Soluble amyloid-β precursor peptide does not regulate GABAB receptor activity
Figures
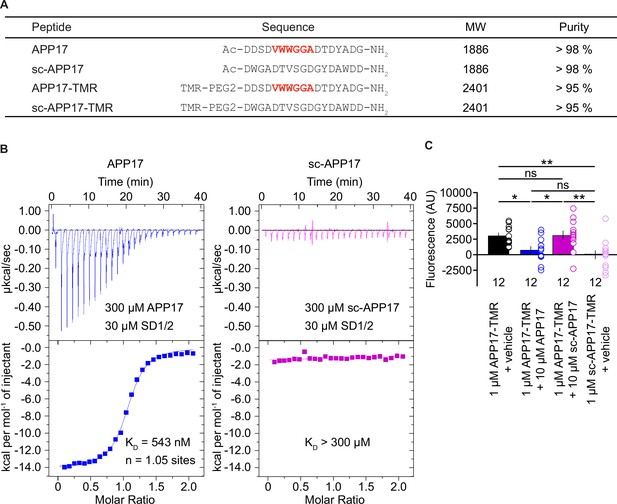
Characterization of synthetic APP17 and sc-APP17 peptides.
(A) Sequence alignment of APP17, sc-APP17, APP17-TMR, and sc-APP17-TMR peptides. Residues critical for sushi domain 1 (SD1) binding are shown in red. (B) Representative isothermal titration calorimetry (ITC) diagrams of the titrations of SD1/2 protein in solution (30 μm) with APP17 (blue) or sc-APP17 (magenta) (300 μm in the syringe); raw heat signature (top) and integrated molar heat release (bottom). The calculated stoichiometry of APP17:SD1/2 protein is 1.05, the KD 543 nM. sc-APP17 showed no binding to SD1/2 protein. (C) Bar graph showing APP17-TMR (1 μM) binding to GB1a/2 receptors in HEK293T cells in the presence of vehicle (black), 10 μM APP17 (blue), and 10 μM sc-APP17 (magenta). sc-APP17-TMR (1 μM) served as a negative control. The background fluorescence of sc-APP17-TMR (1 μM) at HEK293T cells transfected with empty vector was subtracted. Data are means ± SEM. The number of independent experiments is indicated. ns = not significant, *p<0.05, **p<0.01, one-way ANOVA with Holm-Sidak’s multiple comparisons test. Source file containing ICT and TMR fluorescence data is available in Figure 1—source data 1.
-
Figure 1—source data 1
Characterization of synthetic APP17 peptides.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig1-data1-v2.xlsx
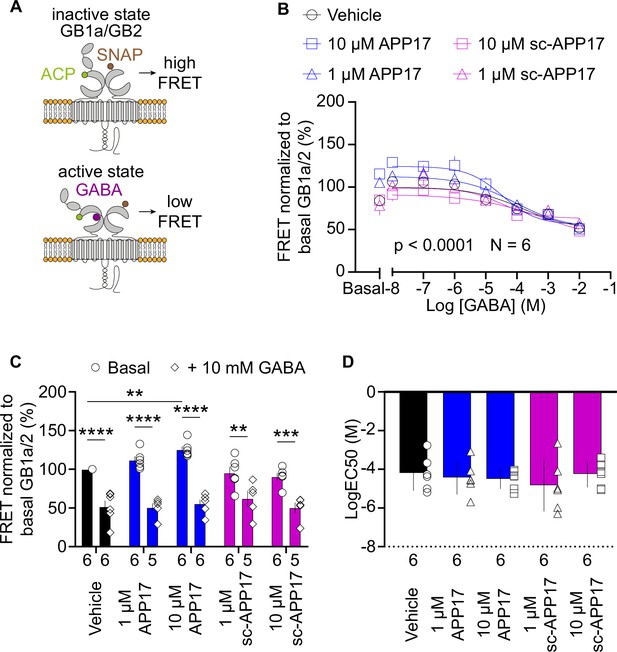
APP17 does not induce the active state of GB1a/2 receptors.
