Gasotransmitter modulation of hypoglossal motoneuron activity
Figures

Disruption of hemeoxygenase-2 (HO-2) impairs inspiratory activity from the hypoglossal nucleus and the preBötC.
(A) HO-2 (red bottom left) expression co-localized to ChAT+ cells (green bottom right) of the hypoglossal nucleus (XIIn, overlay top, n=3). (B–F) Population recordings of rhythmic brain slices were made from ipsilateral preBötC and XIIn simultaneously, analyses were performed in baseline and during bath application of 20 µM ChrMP459. (B) Integrated traces of network activity in spontaneously rhythmic brainstem slices (n=34) recorded from XIIn (gray) and preBötC (black) before (top) and during (bottom) ChrMP459. Failed transmission events are highlighted (pink box) and subnetwork preBötC activity (#, defined as preBötC events with an integrated burst area ≤50% of the mean integrated burst area in baseline and are #) are evident in ChrMP459. Scale bar: 5 s. (C) Comparison of integrated amplitude irregularity score (IrSAMP) during baseline and in ChrMP459 from the XIIn (top) and the preBötC (bottom, n=34). Solid lines within violin plots illustrate IrSAMP from individual experiments. Thick dashed line illustrates mean IrSAMP. (D) Heat maps of I/O ratios from 25 consecutive cycles in baseline and ChrMP459. Each row reflects an individual experiment while each cell represents the I/O ratio for a given cycle. Gray boxes indicate non-events from recordings from slower rhythms where less than 25 cycles occurred during the analysis window. (E) Comparison of transmission from preBötC to XIIn between Baseline (gray) and ChrMP459 (purple). Solid lines within violin plots illustrate transmission from individual slices. Thick dashed line illustrates mean transmission value. (F) Distribution of transmitted (blue) and untransmitted (purple) preBötC bursts in ChrMP459. These values are expressed as a percentage of the total of preBötC events detected from all experiments and are binned. Bin interval = 0.1 intervals of the normalized integrated burst area. preBötC bursts were normalization to the mean integrated burst area during baseline. Statistical analysis for all comparisons via paired t-test; error bars: Standard Error Measurement (SEM); significance level P<0.05.

Genetic deletion of HO-2 reduces the I/O relationship between preBötC and the hypoglossal nucleus and uncouples of motor output from inspiratory rhythmogenesis.
(A) Representative integrated traces of network rhythms in the preBötC and XIIn from wild-type (WT; left, n=18) and HO-2 null (right, n=11) slices. Failed transmissions (pink box) and subnetwork preBötC activity (#) are evident in HO-2 null slices. Scale bar: 4 s. (B) Comparison of IrSAMP in the preBötC of WT (blue) and HO-2 null (red) slices. (C) Comparison of IrSAMP in the XIIn of WT (blue) and HO-2 null (red) slices. (D) Heat maps of cycle-to-cycle I/O ratios from individual experiments performed in WT (left) and HO-2 null (right) slices. Gray boxes indicate non-events in recordings from slower rhythms where less than 25 events occurred during the analysis window. (E) Comparison of transmission from preBötC to XIIn between WT (blue) and HO-2 null (red) slices. Thin gray and purple lines illustrate transmission from individual slices. Thick dashed lines illustrate mean transmission value. (F) Distribution of transmitted (gray) and untransmitted (red) preBötC bursts in HO-2 null slices. These values are expressed as a percentage of the total of preBötC events detected from all experiments and are binned. Bin interval = 0.1 intervals of the normalized integrated burst area. preBötC bursts were normalization to the mean integrated burst area from each individual recording. Statistical analysis for all comparisons via unpaired t-test; error bars: SEM; significance level P<0.05.

While ChrMP459 does not change transmission from the preBötC to the premotor area, ChrMP459 increases transmission failure from the premotor area to the hypoglossal nucleus.
