Beta human papillomavirus 8E6 promotes alternative end joining
Figures
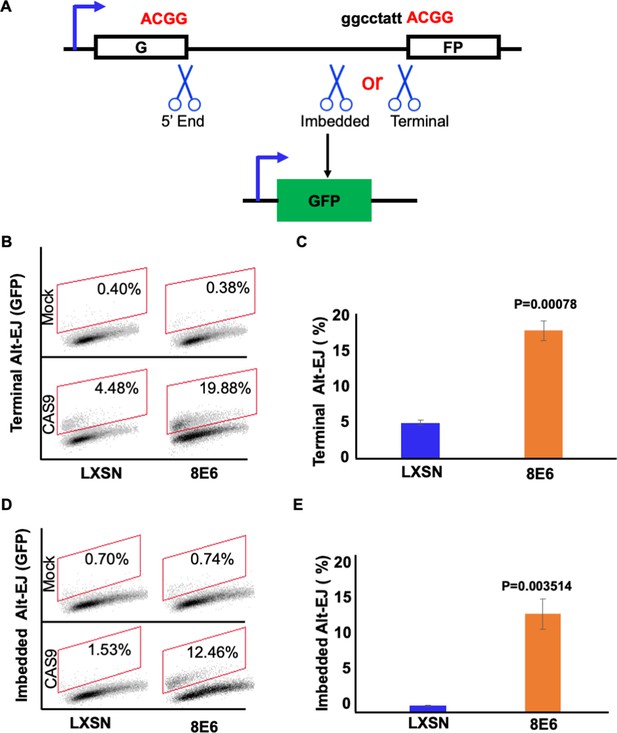
8E6 promotes alternative end joining (Alt-EJ) frequency.
(A) Schematic of Alt-EJ reporter. GFP is disrupted by a 46 nt insertion. One CAS9 is used to induce an upstream double strand break (DSB) (5’ end) and another CAS9 is used to induce a downstream DSB (either imbedded or terminal). Following CAS9 expression, a 4 nt microhomology (ACGG) mediated Alt-EJ event can restore GFP expression. (B) Representative images of flow cytometry results of HFK cells that are GFP positive 24 hr after transfection with terminal Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (C) Percentage of HFK cells that are positive for GFP following transfection with terminal Alt-EJ determined by flow cytometry. (D) Representative images of flow cytometry results of HFK cells that are GFP positive 24 hr after transfection with imbedded Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (E) Percentage of HFK cells that are positive for GFP following transfection with imbedded Alt-EJ determined by flow cytometry. All values are represented as mean ± standard error. The statistical significance of differences between cell lines were determined using Student’s t-test. p-Values indicate significant difference between cell lines. Twenty thousand cells were counted for each of three independent flow cytometry experiments.
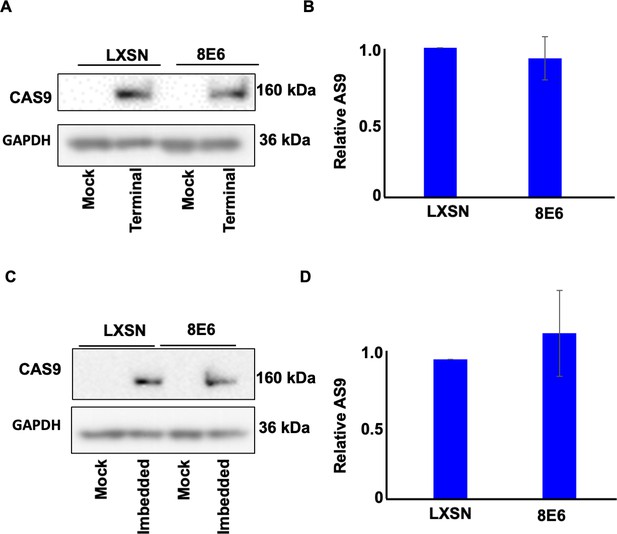
Transfection efficiency represented by CAS9 expression in hTERT human foreskin keratinocyte (HFK).
(A) Representative immunoblotting of CAS9 after transfection with terminal alternative end joining (Alt-EJ). GAPDH is used as a loading control. (B) Densitometry of CAS9 level in cells transfected with terminal Alt-EJ. (C) Representative immunoblotting of CAS9 after transfection with imbedded Alt-EJ. GAPDH is used as a loading control. (D) Densitometry of CAS9 level in cells transfected with imbedded Alt-EJ. All values are represented as mean ± standard error (n=3). The statistical significance of differences between cell lines were determined using Student’s t-test. No significant differences were obtained.
-
Figure 1—figure supplement 1—source data 1
Original blots with and without labels in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/81923/elife-81923-fig1-figsupp1-data1-v3.zip
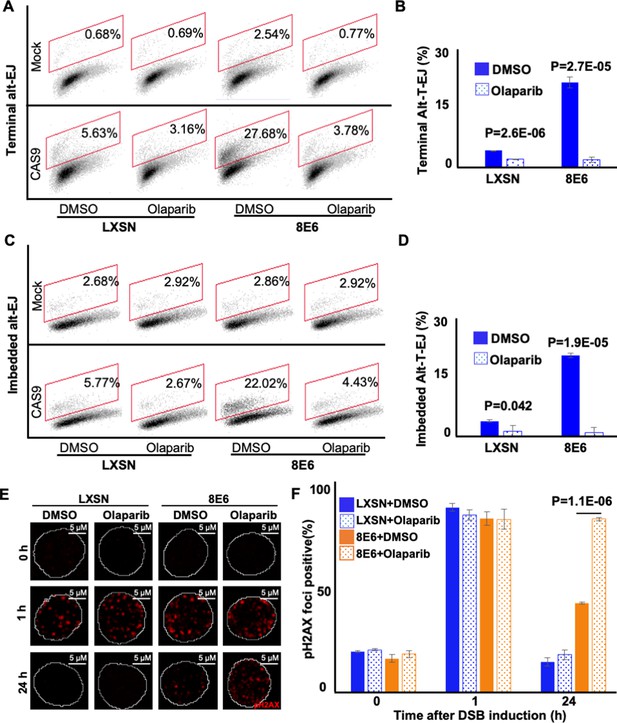
Olaparib abrogates alternative end joining (Alt-EJ) frequency and increases persistent pH2AX.