(A) Assay measuring inter-subunit fluorescence resonance energy transfer (FRET) between fluorophore labeled ACP and SNAP tags in the GB1a and GB2 subunits, respectively. In the absence of receptor agonists, the ACP and SNAP tags are in close proximity resulting in high FRET. Activation of GB1a/2 receptors induces a conformational change in the extracellular domains leading to a reduction in FRET. (B) GABA dose-response curves in the presence of APP17 (blue) or sc-APP17 (magenta) at 1 μM (triangles) and 10 μM (squares) or vehicle (black) exhibit significant differences. (C) Bar graphs showing FRET in the presence of 1 or 10 μM APP17 (blue) and sc-APP17 (magenta) or vehicle (black). Under basal conditions (circles), the presence of 10 μM APP17 resulted in a significant increase of FRET, whereas no significant changes in FRET were observed for all other conditions when compared to vehicle. In the presence of 10 mM GABA (diamonds) no significant differences in FRET were detected with APP17 or sc-APP17 at 1 or 10 μM compared to vehicle. In all conditions, the presence of 10 mM GABA induced a significant reduction in FRET compared to basal. (D) LogEC50 values of individual GABA dose-response curves exhibit no significant differences between conditions. Data are means ± SEM. Three outliers were identified in (C) using the ROUT method (PRISM) with Q=1% (source file). The number of independent experiments is indicated. **p<0.01, ***p<0.001, ****p<0.0001, two-way ANOVA with Sidak’s multiple comparisons test. Source file containing FRET data is available in Figure 2—source data 1.
-
Figure 2—source data 1
FRET analysis of GBR conformational changes.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig2-data1-v2.xlsx
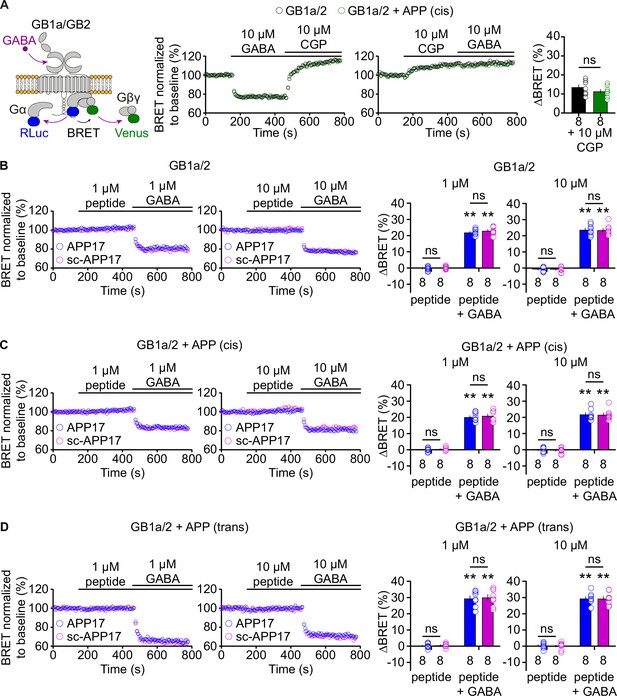
APP17 is not an agonist, inverse agonist, antagonist, or allosteric modulator at GB1a/2 receptors expressed in HEK293T cells in a bioluminescent resonance energy transfer (BRET) assay monitoring G protein activation.
(A) Left: Assay measuring BRET between Gαo-RLuc and Venus-Gγ2. GB1a/2 receptor activation leads to dissociation of the heterotrimeric G protein and a consequent decrease in BRET. Right: Individual experiments showing GABA-induced BRET changes at GB1a/2 receptors. The inverse agonist CGP54626 reverses GABA-induced BRET changes above baseline, indicating constitutive GB1a/2 receptor activity. Likewise, direct application of CGP54626 increased BRET levels above baseline. Subsequent application of GABA did not overcome receptor inhibition. Bar graphs summarize CGP54626-induced BRET changes. Note that application of CGP54626 resulted in similar inhibition of constitutive GB1a/2 receptor activity in the absence (black) or presence (green) of APP695 in cis. (B) Neither APP17 (blue) nor sc-APP17 (magenta) at 1 μM (left) or 10 μM (right) altered BRET in cells expressing GB1a/2 receptors. In the same cells, GABA at 1 μM (left) and 10 μM (right) induced the expected decrease in BRET. The GABA-induced BRET change is similar in the presence of APP17 and scAPP17, indicating the absence of allosteric properties of the peptides at GB1a/2 receptors. Bar graphs summarize BRET changes determined in experiments as shown to the left. (C,D) Neither APP17 (blue) nor sc-APP17 (magenta) at 1 μM (left) or 10 μM (right) altered BRET in cells expressing GB1a/2 receptors together with APP695 in cis (C) or in trans (D). Bar graphs summarize BRET changes. Data are means ± SEM. The number of independent experiments is indicated in the bar graphs. ns = not significant, two-way ANOVA with Sidak’s multiple comparisons test. **p<0.01, one-sample Wilcoxon test (non-parametric) against 0. Source file containing BRET data is available in Figure 3—source data 1.
