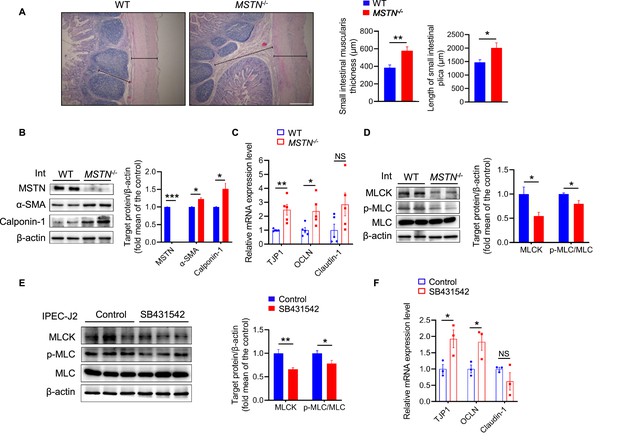
MSTN deletion alters intestinal structure and tight junction in pigs (n=5).
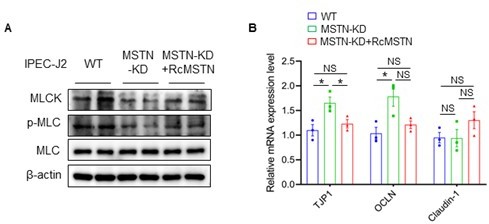
(A) Hematoxylin eosin staining of intestinal morphology. The dotted line indicates the length of the plica and the solid line indicates the thickness of muscularis. Magnification is 40×. Scale bar, 500 μm. MSTN−/− pigs showed an increase of muscularis thickness and plica length in small intestine. (B) Relative expression of tight junction genes TJP1 and OCLN were enhanced in small intestine (Int) of MSTN−/− pigs. (C) The protein expression of MSTN was not detected in intestine (Int) while the α-SMA and Calponin-1 were increased in MSTN−/− pigs compared with the WT pigs. (D) The protein expression of MLCK and phosphorylation of MLC in intestine (Int) were decreased in MSTN−/− pigs compared with the WT pigs. (E) The protein expression of MLCK and phosphorylation of MLC were decreased in IPEC-J2 after SB431542 treatment. (F) The expression of tight junction factors TJP1 and OCLN were enhanced in IPEC-J2 after SB431542 treatment. Statistical analysis is performed using Student’s t-test. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 2—source data 1
Raw data for Figure 2.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig2-data1-v2.zip
-
Figure 2—source data 2
Raw western blot images for Figure 2B, D and E.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig2-data2-v2.zip
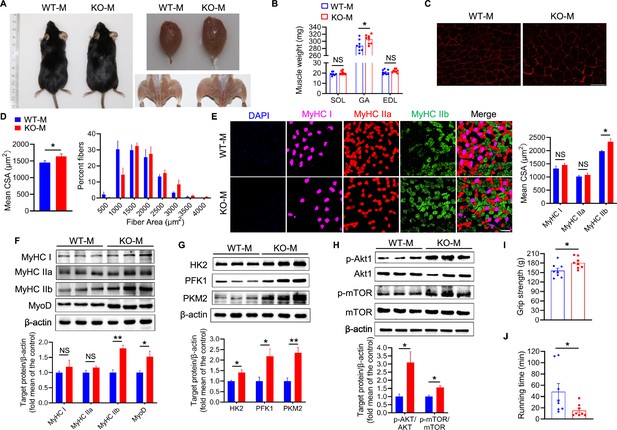
Mice fecal microbiota transplantation from MSTN deletion pigs induces type IIb myofiber growth.
Mice were treated with porcine fecal microbiota for eight weeks by daily oral gavage after combined antibiotics treatment for a week. WT-M, WT pigs fecal microbiota-received mice (n=8); KO-M, MSTN−/− pigs fecal microbiota-received mice (n=8). (A) Representative images of gross appearance and GA of WT-M and KO-M. (B) GA mass was increased in KO-M while SOL and EDL were not different between WT-M and KO-M. (C) Representative images of GA sections stained with laminin. Magnification is 400×. Scale bar, 50 μm. (D) Quantification analysis of myofiber CSA showed that KO-M was larger than WT-M. (E) Representative images of GA sections stained with MyHC I (pink), IIa (red), IIb (green) antibodies and nucleuses were stained with DAPI (blue). Magnification is ×200. Scale bar, 100 μm. Quantification of myofiber displayed MyHC IIb CSA were increased in KO-M. (F) KO-M showed upregulate the level of MyHC IIb and MyoD in GA. (G) The expression of glycolysis enzymes HK2, PFK1, and PKM2 were increased in KO-M GA. (H) The Akt/mTOR pathway was activated in KO-M GA. (I and J) Grip strength was enhanced while running time was reduced in KO-M compared with WT-M. Statistical analysis is performed using Student’s t-test between WT-M and KO-M groups. Data are means ± SEM. *p<0.05; **p<0.01; NS, not statistically significant.