(A) Representative images of flow cytometry results of human foreskin keratinocyte (HFK) cells treated with DMSO or olaparib (1 μM) that are GFP positive 24 hr after transfection with terminal Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (B) Percentage of HFK cells that are positive for GFP following transfection with terminal Alt-EJ determined by flow cytometry. (C) Representative images of flow cytometry results of HFK cells treated with DMSO or olaparib that are GFP positive 24 hr after transfection with imbedded Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (D) Percentage of HFK cells that are positive for GFP following transfection with imbedded Alt-EJ determined by flow cytometry. (E) Representative images of pH2AX in HFK LXSN and HFK 8E6 treated with DMSO or olaparib (1 μM) following zeocin treatment (10 μg/mL, 10 min). (F) Percentage of pH2AX foci positive cells in HFK LXSN and HFK 8E6 treated with DMSO or olaparib following zeocin treatment. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and olaparib with same cell line (p<0.05). Twenty thousand cells were counted for each of three independent flow cytometry experiments.
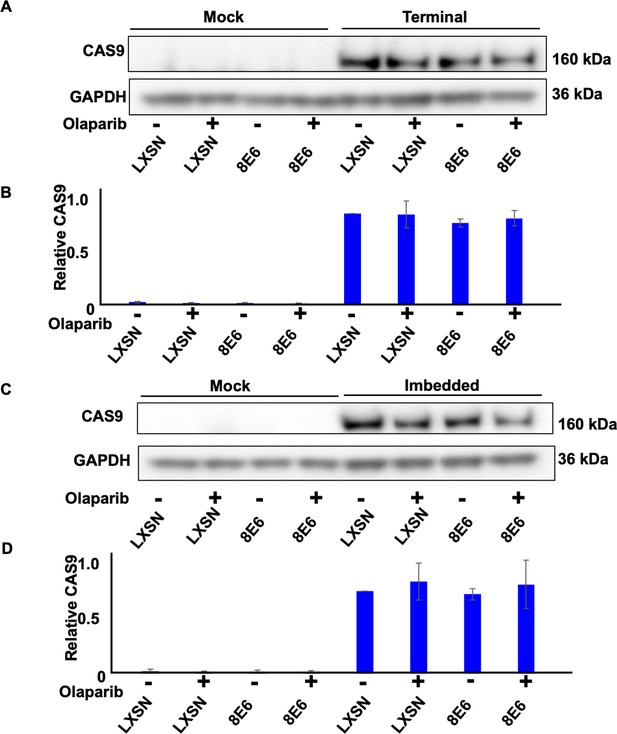
Transfection efficiency represented by CAS9 expression in hTERT human foreskin keratinocyte (HFK).
(A) Representative immunoblotting of CAS9 in cells treated with DMSO or olaparib (1 μM) after transfection with terminal alternative end joining (Alt-EJ). GAPDH is used as a loading control. (B) Densitometry of CAS9 level in cells tranfected with terminal Alt-EJ. (C) Representative immunoblotting of CAS9 in cells treated with DMSO or olaparib after transfection with imbedded Alt-EJ. (D). Densitometry of CAS9 level in cells tranfected with imbedded Alt-EJ. All values are represented as mean ± standard error (n=3). The statistical significance of differences between cell lines were determined using Student’s t-test. No significant differences were obtained.
-
Figure 2—figure supplement 1—source data 1
Original blots with and without labels in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/81923/elife-81923-fig2-figsupp1-data1-v3.zip
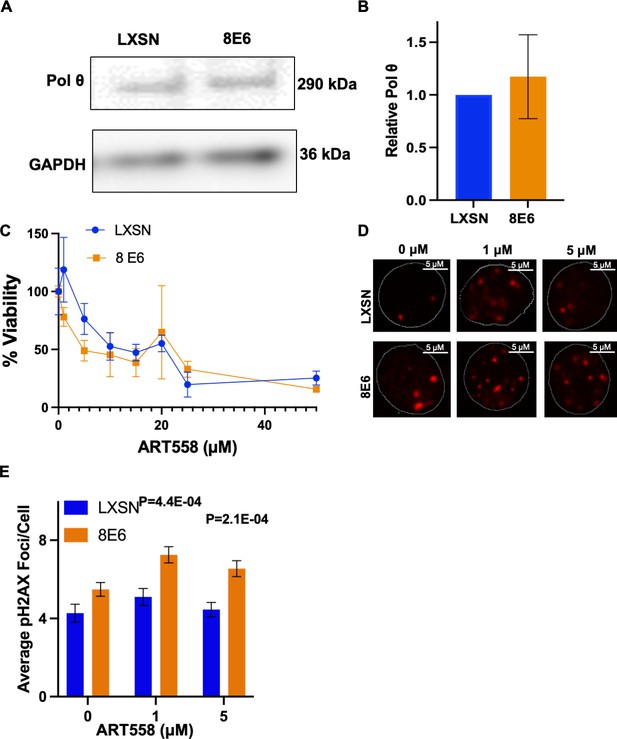
8E6 promotes polymerase theta (POLθ)-dependent double strand break (DSB) repair.