-
Figure 3—source data 1
BRET analysis of GBR-mediated G protein activation.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig3-data1-v2.xlsx
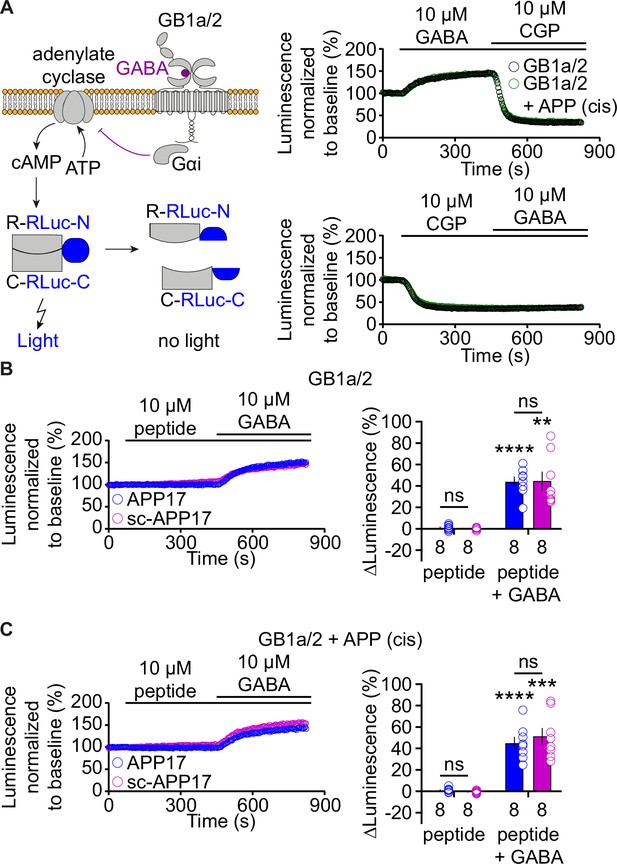
APP17 is not an agonist, inverse agonist, antagonist, or allosteric modulator at GB1a/2 receptors expressed in HEK293T cells in an assay monitoring Gαi signaling.
(A) Left: Assay monitoring dissociation of the regulatory (R) and catalytic (C) subunits of the tetrameric protein kinase A (PKA) holoenzyme upon cAMP binding. PKA subunits were tagged with N- or C-terminal fragments of RLuc (R-RLuc-N, C-RLuc-C). GB1a/2 receptor activation by GABA reduces intracellular cAMP levels, promotes reconstitution of RLuc activity, and increases luminescence. Right: Individual experiments showing GABA-induced luminescence changes. Blockade of GB1a/2 receptor activity with CGP54626 decreased luminescence below baseline, indicating constitutive GB1a/2 receptor activity. Subsequent application of GABA did not overcome receptor inhibition. (B) Neither APP17 (blue) nor sc-APP17 (magenta) altered luminescence in HEK293T cells expressing GB1a/2 receptors. In the same cells, GABA induced the expected luminescence increases. GABA-induced luminescence changes are similar in the presence of APP17 and sc-APP17. Bar graphs summarize the luminescence changes. (C) Neither APP17 (blue) nor sc-APP17 (magenta) induced luminescence changes in HEK293T cells expressing GB1a/2 receptors together with APP695 in cis. Application of GABA to the same cells resulted in the expected luminescence increases. GABA-induced luminescence changes are similar in the presence of APP17 or sc-APP17. Bar graphs summarize the luminescence changes. Data are means ± SEM. The number of independent experiments is indicated. ns = not significant, two-way ANOVA with Sidak’s multiple comparison test. **p<0.01, ***p<0.001, ****p<0.0001, one-sample t-test against 0. Source file containing PKA luminescence data is available in Figure 4—source data 1.