(A) Diagram of medullary brain slice illustrating relative electrode placement for simultaneous triple extracellular recordings (n=5) from the XIIn (light gray, 1), premotor field (dark gray, 2), and preBötC (black, 3). Corresponding representative traces of integrated network activity in Baseline (left) and in 20 μM ChrMP459 (right). Failed transmission (pink box) from preBötC to XIIn and preBötC subnetwork burst activity (#) are evident in ChrMP459; scale bar: 5 s. (B) Heat maps of the cycle to cycle I/O ratio from individual slices (left) and transmission (right) between preBötC and the premotor field. (C) Heat maps of the cycle to cycle I/O ratio from individual slices (left) and transmission (right) between the premotor field and XIIn. Statistical analysis for all comparisons via paired t-test; error bars: SEM; significance level P<0.05.

Heme oxygenase inhibition reduces inspiratory drive currents in hypoglossal neurons.
Whole cell patch clamp recordings were made from hypoglossal neurons in rhythmic brain slices while simultaneously recording ipsilateral preBötC activity in Baseline and in ChrMP459. Neurons were disinhibited from fast synaptic inhibition using 50 μM PTX and 1 μM Strychnine (DI). (A) (left) Representative voltage clamp recordings from a XIIn neuron (Vholding = –60 mV) aligned with corresponding integrated network activity from preBötC before (DI Baseline, top, gray) and after 20 μM ChrMP459 (DI ChrMP459, bottom, purple). Scale bar: 1 s x 10 pA. (middle) Magnification of highlighted (red dotted box) drive currents from DI Baseline (gray) and DI ChrMP459 (purple). Scale bar: 100 ms x 20 pA. (right). Comparison of XIIn inspiratory drive current magnitude distribution in DI Baseline (gray) and DI ChrMP459 (n=19, purple). Thin solid lines illustrate individual neuron response. Dashed black line illustrates mean drive current. (B) (left) Representative current clamp recordings from a spontaneously active XIIn neuron with the preBötC network rhythm in DI Baseline (top, gray) and DI ChrMP459 (bottom, purple); skipped transmission of action potentials in DI ChrMP459 are highlighted (pink box). Scale bar 2 s x 20 mV. (middle) Magnification of highlighted neuronal activity (red dashed box in trace, left). Scale bars: 100 msec x 25 mV. (right) Distribution of the average number of action potentials generated per inspiratory burst in DI Baseline (gray) and in DI ChrMP459 (n=17, purple). Thin solid lines illustrate individual neuron response. Dashed black line illustrates mean action potentials per drive. (C) (left) Representative trace of current clamp recording in response to ramp current injection during DI Baseline (gray trace) and in DI ChrMP459 (purple trace); scale bar: 500 ms. (right) Comparison of rheobase distributions found in inspiratory XIIn neurons during DI Baseline (gray) and in DI ChrMP459 (n=19, purple). Thin solid lines illustrate individual neuron response. Dashed black line illustrates mean Rheobase.Statistical analysis for all comparisons via paired t-test; error bars: SEM; significance level P<0.05.
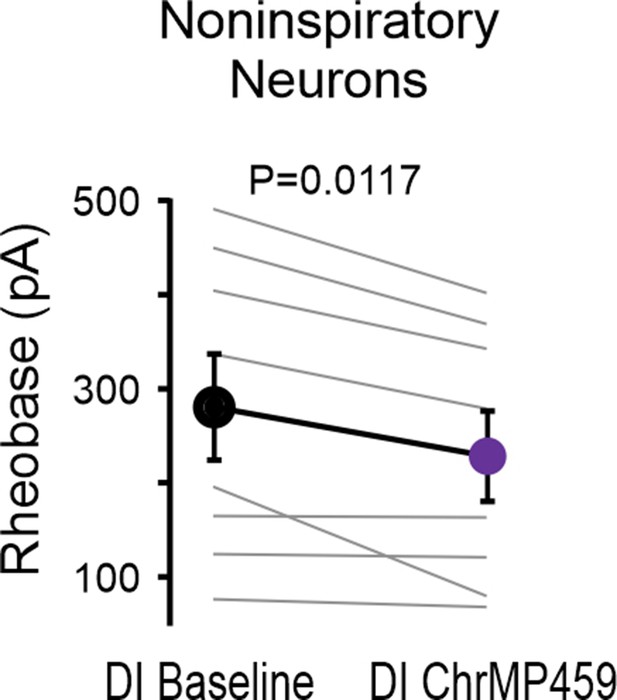
ChrMP459 decreases rheobase of non-inspiratory hypoglossal neurons.