(A) Representative immunoblotting of POLθ in human foreskin keratinocyte (HFK) LXSN and HFK 8E6 cells. (B) Densitometry of POLθ level in HFK LXSN and HFK 8E6 cells. (C) Relative cell viability at various ART558 concentrations in HFK LXSN and HFK 8E6 following zeocin treatment. (D) Representative images of pH2AX in HFK LXSN and HFK 8E6 treated with DMSO or ART588 (1 or 5 μM) 24 hr following zeocin treatment (10 μg/mL, 10 min). (E) Average number of pH2AX foci per cell in HFK LXSN and HFK 8E6 treated with DMSO or ART558 following zeocin treatment. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between HFK LXSN and HFK 8E6 with same ART588 treatment (p<0.05). At least 40 cells were counted for each of three independent microscopy experiments.
-
Figure 3—source data 1
Original blots with and without labels in Figure 3.
- https://cdn.elifesciences.org/articles/81923/elife-81923-fig3-data1-v3.zip
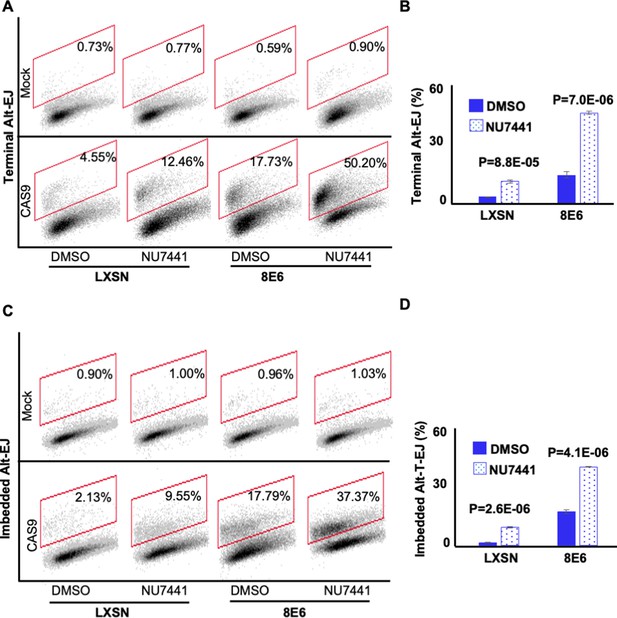
NU7441 promotes alternative end joining (Alt-EJ).
(A) Representative images of flow cytometry results of human foreskin keratinocyte (HFK) cells treated with DMSO or NU7441 (1 μM) that are GFP positive 24 hr after transfection with terminal Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (B) Percentage of HFK cells that are positive for GFP following transfection with terminal Alt-EJ determined by flow cytometry. (C) Representative images of flow cytometry results of HFK cells treated with DMSO or NU7441 that are GFP positive 24 hr after transfection with imbedded Alt-EJ. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (D) Percentage of HFK cells that are positive for GFP following transfection with imbedded Alt-EJ determined by flow cytometry. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and NU7441 within the same cell line. Twenty thousand cells were counted for each of three independent flow cytometry experiments.
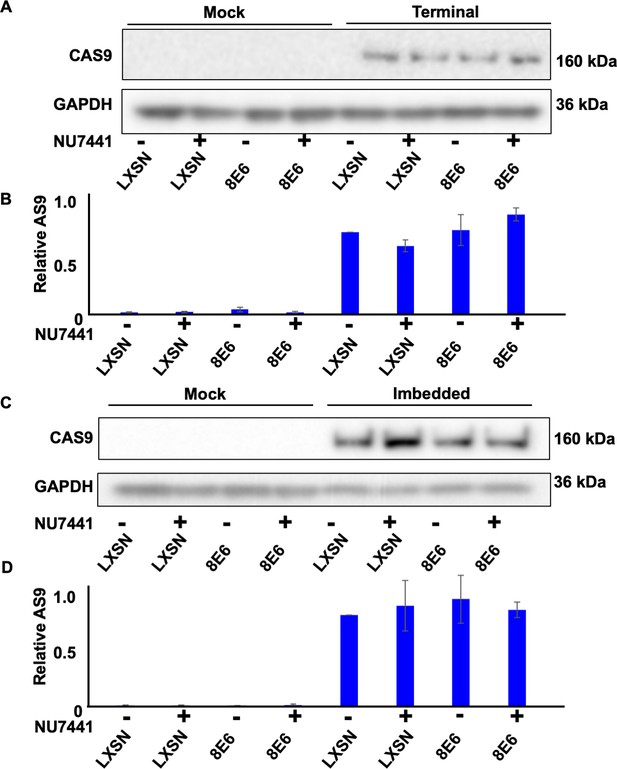
Transfection efficiency represented by CAS9 expression in hTERT human foreskin keratinocyte (HFK).
(A) Representative immunoblotting of CAS9 in cells treated with DMSO or NU7441 (1 μM) after transfection with terminal alternative end joining (Alt-EJ). GAPDH is used as a loading control. (B) Densitometry of CAS9 level in cells tranfected with terminal Alt-EJ. (C) Representative immunoblotting of CAS9 in cells treated with DMSO or NU7441 after transfection with imbedded Alt-EJ. GAPDH is used as a loading control. (D) Densitometry of CAS9 level in cells tranfected with imbedded Alt-EJ. All values are represented as mean ± standard error (n=3). The statistical significance of differences between cell lines were determined using Student’s t-test. No significant differences were obtained.