-
Figure 3—source data 1
Raw data for Figure 3.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig3-data1-v2.zip
-
Figure 3—source data 2
Raw western blot images for Figure 3F–H.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig3-data2-v2.zip
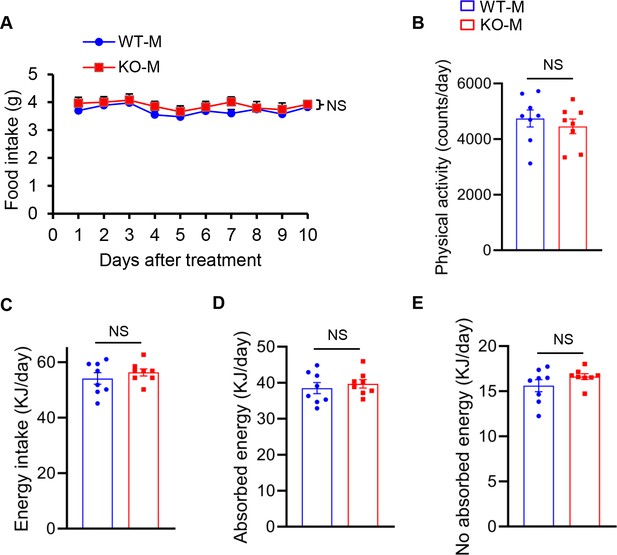
Food intake, physical activity and energy in mice after fecal microbiota transplantation.
Mice were treated with porcine fecal microbiota for 8 weeks by daily oral gavage after combined antibiotics treatment for a week. WT-M, WT pigs fecal microbiota-received mice (n=8); KO-M, MSTN−/− pigs fecal microbiota-received mice (n=8). There were no differences between WT-M and KO-M in (A) Food intake, (B) physical activity, (C) energy intake, (D) absorbed energy, (E) no absorbed energy. For food intake curves, a repeated measure two-way ANOVA, others is performed using Student’s t-test between WT-M and KO-M groups. Data are means ± SEM. NS, not statistically significant.
-
Figure 3—figure supplement 1—source data 1
Raw data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig3-figsupp1-data1-v2.zip
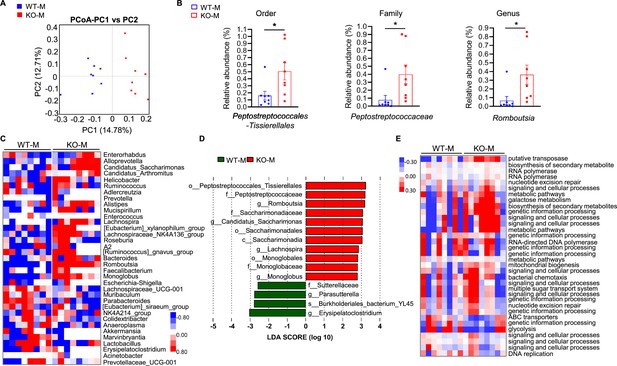
MSTN−/− pigs fecal microbiota transplantation alters microbiota composition in mice.
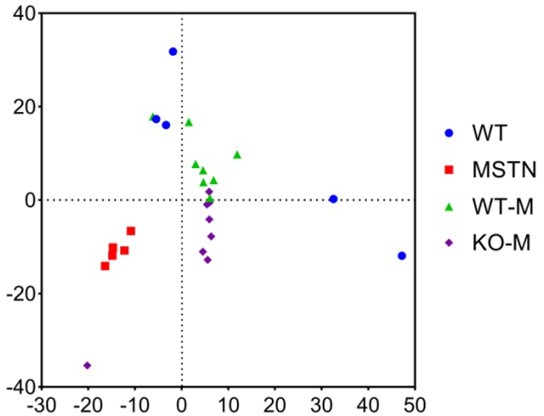
Transplanting fecal microbiota of MSTN−/− pigs and WT pigs separately to mice (n=8). (A) Plots shown were generated using the weighted version of the Unifrac-based PCoA. (B) Comparison proportion of order, family and genus levels of Romboutsia in feces detected by pyrosequencing analysis. (C) Heatmap shows the abundance of top 35 microbial genuses levels was significantly altered by WT and MSTN−/− donor pigs between WT-M and KO-M groups. (D) Discriminative taxa determined by LEfSe between two groups (log10 LDA >3.5). (E) Functional prediction shows that intestinal microbial functions are concentrated in functional pathways related to metabolite synthesis after fecal microbiota transplantation. Statistical analysis is performed using Student’s t-test between WT-M and KO-M groups. Data are means ± SEM. *p<0.05.