Comparison of rheobase from non-inspiratory hypoglossal neurons in baseline and ChrMP (n=8). Non-inspiratory hypoglossal neurons were defined as neurons that did not receive synaptic drive in-phase with preBötC bursts. Neurons were disinhibited from fast synaptic inhibition using 50 μM PTX and 1 μM Strychnine.Statistical analysis for all comparisons via paired t-test; error bars: SEM; significance level P<0.05.
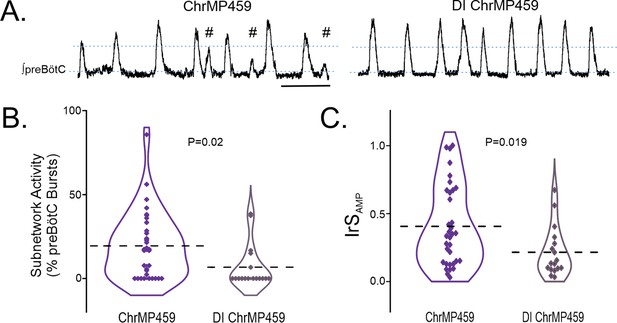
Disinhibition reduces ChrMP459-induced subnetwork activity and amplitude irregularities in preBötC population activity.
(A) Representative integrated trace of preBötC activity from slices with bath application of ChrMP459 (top, n=34) and disinhibited slices with ChrMP459 and 50 μM PTX and 1 μM Strychnine for fast synaptic inhibition (DI ChrMP459, bottom, n=17). Subnetwork preBötC activity (#) is identified in ChrMP459. Scale bar 5 sec. (B) Comparison of subnetwork activity in preBötC slices as a percentage of total bursts (subnetwork ≥%50 average burst area in baseline) in ChrMP459 (purple) and DI ChrMP459 (dark purple). Individual values represented by ◊, thick dashed line represents mean subnetwork activity. (C) Comparison of IrSAMP between ChrMP459 (purple, replotted from Figure 1C preBötC) and DI ChrMP459 (dark purple). Individual values represented by ◊, thick dashed line represents mean IrSAMP. Statistical analysis for all comparisons via paired t-test; error bars: SEM; significance level P<0.05.

CSE-dependent H2S is produced in the hypoglossal nucleus and exogenous NaHS application uncouples hypoglossal nucleus activity from the preBötC.
(A) CSE (red, bottom left) expression co-localizes with ChAT+ neurons (green, bottom right) in the XIIn (overlay, top). Scale bar 50 μm. (B) CSE-dependent H2S generation in pooled homogenates from WT and HO-2 null. Homogenates were prepared from tissue punches from the XIIn (red area in slice diagram) and inferior olive (gray area in slice diagram) at bregma between –7.20 mm and –7.90 mm. (WT: XIIn n=6; HO-2 null: XIIn n=6, inferior olive n=4). Each n in B represents a biological replicate consisting of the corresponding anatomical region pooled from two animals. (C) Integrated traces from XIIn (top) and preBötC (bottom) during Baseline (black), and in response to the H2S donor, NaHS, at 50 μM (dark gray) and 100 μM (light gray). NaHS application caused XIIn but not preBötC burst amplitude to diminish (blue dashed box) and in some cases, preBötC drive failed to produce activity in the XIIn (pink boxes). (D) Comparison of transmission from preBötC to XIIn after NaHS application at 10 μM, 50 μM and 100 μM. (E) I/O ratios for each NaHS concentration. (Baseline: n=9; 10 μM n=5; 50 μM n=6; 100 μM n=9). Statistical analysis for all comparisons via one-way ANOVA with Dunnett’s correction; error bars: SEM; significance level P<0.05.