-
Figure 4—figure supplement 1—source data 1
Original blots with and without labels in Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/81923/elife-81923-fig4-figsupp1-data1-v3.zip
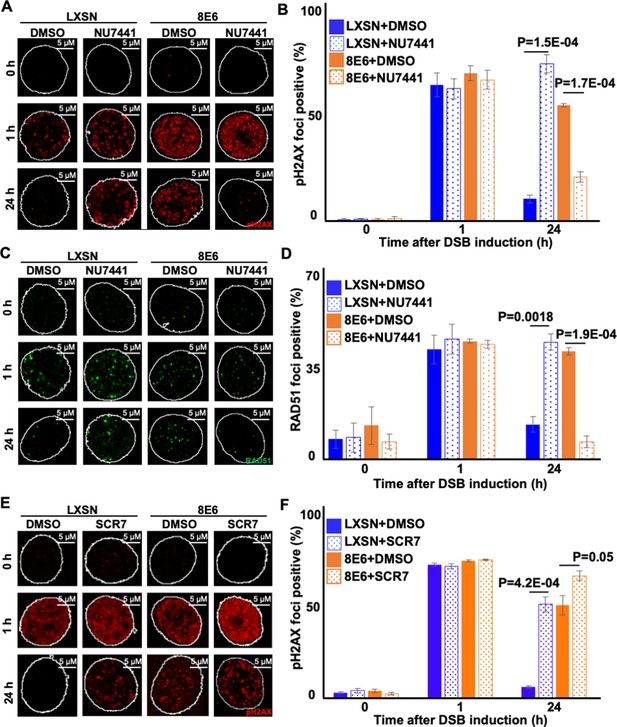
Nu7441 increases double strand break (DSB) repair in cells with 8E6.
(A) Representative images of pH2AX in human foreskin keratinocyte (HFK) LXSN and HFK 8E6 treated with NU7441 (1 μM) following zeocin treatment (10 μg/mL, 10 min). (B) Percentage of pH2AX foci positive cells in HFK LXSN and HFK 8E6 treated with NU7441 following zeocin treatment. (C) Representative images of RAD51 in HFK LXSN and HFK 8E6 treated with NU7441 following zeocin treatment. (D) Percentage of RAD51 foci positive cells in HFK LXSN and HFK 8E6 treated with NU7441 following zeocin treatment. (E) Representative images of pH2AX in HFK LXSN and HFK 8E6 treated with SCR7 (1 μM) following zeocin treatment. (F) Percentage of pH2AX foci positive cells in HFK LXSN and HFK 8E6 treated with SCR7 following zeocin treatment. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and inhibitor treated with the same cell line. At least 150 cells were counted over three independent experiments. Nuclei were determined by DAPI staining. The edge of this staining is shown by a white line depicting the nucleus.
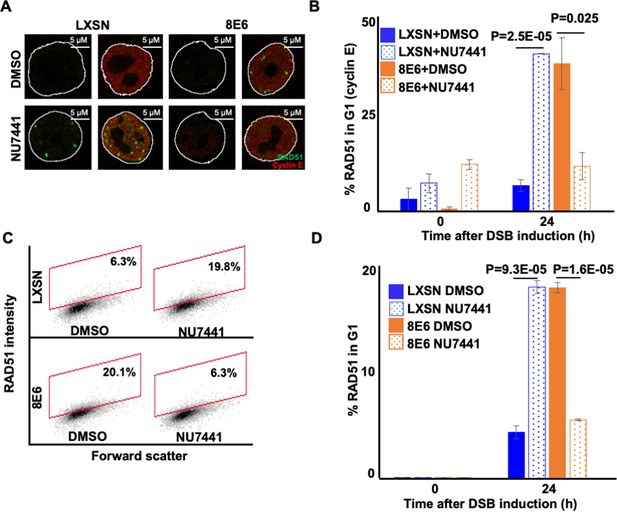
NU7441 abrogates RAD51 in G1 induced by 8E6.
(A) Representative cyclin E negative and positive human foreskin keratinocyte (HFK) LXSN and HFK 8E6 cells stained for RAD51 (green) and cyclin E (red) treated with DMSO or NU7441 (1 μM) 24 hr following zeocin treatment (10 µg/mL, 10 min). (B) Percentage of RAD51 positive HFK cell in G1 determined by cyclin E staining after zeocin treatment. (C) Representative images of flow cytometry results of HFK LXSN and HFK 8E6 cells in G1 stained with RAD51 treated with DMSO or NU7441 24 hr after zeocin treatment. RAD51 intensity is determined by Alexa 488-conjugated secondary antibody and shown on the y-axis. The gating represents RAD51 positive based off secondary only control. The x-axis shows cells distributed by forward scatter to avoid debris. (D) Percentage of HFK cells in G1 that are positive for RAD51 as determined by flow cytometry. Nuclei were determined by DAPI staining. The edge of this staining is shown by a white line depicting the nucleus. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and NU7441 treatment with the same cell line. At least 150 cells were counted over three independent experiments for microscopy. Twenty thousand cells were counted for each of three independent flow cytometry experiments.