-
Figure 4—source data 1
Raw data for Figure 4.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig4-data1-v2.zip
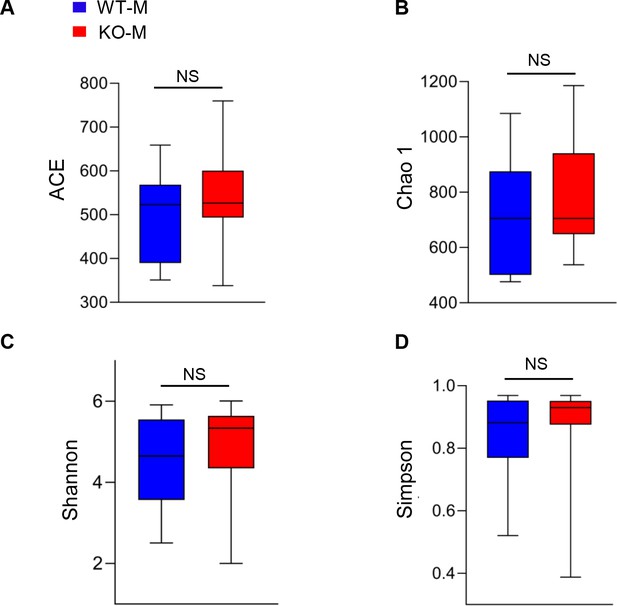
Alpha-diversity in mice after fecal microbiota transplantation from MSTN−/− pigs.
Mice were treated with porcine fecal microbiota for 8 weeks by daily oral gavage after combined antibiotics treatment for a week. WT-M, WT pigs fecal microbiota-received mice (n=8); KO-M, MSTN−/− pigs fecal microbiota-received mice (n=8). There were no differences between WT-M and KO-M in (A) ACE, (B) Chao 1, (C) Shannon and (D) Simpson index. Statistical analysis is performed using Student’s t-test between WT-M and KO-M groups. Data are means ± SEM. NS, not statistically significant.
-
Figure 4—figure supplement 1—source data 1
Raw data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig4-figsupp1-data1-v2.zip
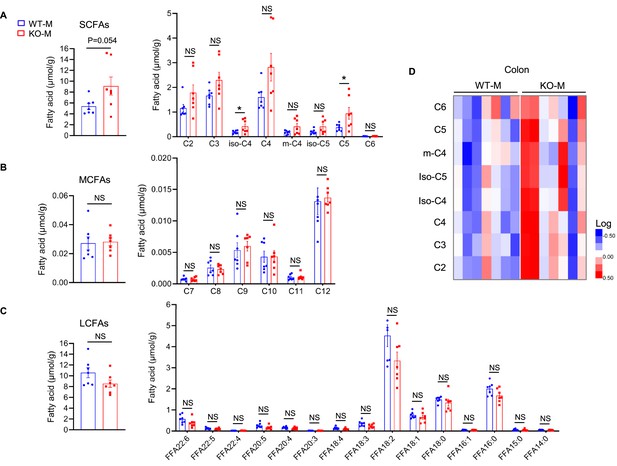
MSTN−/− pigs fecal microbiota transplantation alters the level of fatty acids in mice (n=7).
(A) Fecal microbiota transplantation increased colon total SCFAs (particularly valeric acid and isobutyric acid) in KO-M. (B) Fecal microbiota transplantation has no effect on MCFAs between WT-M and KO-M. (C) Fecal microbiota transplantation has no effect on LCFAs between WT-M and KO-M. (D) Heatmap showed the difference of SCFAs between WT-M and KO-M. Statistical analysis is performed using Student’s t-test. Data are means ± SEM. *p<0.05; NS, not statistically significant.