HO-dependent transmission failures can be recovered with CO-donor CORM-3 and are not present in HO-2:CSE null transmission.
(A) Representative integrated traces from preBotC and XIIn in WT slices during Baseline (left), in ChrMP459 alone (middle) and in ChrMP459 +20 µM CORM-3 (CORM-3, right). Both subnetwork (#) and failed transmissions (pink rectangle) are highlighted. (B) Heat maps of cycle-to-cycle I/O ratios during dysregulated HO-2 (n=8: n=4 HO-2 null and n=4 WT-ChrMP459) before and after CORM-3 application. Gray boxes indicate non-events in recordings from slower rhythms where less than 25 events occurred during the analysis window. (C) Comparison of transmission from preBötC to XIIn from dysregulated HO-2 slices before (red) and after (green) bath application of CORM-3. (D) Representative integrated traces from preBötC and XIIn in slices from HO-2:CSE null; scale bar 2 sec. (E) Heat map of cycle-to-cycle I/O ratio from preBötC to XIIn in HO-2:CSE null. The I/O ratio from HO-2:CSE null is greater than I/O ratios from HO-2 null (n=7, p=0.003). Gray boxes indicate non-events in recordings from slower rhythms where less than 25 events occurred during the analysis window. (F) Comparison of transmission from preBötC to XIIn in HO-2 null (red, n=7, subset replotted from Figure 2) and HO-2:CSE null (light blue, n=6). HO-2 null data used for comparisons in E and F are a subset of the data originally shown in Figure 2. Statistical analysis for B and C via paired t-test, analysis for E and F via unpaireed t-test; error bars: SEM; significance level P<0.05.
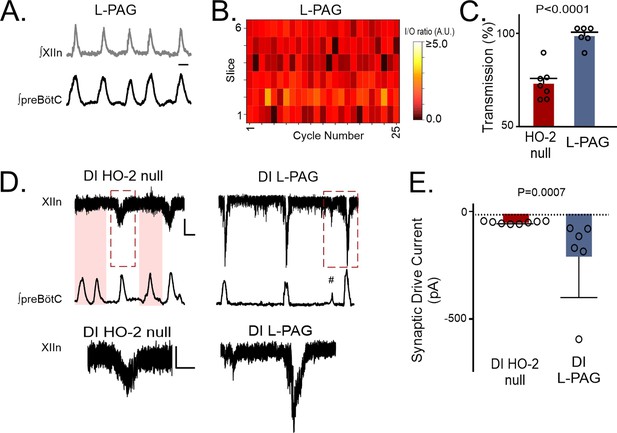
Transmission failures in HO-2 null mice are rescued with CSE inhibitor L-propargylglycine.
(A) Representative integrated traces of preBötC (bottom) and XIIn (top) in slices from HO-2 null mice treated with L- propargylglycine (L-PAG, 30 mg/kg, n=5). Scale bar 2 sec. (B) Heat map of cycle-to-cycle I/O ratio in rhythmic slices from HO-2 null mice treated with L-PAG (n=6). (C) Comparison of transmission between HO-2 null (n=7, subset replotted from Figure 2; red) and L-PAG slices (n=6, blue). (D) Representative voltage clamp recordings of inspiratory drive currents received by hypoglossal neurons from DI HO-2 null (n=8, left) and DI L-PAG (n=6, right). Neurons were disinhibited from fast synaptic inhibition using 50 μM PTX and 1 μM Strychnine. Scale bar 100 ms x 20 pA. Skipped transmission between preBötC (bottom) the XIIn neuron (top) occurs in untreated DI HO-2 null (highlighted pink boxes) but not in neurons from DI L-PAG. Subnetwork transmission to XIIn neuron in DI L-PAG (#). Magnified representative (red dashed box) drive potentials from DI HO-2 null and DI L-PAG. scale bars: 100 msec x 10 pA. (E) Comparison of average synaptic drive currents received by XIIn motoneurons from DI HO-2 null mice (n=8, red) produce smaller drive potentials when compared to DI L-PAG (n=6, blue). Statistical analysis for all comparisons via unpaired t-test; error bars: SEM; significance level P<0.05.