-
Figure 4—source data 1
Luminescence analysis of GBR-mediated Gαi singaling.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig4-data1-v2.xlsx
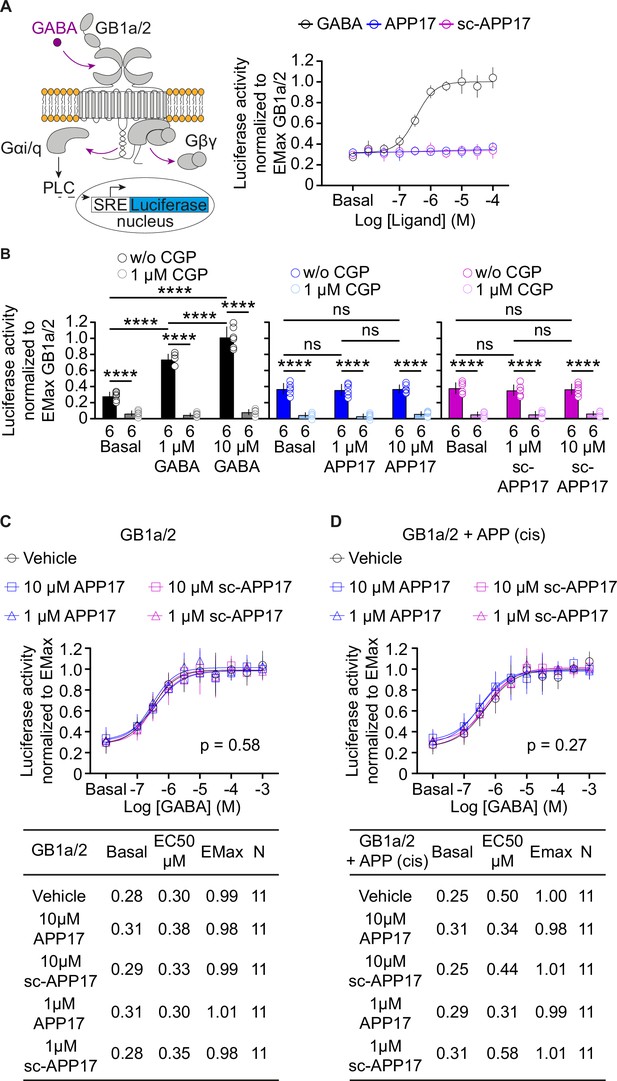
APP17 is not an agonist, inverse agonist, allosteric modulator, or antagonist at GB1a/2 receptors expressed in HEK293T cells when monitoring Gaqi signaling in an accumulation assay.
(A) Left: Assay monitoring phospholipase C (PLC)-dependent FLuc expression under control of the serum response element (SRE). GB1a/2 receptors were artificially coupled to PLC by stably expressing the chimeric G protein subunit Gαqi. GB1a/2 receptors and SRE-FLuc reporter were transiently expressed in HEK293T-Gαqi cells. Right: Dose-response curve showing that GABA (black) but not APP17 (blue) or sc-APP17 (magenta) induces FLuc activity in transfected cells. (B) CGP54626 blocked constitutive and GABA-induced FLuc activity in transfected cells. Constitutive GB1a/2 receptor activity is unchanged in the presence of APP17 (middle) or sc-APP17 (right) at 1 or 10 μM, indicating the absence of inverse agonistic properties of the peptides at GB1a/2 receptors. (C,D) APP17 (blue) or sc-APP17 (magenta) at 1 μM (triangles) or 10 μM (squares) did not significantly alter GABA dose-response curves in the absence (C) or presence (D) of APP695 in cis, indicating that the peptides do not allosterically regulate GB1a/2 receptors. Tables show basal, EC50, and Emax values derived from the curve fits. All data are mean ± SD. The number of independent experiments is indicated in the bar graphs or tables. Linear regression curve fit of 6 (APP17, sc-APP17, A) independent experiments per condition. Non-linear regression curve fits of 6 (GABA, A) or 11 (C,D) independent experiments per condition. p=0.58, p=0.27, extra sum-of-squares F test. Source file containing FLuc activity data is available in Figure 5—source data 1.
-
Figure 5—source data 1
SRE-luciferase analysis of GBR-mediated Gaqi signaling.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig5-data1-v2.xlsx
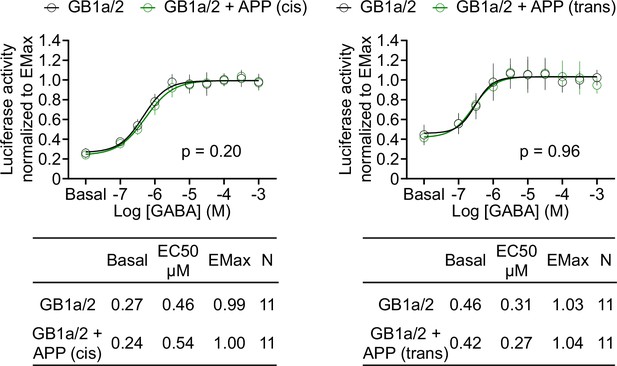
APP695 expressed in cis or in trans with GB1a/2 receptors exerts no allosteric effects on Gαqi signaling in HEK293T cells.