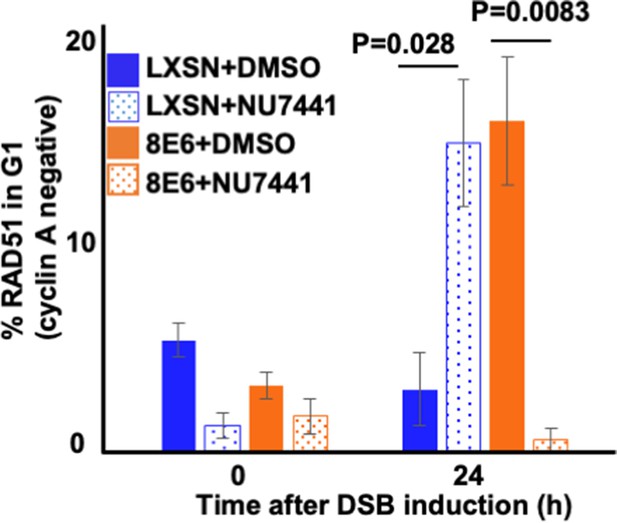
NU7441 abrogates RAD51 in G1 (cyclin A negative) induced by 8E6.
Percentage of RAD51 positive human foreskin keratinocyte (HFK) cell treated by DMSO or NU7441 (1 μM) in G1 determined by cyclin A negative after zeocin treatment (10 μg/mL, 10 min). All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and NU7441 treatment with same cell line. At least 150 cells were counted over three independent experiments for microscopy.
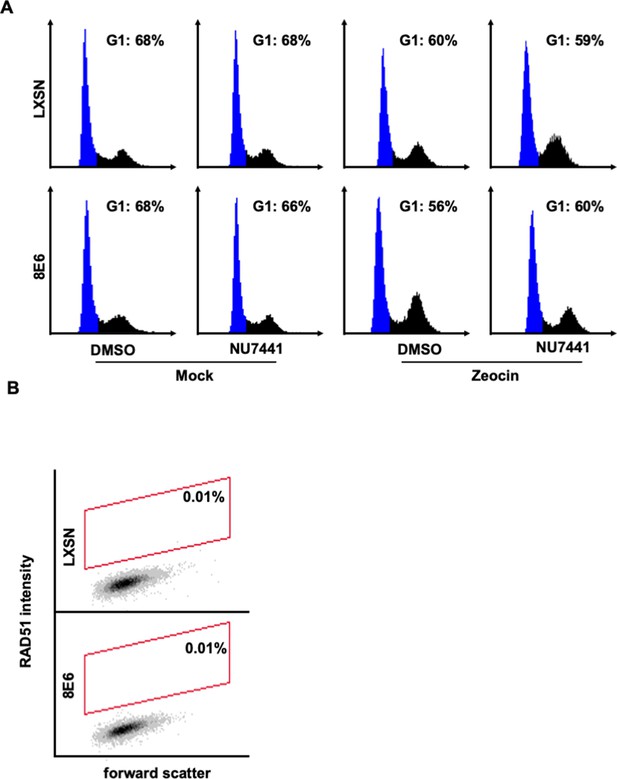
Controls were used to determine RAD51 staining cutoff and G1 gating in human foreskin keratinocyte (HFK) cells by flow cytometry.
(A) Representative images of cell cycle analysis by flow cytometry following zeocin treatment (10 µg/mL, 10 min). NUCLEAR-ID Red DNA stain was used to determine DNA content and G1 (blue). (B) Representative images of flow cytometry results of HFK LXSN and HFK 8E6 cells stained with Alexa 488-conjugated secondary antibody and shown on the y-axis. The gating represents RAD51 positive based off secondary only control. The x-axis shows cells distributed by forward scatter to avoid debris. Twenty thousand cells were counted for each of three flow cytometry experiments.
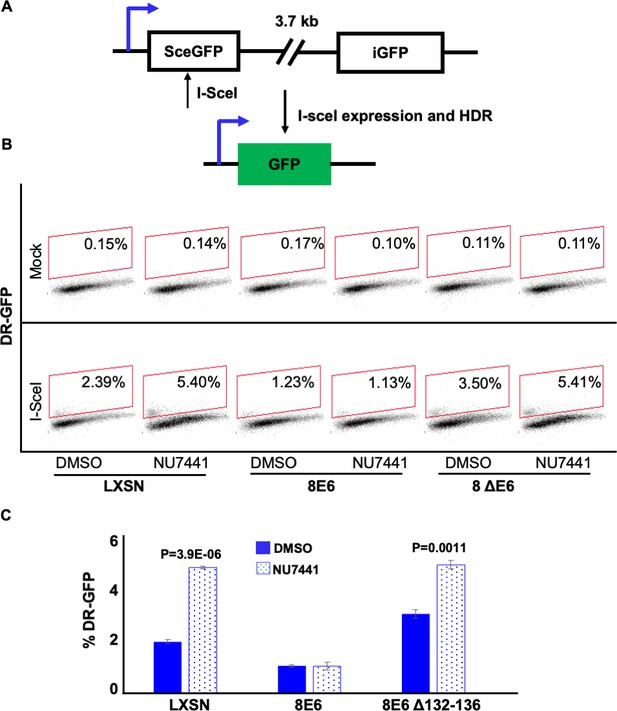
NU7441 does not increase homologous recombination (HR) in cells with 8E6.
(A) Schematic of DR-GFP reporter. GFP open reading frame is disrupted by insertion of ISCE-1 site (SceGFP). Downstream of the reporter is a truncated internal GFP(iGFP) that can be used as a template to remove the ISCE-1 site and restore GFP expression during HR event. (B) Representative images of flow cytometry results of U2OS cells that are GFP positive treated with DMSO or NU7441(1 μM) 24 hr after ISCE-1 transfection. The gating represents GFP positive based off mock transfected control. The x-axis shows cells distributed by forward scatter to avoid debris. (C) Percentage of U2OS cells that are positive for GFP determined by flow cytometry. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and NU7441 with same cell line. Twenty thousand cells were counted for each of three independent flow cytometry experiments.