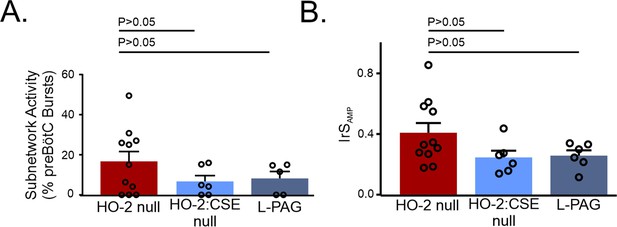
Effects of genetic ablation of CSE and CSE pharmacological inhibition on the preBötC in the HO-2 null slice.
HO-2:CSE null slices and slices from HO-2 null mice treated with L-PAG prior to slice preparation were compared to HO-2 null slices. (A) Comparison of subnetwork preBötC activity in HO-2 null (red) experiments to HO-2:CSE null (light blue) and L-PAG (dark blue) experiments. (B) Comparison of IrS in ChrMP459 and DI ChrMP459. HO-2: n=11; HO-2:CSE null: n=6; and L-PAG: n=5. Statistical analysis for all comparisons via one-way ANOVA with Dunnett’s correction; error bars: SEM; significance level P<0.05.
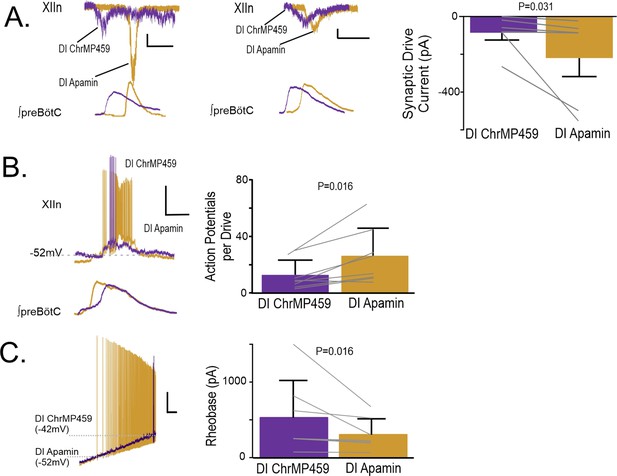
Apamin reverses changes to hypoglossal neurons’ intrinsic and synaptic excitability caused by HO dysregulation.
(A) Representative synaptic drive current received by hypoglossal neurons in DI ChrMP459 (purple) and in DI Apamin (200 μM, gold). Left, depicts an example of apamin increasing the drive current >100 pA. Scale bars: 1 s x 50 pA., whereas middle, representative inspiratory drive current hypoglossal neuron in DI ChrMP459 (purple) and in DI Apamin (gold) where apamin increased the drive current by less than <100 pA Scale bars: 1 s x 50 pA. Comparison of inspiratory drive currents from hypoglossal neurons exposed to DI ChrMP459 (purple) to DI Apamin (n=6, gold). The effect of ChrMP459 on baseline disinhibited drive current for each of these neurons were reported in Figure 4A. (B). (left) Representative current clamp recordings from a spontaneously active XIIn neuron with the preBötC network rhythm during DI ChrMP459 (purple) and in DI Apamin (n=8, gold). Scale bars: 20 mV x 500 ms. (right) Comparison of action potentials generated per preBötC burst during DI ChrMP459 (purple) and DI Apamin (n=8, gold). The effect of DI ChrMP459 on baseline action potential generated per preBötC burst for each of these neurons were reported in Figure 4D. (C) (left) Representative traces of current clamp recordings in response to ramp current injection in DI (purple) and in DI Apamin (gold). Scale bar: 500 ms x 10 mV. (right) Rheobase comparison from inspiratory XIIn neurons during DI ChrMP459 (purple) and DI Apamin (n=7, gold). The effect of DI ChrMP459 on baseline rheobase for each of these neurons were reported in Figure 4F. Statistical analysis for all comparisons via paired t-test with Wilcoxon Correction; error bars: SEM; significance level P<0.05.