GABA dose-response curves show no difference in the absence (black) or presence of APP695 (green) in cis (left) or in trans (right). Tables show basal, EC50, and Emax values derived from the curve fits. All data are means ± SD. The number of independent experiments is indicated in the tables. Non-linear regression curve fits of 11 independent experiments per condition. p=0.20, p=0.96, extra sum-of-squares F test. Source file containing FLuc activity data is available in Figure 5—figure supplement 1—source data 1.
-
Figure 5—figure supplement 1—source data 1
SRE-luciferase analysis of GBR-mediated Gaqi signaling in the presence of APP695 in cis or in trans.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig5-figsupp1-data1-v2.xlsx
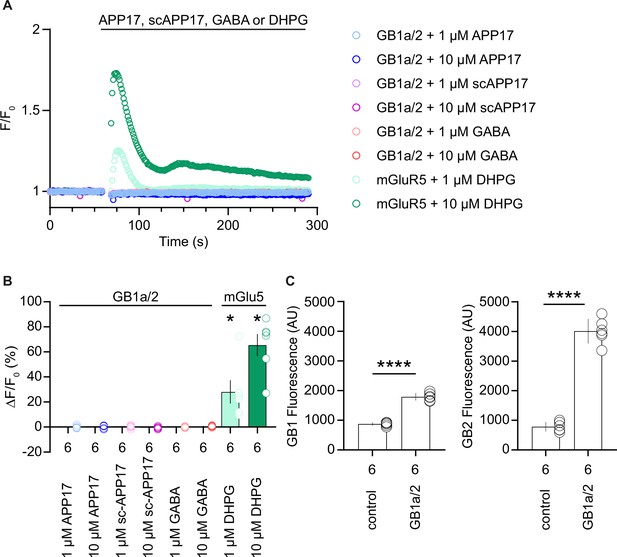
APP17 does not increase intracellular Ca2+ in HEK293T cells expressing GB1a/2 receptors.
(A) APP17, sc-APP17, and GABA at 1 or 10 µM did not increase intracellular Ca2+ in HEK293T cells expressing GB1a/2 receptors. Cells were loaded with the Ca2+ indicator Calcium 6. Control experiments with (S)-–3,5-DHPG at 1 and 10 µM showed concentration-dependent Ca2+ increases in cells expressing mGlu5. The fluorescence intensity relative to basal levels (F/F0) over time is depicted. (B) Bar graphs showing maximal fluorescence intensity changes in response to drug application to GB1a/2 or mGlu5 expressing cells. (C) Bar graphs showing GB1 and GB2 fluorescence levels (Alexa Fluor 647) in control or GB1a/2 receptor-expressing HEK293T cells. Data are mean ± SEM. The number of independent transfections is indicated. *p<0.05, one-sample Wilcoxon test (non-parametric) against 0 (B), ****p<0.0001, unpaired t-test (C). Source file containing Ca2+ responses and GB1 and GB2 immunofluorescence data is available in Figure 5—figure supplement 2—source data 1.
-
Figure 5—figure supplement 2—source data 1
Analysis of GBR and mGlu5 receptor mediated intracellular Ca2+ increases.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig5-figsupp2-data1-v2.xlsx
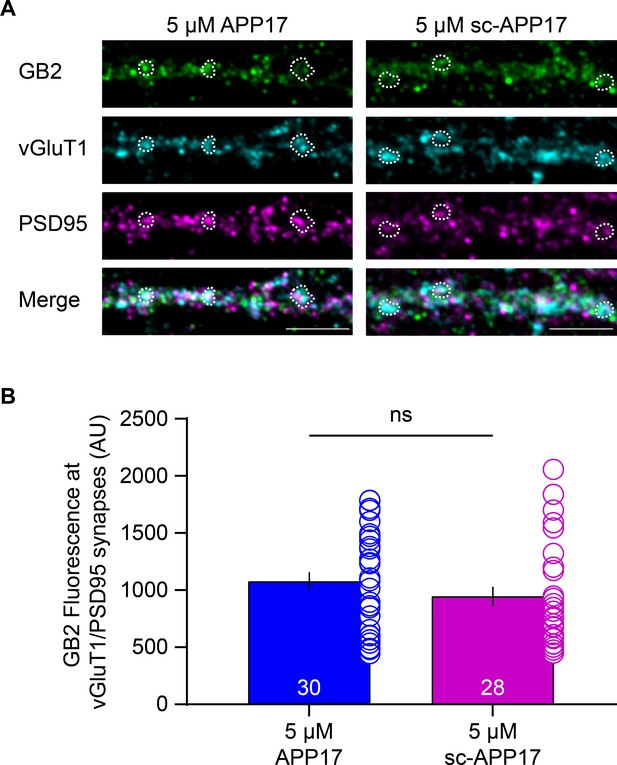
APP17 does not influence GBR levels at glutamatergic synapses.