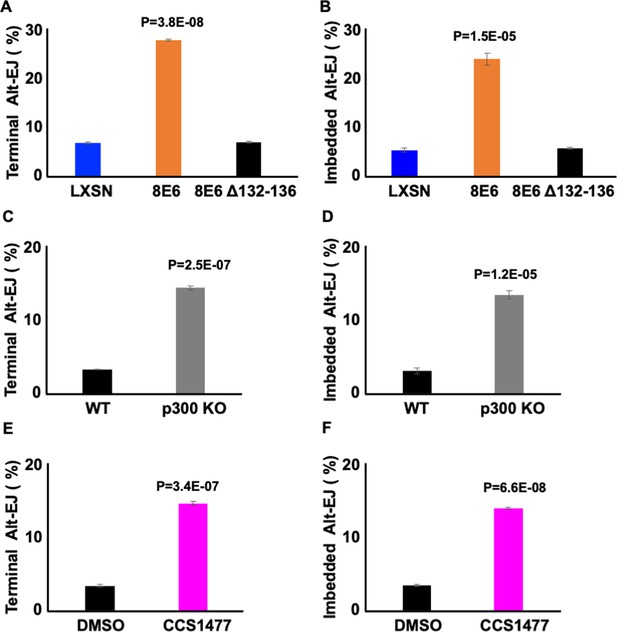
Losing p300 activity promotes alternative end joining (Alt-EJ) frequency.
(A–B) Percentage of U2OS cells that are positive for Alt-EJ following transfection with (A) terminal or (B) imbedded determined by flow cytometry. (C–D) Percentage of human foreskin keratinocyte (HFK) WT and HFK p300 KO cells that are positive for Alt-EJ following transfection with (C) terminal or (D) imbedded determined by flow cytometry. (E–F) Percentage of HFK cells treated with DMSO or CCS1477 (1 μM) that are positive for Alt-EJ following transfection with (E) terminal or (F) imbedded determined by flow cytometry. All values are represented as mean ± standard error. The statistical significance of differences between cell lines and treatments were determined using Student’s t-test. p-Values indicate significant difference between LXSN and 8E6 (A–B); WT and p300KO (C–D); and DMSO and CCS1477 treatment (E–F). Twenty thousand cells were counted for each of three independent flow cytometry experiments.
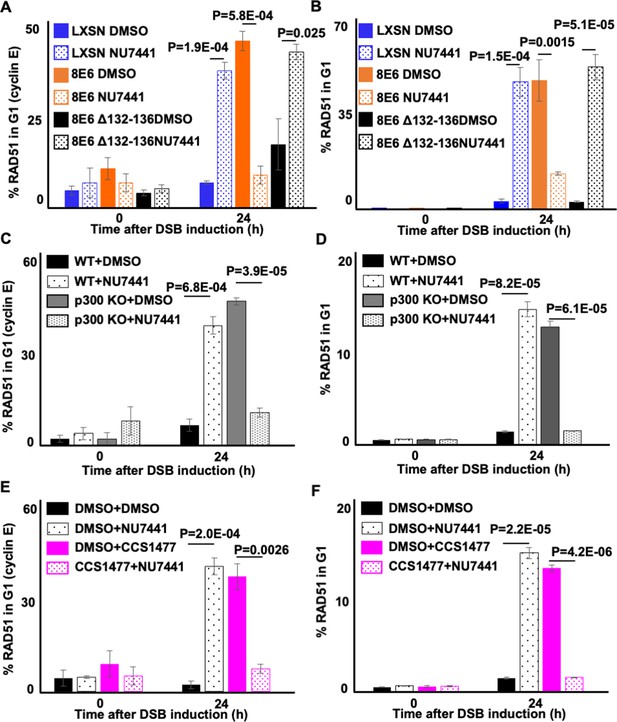
NU7441 abrogates RAD51 in G1 induced by losing p300 activity.
(A–B) Percentage of U2OS treated with DMSO or NU7441 (1 μM) in G1 that RAD51 staining after zeocin treatment (10 µg/mL, 10 min) as determined by (A) cyclin E staining or (B) flow cytometry. (C–D) Percentage of human foreskin keratinocyte (HFK) cells treated with DMSO or NU7441 in G1 that RAD51 staining after zeocin treatment determined by (C) cyclin E staining or (D) flow cytometry. (E–F) Percentage of HFK cells treated with DMSO, CCS1477 (1 μM), or NU7441 in G1 that RAD51 staining after zeocin treatment determined by (E) cyclin E staining or (F) flow cytometry. All values are represented as mean ± standard error. The statistical significance of differences between treatments were determined using Student’s t-test. p-Values indicate significant difference between DMSO and NU7441 with same cell line. At least 150 cells were counted over three independent experiments for microscopy. Twenty thousand cells were counted for each of three independent flow cytometry experiments.