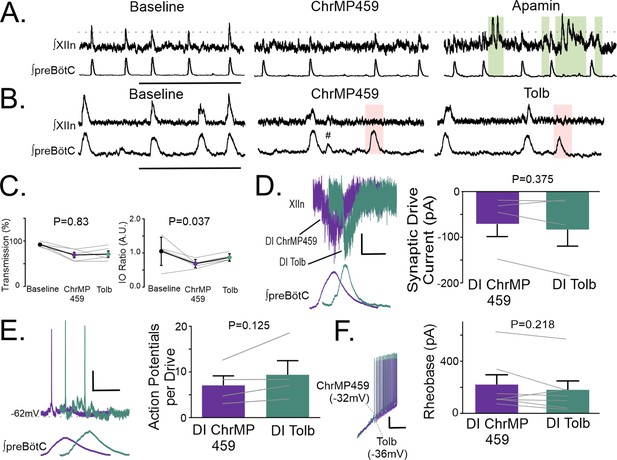
Apamin produce ectopic hypoglossal network activity during ChrMP459 and tolbutamide has limited effects on network and inspiratory hypoglossal neurons activity during ChrMP459.
(A) Representative traces of integrated network activity of preBötC (top) and XIIn (bottom) in slices from WT mice. Baseline (left), ChrMP459 (middle), and ChrMP459 with 200 μM Apamin (right, Apamin). Green box indicates ectopic network bursting. Scale bar 10 s. Due to ectopic bursting within and around rhythmic burst transmissions, analysis of apamin at the network level could not be accurately detected. (B) Representative traces of integrated network activity of preBötC (top) and XIIn (bottom) in slices from WT mice. Baseline (left), ChrMP459 (middle), and ChrMP459 with tolbutamide (right, 100 μM, Tolb). Pink box indicates cycles where preBötC drive failed to produce activity in the XIIn and # demarcates subnetwork bursts. (C) Comparisons of Transmission (left) and I/O ratio (right) in Baseline, ChrMP459, and Tolb (n=5). (D–F) Hypoglossal neurons were recorded under DI conditions to determine the effect of Tolb (DI Tolb) on ChrMP459 dysregulation. (D) (top) Representative traces of inspiratory drive currents in DI ChrMP459 (purple) and in DI Tolb (green). Scale bar 1 s x 50 pA. (bottom) Comparison synaptic drive currents in DI ChrMP459 (purple) and DI Tolb (n=4, green). The effect of DI ChrMP459 on baseline drive current for each of these neurons were reported in Figure 4B. (E). (left) Representative current clamp recordings from a spontaneously active XIIn neuron with the preBötC network rhythm during DI ChrMP459 (purple) and in DI Tolb (n=4, green). Scale bars: 10 mV x 500 ms. (right) Comparison of action potentials generated per preBötC burst during DI ChrMP459 (purple) and DI Tolb (n=4, green). The effect of DI ChrMP459 on baseline action potentials generated per preBötC burst for each of these neurons were reported in Figure 4C. (F) (left) Representative traces of current clamp recordings in response to ramp current injection in DI ChrMP459 (purple) and in DI Tolb (green). Scale bar: 1 sec x 10 mV. (right) Rheobase comparison from inspiratory hypoglossal neurons during DI ChrMP459 (purple) and DI Tolb (n=7, green). The effect of DI ChrMP459 on rheobase for each of these neurons were reported in Figure 4C. Statistical analysis for all comparisons via paired t-test with Wilcoxon Correction; error bars: SEM; significance level P<0.05.