(A) Images of dendrites of cultured hippocampal neurons (DIV14) exposed to 5 µM APP17 or sc-APP17 for 1 hr. Immunofluorescence labeling of GB2 (Alexa Fluor 488), vGluT1 (Alexa Fluor 647), PSD95 (Alexa Fluor 555), and their overlay (merged) is shown. Dashed areas indicate examples of vGluT1/PSD95 synapses. Scale bar: 5 µM. (B) Bar graphs showing GB2 (Alexa Fluor 488) immunofluorescence at vGluT1/PSD95 synapses. Data are mean ± SEM. The number of analyzed images from three independent preparations is indicated. ns = not significant, Mann-Whitney test (non-parametric). Source file containing GB2 immunofluorescence data is available in Figure 6—source data 1.
-
Figure 6—source data 1
Analysis of synaptic GBR levels.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig6-data1-v2.xlsx
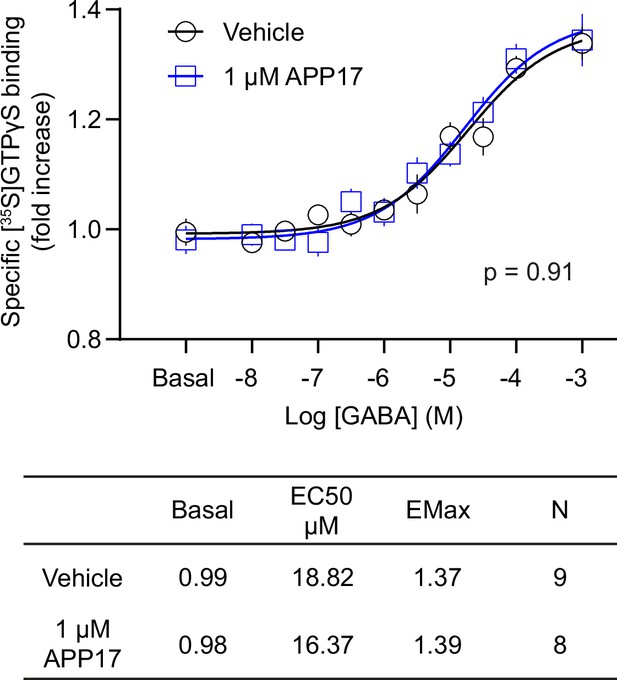
APP17 is not an agonist, antagonist, or allosteric modulator at native GB1a/2 receptors in [35S]GTPγS binding experiments.
[35S]GTPγS binding in brain membrane preparations of WT mice induced by increasing concentrations of GABA is not altered in the presence of APP17 (blue). The table shows basal, EC50, and Emax values derived from non-linear regression curve fits. Experiments with vehicle and APP17 were performed with membrane preparations from the same mouse. Data are mean ± SEM. Non-linear regression curve fit of nine (vehicle) and eight (APP17) independent experiments with nine different mice. p=0.91, extra sum-of-squares F test. Source file containing [35S]GTPγS data is available in Figure 7—source data 1.
-
Figure 7—source data 1
[35S]GTPγS analysis of GBR activity in mouse brain membrane preparations.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig7-data1-v2.xlsx
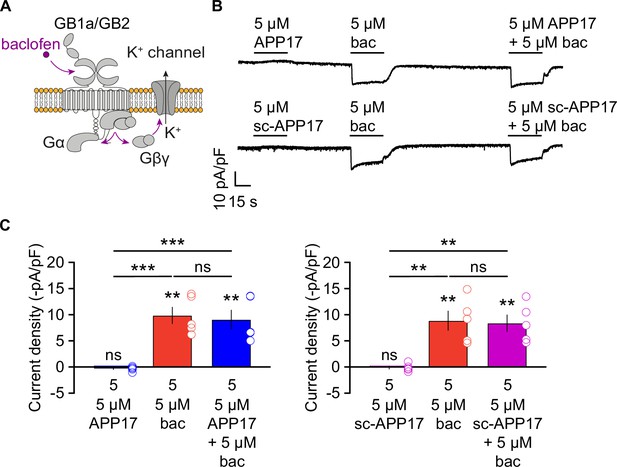
APP17 does not evoke or influence GB1a/2 receptor-mediated K+ currents in cultured hippocampal neurons.