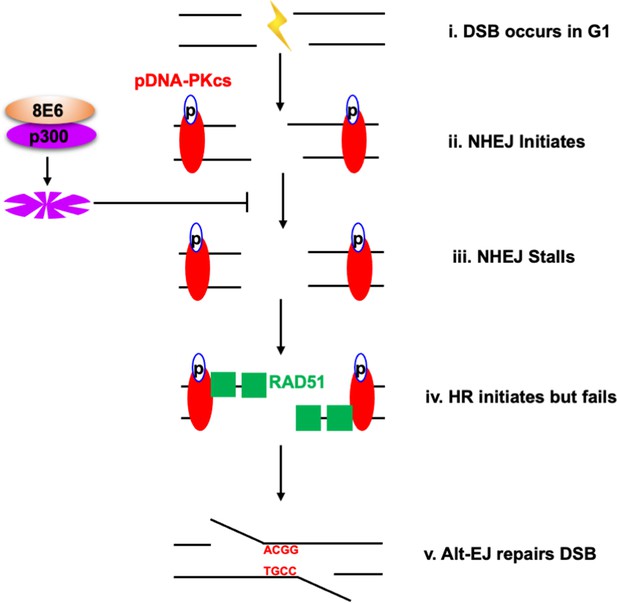
Alternative end joining (Alt-EJ) repairs double strand breaks (DSBs) in cells expressing 8E6.
(i) DSB occurs in G1 phase in cells expressing 8E6. (ii) Non-homologous end joining (NHEJ) initiates with auto-phosphorated DNA-dependent protein kinase catalytic subunit (DNA-PKcs). (iii) 8E6 stalls NHEJ by degrading p300 (Hu et al., 2020). (iv) Homologous recombination (HR) initiates and fails at the site of failed NHEJ (Hu and Wallace, 2022c). (v) Finally, PARP-1-dependent Alt-EJ repairs the DSB, which lead to microhomology mediated indels.
Tables
Read counts.
| LXSN | Reads | Quality reads | 8E6 | Reads | Quality reads |
|---|---|---|---|---|---|
| R1 | 480,023,697 | 466,581,986 | R1 | 326,902,764 | 312,236,357 |
| R2 | 480,023,697 | 466,581,986 | R2 | 326,902,764 | 312,236,357 |
Genome coverage of reads normalized to 624,472,714.
| Samples | Mean read depth | Coverage (%) | Reads mapped (%) |
|---|---|---|---|
| LXSN | 45.4 | 92.4 | 99.74 |
| 8E6 | 44.6 | 92.4 | 99.76 |
Summary of variant analysis reads normalized to 624,472,714.
| Genome (GRCh37) | LXSN | 8E6 |
|---|---|---|
| Number of variants processed | 1,031,891 | 928,763 |
| Number of effects | 2,514,053 | 2,251,061 |
| Genome total length | 3,234,834,690 | 3,234,834,690 |
| Genome effective length | 3,095,677,413 | 3,095,677,413 |
| Variant rate | 1 variant every 3000 bases | 1 variant every 3333 bases |
| Number of annotated genes | 71,845 | 71,078 |
| Insertions | 476,516 | 431,055 |
| Deletions | 555,375 | 497,708 |
Characteristics of microhomology (Mh) mediated short deletions (2–29 bp) in HFK 8E6 cells.
| LXSNtotal | Minimum Mh (bp) | Alt-EJ | Frequency(%) | E6total | Minimum Mh (bp) | Alt-EJ | Frequency(%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| 49,119 | 2 | 40,440 | 82 | 32,763 | 2 | 28,425 | 87 | 2.2e-16 |
| 3 | 22,580 | 46 | 3 | 15,512 | 47 | 1.13e-4 | ||
| 4 | 16,370 | 33 | 4 | 11,337 | 35 | 1.61e-4 | ||
| 5 | 10,826 | 22 | 5 | 7664 | 23 | 6.06e-6 | ||
| 6 | 8918 | 18 | 6 | 6468 | 20 | 1.33e-8 | ||
| 7 | 7076 | 14 | 7 | 5037 | 15 | 1.37e-4 | ||
| 8 | 6243 | 13 | 8 | 4557 | 14 | 7.16e-7 | ||
| 9 | 4870 | 10 | 9 | 3507 | 11 | 2.72e-4 | ||
| 10 | 4392 | 9 | 10 | 3165 | 10 | 5.22e-4 | ||
| 11 | 3621 | 7 | 11 | 2498 | 8 | 0.1825 | ||
| 12 | 3234 | 7 | 12 | 2292 | 7 | 0.0222 | ||
| 13 | 2448 | 5 | 13 | 1700 | 5 | 0.1957 | ||
| 14 | 2211 | 5 | 14 | 1522 | 5 | 0.3412 | ||
| 15 | 1847 | 4 | 15 | 1185 | 4 | 0.2958 | ||
| 16 | 1602 | 3 | 16 | 1060 | 3 | 0.8522 | ||
| 17 | 1238 | 3 | 17 | 763 | 2 | 0.0861 | ||
| 18 | 1083 | 2 | 18 | 682 | 2 | 0.2440 | ||
| 19 | 866 | 2 | 19 | 509 | 2 | 0.0240 | ||
| 20 | 765 | 2 | 20 | 467 | 1 | 0.1358 |
Characteristics of microhomology (Mh) long deletions (30–500 bp) in human foreskin keratinocyte (HFK) 8E6 cells.