-
Figure 5—source data 1
Raw data for Figure 5.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig5-data1-v2.zip
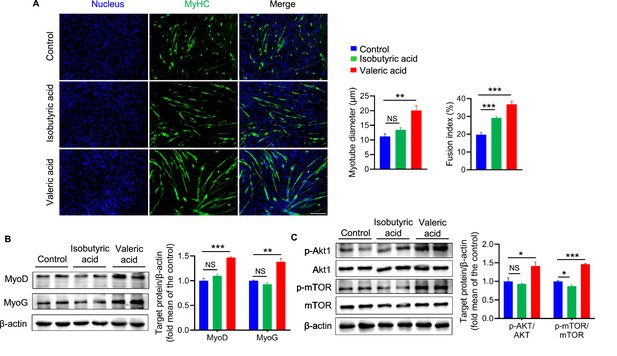
Valeric acid treatment promotes myogenic differentiation of myoblast (n=6).
(A) Representative images of immunofluorescence stained with a specific antibody to identify MyHC (green) of myotubes and the nucleuses were stained with DAPI (blue). Magnification is ×100. Scale bar, 200 μm. Quantification analysis displayed valeric acid treatment increased the diameter and fusion index of myotube, while isobutyric acid only increased the myotube fusion index. (B) Valeric acid treatment increased the expression of MyoD and MyoG in C2C12 myoblasts. (C) Valeric acid treatment activated the Akt/mTOR pathway. Statistical analysis is performed using one-way ANOVA. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 6—source data 1
Raw data for Figure 6.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig6-data1-v2.zip
-
Figure 6—source data 2
Raw western blot images for Figure 6B and C.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig6-data2-v2.zip
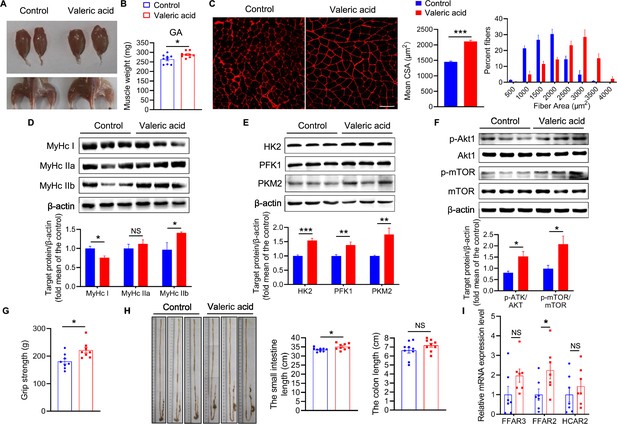
Valeric acid induces type IIb myofiber growth and increased GA mass in mice.
Mice were treated with valeric acid (100 mg/kg) for 5 weeks by daily oral gavage (n=8–9). (A) Representative images of gross appearance and GA of control and valeric acid treated mice. (B) Valeric acid treatment increased GA mass. (C) Representative images of GA sections stained with laminin, showed valeric acid treatment increased CSA of myofiber. Magnification is ×200. Scale bar, 100 μm. Western blot analysis showed that valeric acid treatment increased the levels of (D) MyHC IIb, (E) glycolysis enzymes HK2, PFK1, and PKM2, and (F) activated the Akt/mTOR pathway in GA compared with control mice. (G) Valeric acid treatment improved grip strength. (H) Representative images of cecum, small intestine, and colon of mice, showed valeric acid treatment inceresed small intestine length. (I) Real-time PCR analysis indicated that valeric acid treatment enhanced relative mRNA expression of FFAR2 in GA. Statistical analysis is performed using Student’s t-test. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 7—source data 1
Raw data for Figure 7.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig7-data1-v2.zip
-
Figure 7—source data 2
Raw western blot images for Figure 7D–F.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig7-data2-v2.zip
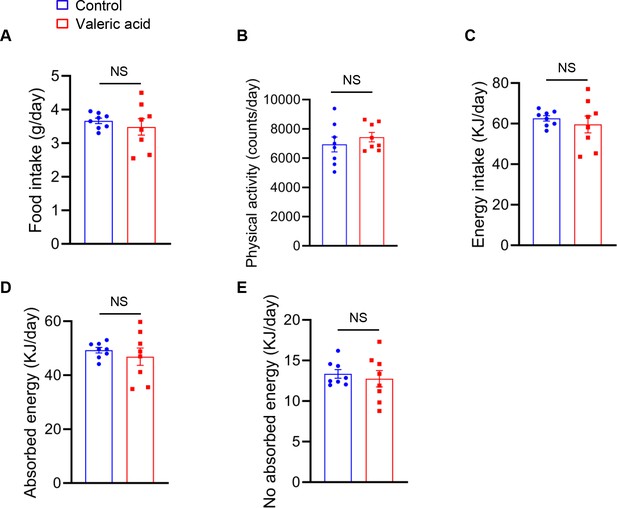
Food intake, physical activity and energy in mice after valeric acid treatment.