Tables
Properties of Network Activity in the preBötC and XIIn during dysregulated HO-2.
Analysis of instantaneous frequency (finst) and integrated burst amplitude of network activity in the preBötC and hypoglossal nucleus. Statistical analysis for all comparisons via paired t-test. Values are displayed as mean ± SEM (n). Significance level P<0.05.
| finst (Hz) | Burst Amplitude (mV) | ||||||
|---|---|---|---|---|---|---|---|
| Experiment | Recording Location | Control* (n) | Dysregulated HO-2 (n) | p-value | Control* (n) | Dysregulated HO-2 (n) | p-value |
| ChrMP459 | preBötC | 0.225±0.014 (34) | 0.242±0.019 (34) | 0.139 | 0.078±0.011 (34) | 0.08±0.012 (34) | 0.626 |
| HO-2 null slice | preBötC | 0.235±0.022 (18) | 0.420±0.049 (11) | 0.0006 | 0.059±0.007 (18) | 0.051±0.010 (11) | 0.463 |
| ChrMP459 | XIIn | 0.239±0.015 (34) | 0.221±0.015 (34) | 0.044 | 0.047±0.008 (34) | 0.046±0.009 (34) | 0.804 |
| HO-2 null slice | XIIn | 0.256±0.018 (18) | 0.432±0.051 (11) | 0.0006 | 0.038±0.006 (18) | 0.021±0.003 (11) | 0.050 |
-
*
= Control in ChrMP459 experiments is defined as baseline activity prior to ChrMP459. Control in HO-2 null experiments is defined as recordings in wild-type slices.
Comparison of ChrMP459 and HO-2 null effects on preBötC and XIIn transmission properties.
Properties of pharmacological effects of HO-2 dysregulation on preBötC and XIIn activity via ChrMP459 application were compared to genetic dysregulation in HO-2 null mouse experiments. Instantaneous frequency (finst), integrated burst amplitude, irregularity of integrated burst amplitude, and frequency of subnetwork bursting (% of total bursts) were analyzed for preBötC activity. I/O ratio of integrated preBötC area input to XIIn output and percent transmission were compared between the two regions. XIIn disinhibited neurons synaptic drive potentials were also assessed. Statistical analysis for all comparisons via unpaired t-test; values presented as mean ± SEM; significance level P<0.05.
| Metric | ChrMP459 (n) | HO-2 null (n) | p-value |
|---|---|---|---|
| finst* (Hz) | 0.24±0.019 (34) | 0.42±0.049 (11) | <0.0001 |
| Burst Amplitude* (mV) | 0.08±0.012 (34) | 0.05±0.010 (11) | 0.189 |
| IrS AMP† (A.U.) | 0.41±0.050 (34) | 0.39±0.070 (11) | 0.127 |
| Subnetwork‡ (%) | 19.43±3.373 (34) | 16.70±4.898 (11) | 0.670 |
| I/O Ratio§ (A.U.) | 0.59±0.064 (34) | 0.79±0.074 (11) | 0.096 |
| Transmission¶ (%) | 75.10±3.425 (34) | 64.11±6.002 (11) | 0.119 |
| Synaptic Drive Current** (pA) | –95.31±21.790 (19) | –36.71±2.141 (8) | 0.097 |
-
*
Data originally reported in Table 1.
-
†
-
‡
data from Figure 5B and Figure 8—figure supplement 1.
-
§
data from Figure 1D and Figure 9—figure supplement 1.
-
¶
data from Figure 1E and Figure 9—figure supplement 1.
-
**