| LXSNtotal | Minimum Mh (bp) | Alt-EJ | Frequency(%) | E6total | Minimum Mh (bp) | Alt-EJ | Frequency(%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| 2612 | 2 | 1959 | 75 | 1168 | 2 | 888 | 76 | 0.5247 |
| 3 | 1844 | 71 | 3 | 842 | 72 | 0.3704 | ||
| 4 | 1735 | 66 | 4 | 782 | 67 | 0.7790 | ||
| 5 | 1689 | 65 | 5 | 765 | 65 | 0.6460 | ||
| 6 | 1635 | 63 | 6 | 722 | 62 | 0.6734 | ||
| 7 | 1594 | 61 | 7 | 693 | 59 | 0.3429 | ||
| 8 | 1536 | 59 | 8 | 673 | 58 | 0.5171 | ||
| 9 | 1487 | 57 | 9 | 656 | 56 | 0.6868 | ||
| 10 | 1433 | 55 | 10 | 634 | 54 | 0.7669 | ||
| 11 | 1381 | 53 | 11 | 616 | 53 | 0.9684 | ||
| 12 | 1332 | 51 | 12 | 596 | 51 | 1.0000 | ||
| 13 | 1286 | 49 | 13 | 568 | 49 | 0.7580 | ||
| 14 | 1245 | 48 | 14 | 543 | 46 | 0.5265 | ||
| 15 | 1202 | 46 | 15 | 530 | 45 | 0.7410 | ||
| 16 | 1168 | 45 | 16 | 505 | 43 | 0.4172 | ||
| 17 | 1131 | 43 | 17 | 485 | 42 | 0.3249 | ||
| 18 | 1089 | 42 | 18 | 468 | 40 | 0.3673 | ||
| 19 | 1051 | 40 | 19 | 451 | 39 | 0.3644 | ||
| 20 | 1010 | 39 | 20 | 433 | 37 | 0.3697 |
Filtered variants for Alt-EJ analyses.
| LXSN | 8E6 | |
|---|---|---|
| Pre-filtered | 879,302 | 820,766 |
| Unique INDELs | 168,005 | 104,322 |
Short deletions bearing microhomology signatures of Alt-EJ.
| Sample | Short deletions (2–29 bp) | Matching Alt-EJ | Frequency (%) | p-Value |
|---|---|---|---|---|
| LXSN | 49,119 | 40,440 | 82.33 | |
| 8E6 | 32,763 | 28,425 | 86.75 | 2.2e-16 |
Long deletions bearing microhomology signatures of Alt-EJ.
| Sample | Long deletions (≥30 bp) | Matching Alt-EJ | Frequency (%) | p-Value |
|---|---|---|---|---|
| LXSN | 2612 | 1959 | 75.00 | |
| 8E6 | 1168 | 888 | 76.03 | 0.5247 |
Short insertions bearing microhomology signatures of Alt-EJ.
| Sample | Short insertions (<5 bp) | Matching Alt-EJ | Frequency (%) | p-Value |
|---|---|---|---|---|
| LXSN | 50,390 | 959 | 1.90 | 5.62e-10 |
| 8E6 | 27,611 | 367 | 1.33 |
Long insertions bearing microhomology signatures of Alt-EJ.
| Sample | Long insertions (≥18 bp) | Matching Alt-EJ | Frequency % | p-Value |
|---|---|---|---|---|
| LXSN | 5485 | 1068 | 19.47 | 1.547e-05 |
| 8E6 | 2545 | 392 | 15.40 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HFK | This paper | Derived from neonatal foreskins | |
| Cell line (Homo sapiens) | N/TERT HFK | Michael Underbrink (PMID:18256157) | N/TERT immortalized HFK | |
| Cell line (Homo sapiens) | U2OS | PMID:10541549 | Cell line used to measure HR frequency | |
| Recombinant DNA reagent | Alt-EJ reporter | Addgene | #113619 | Alt-EJ reporter using 4 nt microhomology |
| Recombinant DNA reagent | Alt-EJ reporter (5’ end) | Addgene | #113620 | sgRNA/CAS9 to induce the 5’ end DSB |
| Recombinant DNA reagent | Alt-EJ reporter (terminal) | Addgene | #113625 #113625 | sgRNA/CAS9 to induce the DSB at the edge of the microhomology |
| Recombinant DNA reagent | Alt-EJ reporter (imbedded) | Addgene | #113626 | sgRNA/CAS9 to induce the DSB 8 nt upstream of the microhomology |
| Antibody | Anti-RAD51 (Mouse monoclonal) | Abcam | ab1837 | IF (1:200) |
| Antibody | Anti-cyclin E (Rabbit monoclonal) | Cell Signaling | 4132S | IF (1:200) |
| Antibody | Anti-pH2AX S139 (Rabbit monoclonal) | Cell Signaling | 9718S | IF (1:200) |
| Antibody | Anti-cyclin A (Mouse monoclonal) | Abcam | ab39 | IF (1:200) |
| Antibody | Alexa Fluor 594 (Goat polyclonal) | Thermo Fisher Scientific | A11012 | IF (1:500) |
| Antibody | Alexa Fluor 488 (Goat polyclonal) | Thermo Fisher Scientific | A11001 | IF (1:500) |
| Chemical compound, drug | CCS1477 | Chemietek | CT-CCS1477 | P300 inhibitor |
| Chemical compound, drug | NU7441 | Selleckchem | S2638 | DNA-PKcs inhibitor |
| Chemical compound, drug | Zeocin | Alfa Aesar | J67140-XF | Used to induce DSBs |
| Chemical compound, drug | ART558 | MedChem Express | HY-141520 | Pol Theta inhibitor |
| Chemical compound, drug | DAPI stain | Invitrogen | D1306 | IF (10 µM) |
| Chemical compound, drug | NUCLEAR-ID Red | Enzo Life Science | ENZ-52406 | Flow cytometry (1:1000) |
| Software, algorithm | ImageJ | ImageJ (https://imagej.nih.gov/ij/) | Version 2.3.0 | |
| Software, algorithm | GraphPad Prism | GraphPad Prism (https://graphpad.com) | Version 9.0.0 |