(A) GBR activation with baclofen results in the dissociation of the heterotrimeric G protein and the subsequent activation of K+ channels by Gβγ. (B) Representative traces showing that neither APP17 (top) nor sc-APP17 (bottom) evoke GB1a/2 receptor-induced K+ currents in cultured hippocampal neurons. Application of baclofen alone or in the presence of APP17 or sc-APP17 yielded similar current amplitudes, showing that APP17 does not allosterically modulate baclofen-induced currents. (C) Bar graphs showing K+ current densities determined in experiments as shown to the top. Data are means ± SEM. The number of independent experiments is indicated in the bar graphs. ns = not significant, **p<0.01, ***p<0.001, paired one-way ANOVA with Holm-Sidak’s multiple comparisons test (to compare different means) and one-sample t-test against 0. Source file containing K+ current data is available in Figure 8—source data 1.
-
Figure 8—source data 1
Analysis of GBR-mediated K+ currents in cultured hippocampal neurons.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig8-data1-v2.xlsx
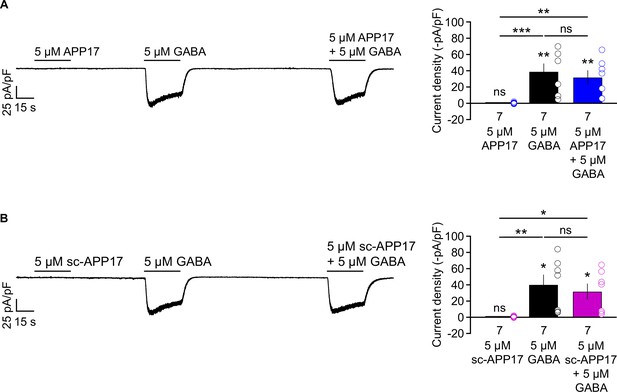
APP17 does not evoke or influence GB1a/2 receptor-induced Kir3 currents in transfected HEK293T cells.
(A,B) Left: Representative traces showing that neither APP17 (A) nor sc-APP17 (B) evoke GB1a/2 receptor-induced K+ currents in transfected HEK293T cells. Application of GABA alone or in the presence of APP17 (A) or sc-APP17 (B) yielded similar current amplitudes, showing that the peptides do not allosterically modulate GABA-induced currents. Right: Bar graphs showing K+ current densities determined in experiments as shown to the left. Data are means ± SEM. The number of independent experiments is indicated in the bar graphs. ns = not significant, *p<0.05, **p<0.01, ***p<0.001, paired one-way ANOVA with Holm-Sidak’s multiple comparisons test (to compare different means) and one-sample t-test against 0. Source file containing K+ current data is available in Figure 8—source data 1.
-
Figure 8—figure supplement 1—source data 1
Analysis of GBR-mediated Kir3 currents in HEK293T cells.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig8-figsupp1-data1-v2.xlsx
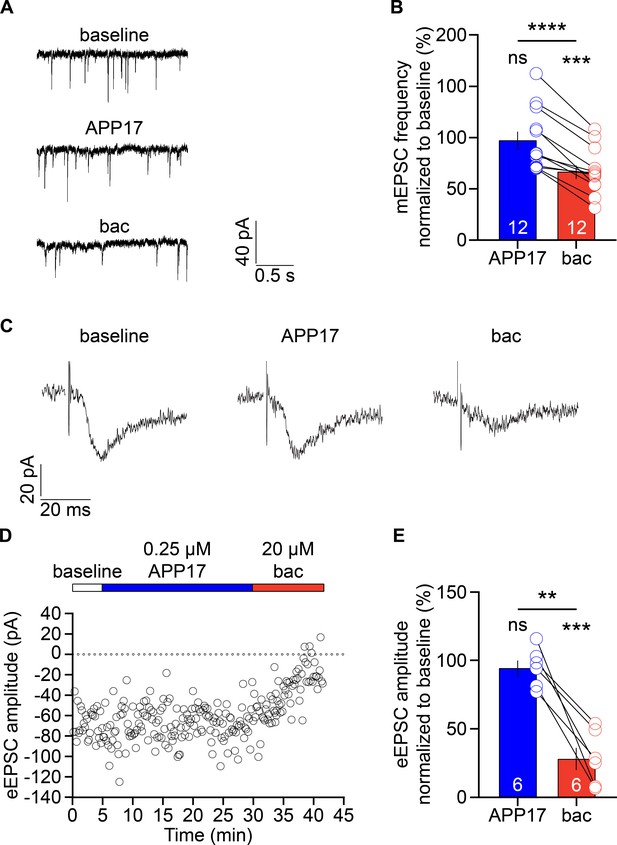
APP17 does not influence excitatory synaptic transmission.