Mice were treated with valeric acid (100 mg/kg) for 5 weeks by daily oral gavage (n=8). There were no differences between valeric acid treatment and control in (A) Food intake, (B) Physical activity, (C) Energy intake, (D) Absorbed energy, (E) No absorbed energy. Statistical analysis is performed using Student’s t-test. Data are means ± SEM. *p<0.05; NS, not statistically significant.
-
Figure 7—figure supplement 1—source data 1
Raw data for Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig7-figsupp1-data1-v2.zip
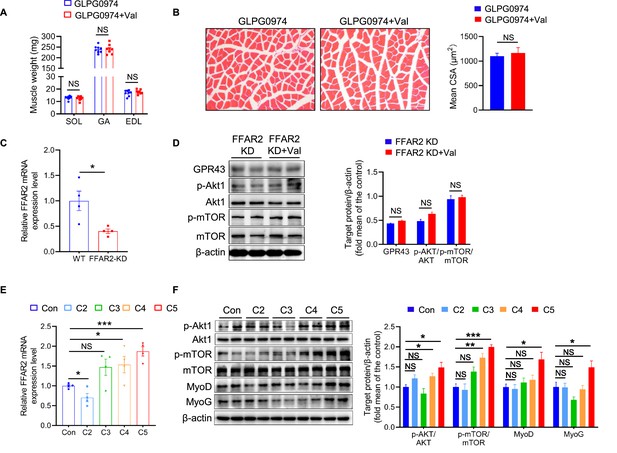
Valeric acid promotes skeletal muscle growth by activating the Akt/mTOR pathway through GPR43.
(A) Mice were treated with valeric acid and GPR43-specific inhibitor GLPG0974 for 5 weeks by oral gavage (n=8), and valeric acid treatment did not increase muscle mass. (B) Representative images of GA sections stained with hematoxylin eosin, showed valeric acid treatment did not increase CSA of myofiber when GPR43 was inhibited. Magnification is ×200. Scale bar, 100 μm. (C) The mRNA expression of FFAR2 was knockdown (FFAR2-KD) in C2C12 myoblast. (D) Western blot analysis showed that valeric acid treatment did not activate the Akt/mTOR pathway after FFAR2-KD. (E) Valeric acid (C5) and butyric acid (C4) significantly increased the mRNA expression of FFAR2, whereas acetic acid (C2) and propionic acid (C3) did not. (F) Valeric acid (C5) and butyrate acid (C4) activated the Akt and mTOR, and only valeric acid induced high expression of MyoD and MyoG. Statistical analysis is performed using Student’s t-test. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 8—source data 1
Raw data for Figure 8.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig8-data1-v2.zip
-
Figure 8—source data 2
Raw western blot images for Figure 8D and F.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig8-data2-v2.zip
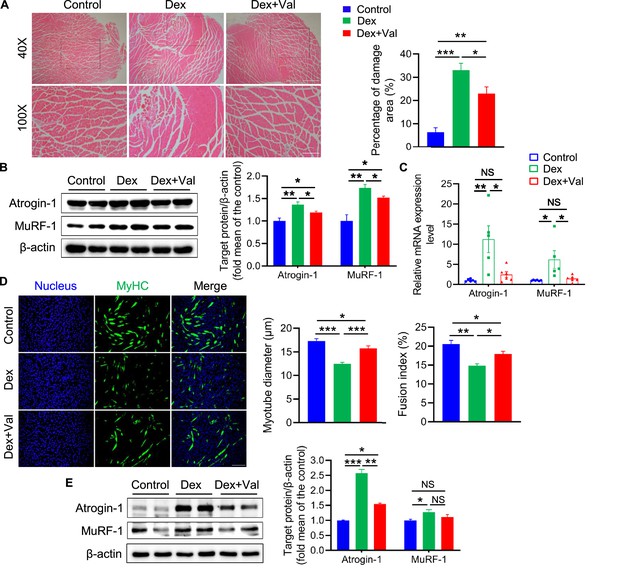
Valeric acid ameliorates Dex-induced skeletal muscle and myotube atrophy.