(A) Sample mEPSCs at baseline and in the presence of 0.25 µM APP17 or 30 µM baclofen (bac) recorded from cultured hippocampal neurons (DIV13-16). (B) Bar graphs showing the mEPSC frequency normalized to baseline in the presence of APP17 (blue) or baclofen (red). Data are means ± SEM. The number of recorded neurons is indicated. ns = not significant; ****p<0.0001, paired Student’s t-test; ***p<0.001, one-sample t-test against 100. (C) Sample eEPSCs at baseline and in the presence of APP17 and baclofen recorded in CA1 pyramidal neurons of acute hippocampal slices. (D) Time course of eEPSC amplitudes in a CA1 pyramidal neuron. 0.25 µM APP17 and 20 µM baclofen were bath applied as indicated. (E) Bar graphs of the EPSC amplitude reduction in the presence of APP17 and baclofen. Data are means ± SEM. The number of recorded neurons from six different mice is indicated. ns = not significant; **p<0.01, paired t-test; ***p<0.001, one sample t-test against 100. Source file containing mEPSC and eEPSC data is available in Figure 9—source data 1.
-
Figure 9—source data 1
Analysis of mEPSCs and eEPSCs in hippocampal neurons.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig9-data1-v2.xlsx
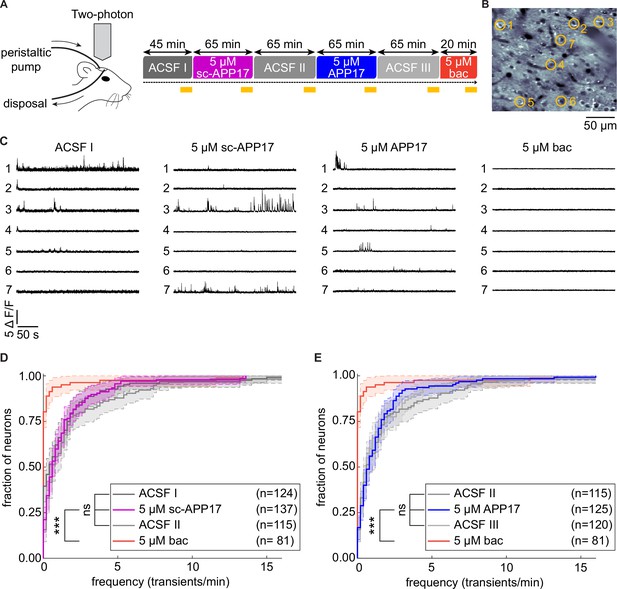
APP17 does not influence spontaneous neuronal activity in the auditory cortex of mice.
(A) Left: Two-photon imaging of Ca2+ transients in the auditory cortex of anesthetized mice during perfusion of artificial cerebrospinal fluid (ACSF), APP17, sc-APP17, and baclofen. Right: Scheme of the experimental design. Time specifications denote the durations of the perfusions. Yellow lines indicate the two-photon Ca2+ imaging periods (5 min each). (B) In vivo two-photon image of neurons expressing GCaMP6f. Representative neurons selected to illustrate Ca2+ transients in (C) are marked with yellow circles. (C) Ca2+ transients of neurons shown in (B) across the entire 5 min imaging period of a given condition. (D) Cumulative distribution of the frequency of Ca2+ transients, comparing sc-APP17 with baseline (ACSF I) and washout (ACSF II) and perfusion with baclofen (bac). (E) Cumulative distribution of the frequency of Ca2+ transients, comparing APP17 with baseline (ACSF II) and washout (ACSF III) and baclofen. (D,E) 95% confidence intervals are shown as shaded areas. The number of neurons recorded in each condition are indicated. Kruskal-Wallis multicomparison test: APP17 versus ACSF I, II, III, and sc-APP17 are not significantly different (p>0.05); bac versus ACSF I, II, III, sc-APP17, and APP17 are all significantly different (p<0.0001). For p-values, see Figure 10—source data 2. Source file containing Ca2+ transient data is available in Figure 10—source data 1.
-
Figure 10—source data 1
Analysis of neuronal activity in anesthetized mice.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig10-data1-v2.xlsx
-
Figure 10—source data 2
Analysis of neuronal activity in anesthetized mice: p-values between experimental conditions.
- https://cdn.elifesciences.org/articles/82082/elife-82082-fig10-data2-v2.docx
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/82082/elife-82082-mdarchecklist1-v2.docx
-
Reporting standard 1
ARRIVE guidelines 2.0 checklist.
- https://cdn.elifesciences.org/articles/82082/elife-82082-repstand1-v2.pdf