Mice were treated with intraperitoneal injection of 20 mg/kg Dex every 2 day for 2 weeks and 100 mg/kg of valeric acid was fed orally every day before 2 weeks of Dex injection (n=5). Myotube atrophy was induced with 100 μM/L of Dex, and 5 mM/L of valeric acid was supplied at the same time (n=6). (A) Hematoxylin eosin staining of GA morphology. Magnification is ×40. Scale bar, 500 μm. Quantification analysis showed that Dex induced the myofiber damage, and valeric acid treatment decreased the percentage of damage area. (B) Western blot analysis showed that Dex induced the expression of Atrogin-1 and MuRF-1 in GA, while valeric acid treatment reduced the level of these. (C) Real-time PCR analysis of relative expression of atrophy genes (Atrogin-1 and MuRF-1) in GA, showed valeric acid treatment could inhibit the expression of these genes induced by Dex. (D) Immunofluorescence stained with a specific antibody was used to identify MyHC (green) of myotube and the nucleus were stained with DAPI (blue). Magnification is ×100. Scale bar, 200 μm. Quantification analysis showed valeric acid treatment could improve the reduction of myotubes diameter and fusion index induced by Dex. (E) Western blot analysis showed valeric acid treatment could inhibit the expression of Atrogin-1 induced by Dex in C2C12 myotubes and had no effect on MuRF-1 induced by Dex. Statistical analysis is performed using one-way ANOVA with Least Significant Difference test. Data are expressed as means ± SEM. *p<0.05; **p<0.01; ***p<0.001; NS, not statistically significant.
-
Figure 9—source data 1
Raw data for Figure 9A.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig9-data1-v2.zip
-
Figure 9—source data 2
Raw data for Figure 9B–E.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig9-data2-v2.zip
-
Figure 9—source data 3
Raw western blot images for Figure 9B and E.
- https://cdn.elifesciences.org/articles/81858/elife-81858-fig9-data3-v2.zip
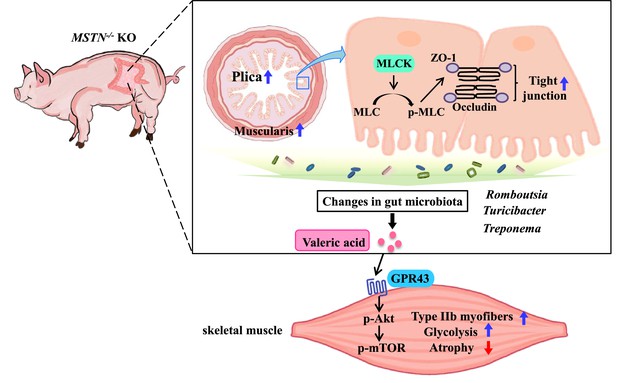
Schematic illustration of the results.
Intestine MSTN deficiency inhibited MLCK/MLC, altered the intestinal structure and barrier, and reshaped gut microbiota; gut microbiota metabolite-valeric acid activates Akt/mTOR pathway via GPR43 to stimulate fast-twitch glycolytic skeletal muscle growth.
Tables
| Body Weight(g) | GA(mg) | SOL(mg) | EDL(mg) | TA(mg) | GA/BW(%) | SOL/BW(%) | EDL/BW(%) | TA/BW(%) | Muscle/BW(%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT-M | 25.65 | 287.73 | 19.16 | 20.93 | 94.72 | 11.22 | 0.75 | 0.82 | 3.69 | 16.47 |
| KO-M | 26.7 | 305.77 | 20.22 | 22.05 | 105.42 | 11.45 | 0.76 | 0.83 | 3.94 | 16.98 |
| TTEST | 0.02 | 0.02 | 0.22 | 0.28 | 0.05 | 0.37 | 0.70 | 0.79 | 0.11 | 0.04 |
| Body Weight(g) | GA(mg) | SOL(mg) | EDL(mg) | TA(mg) | GA/BW(%) | SOL/BW(%) | EDL/BW(%) | TA/BW(%) | Muscle/BW(%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT-M | 23.23 | 264.24 | 13.31 | 17.32 | 77.09 | 11.38 | 0.57 | 0.74 | 3.31 | 16.0 |
| KO-M | 25.12 | 288.59 | 14.48 | 19.34 | 86.19 | 11.49 | 0.57 | 0.77 | 3.43 | 16.26 |
| TTEST | 0.01 | 0.02 | 0.12 | 0.05 | 0.05 | 0.64 | 0.75 | 0.41 | 0.38 | 0.04 |
Additional files
-
Supplementary file 1
Primers sequences used for real-time PCR.
- https://cdn.elifesciences.org/articles/81858/elife-81858-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/81858/elife-81858-mdarchecklist1-v2.docx