Synaptic vesicle proteins are selectively delivered to axons in mammalian neurons
Figures
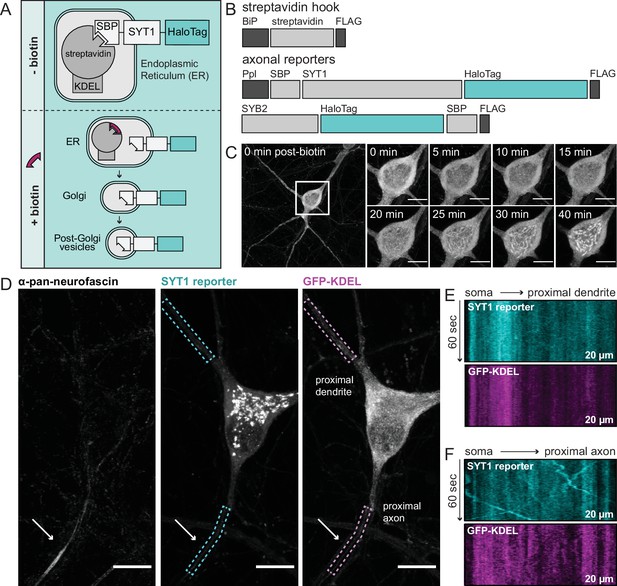
Using retention using selective hooks (RUSH) to study egress of synaptic vesicle (SV) proteins from the soma of cultured rat hippocampal neurons.
(A) A cartoon of RUSH; pre- and post-biotin conditions are shown. (B) Schematic of the streptavidin hook, and SYT1 and SYB2 reporter RUSH constructs: BiP, a signal peptide that drives translocation into the ER; FLAG, provides a means to detect each construct; SBP, streptavidin-binding peptide; Ppl, a pre-prolactin leader sequence to translocate the SBP into the endoplasmic reticulum (ER). In all cases the reporter is a HaloTag. (C) Representative super-resolution fluorescent live-cell MAX projection images from rat neurons at 15 days in vitro (DIV). Images of SYT1 reporter immediately after biotin addition with enlarged insets to detail the time course of release. Inset scale bar is 10 µm in panels (C–D). Since SYT1 and SYB2 behaved similarly, only SYT1 images are shown in panels (C–F). (D) Image of a neuron, 30 min after biotin addition, expressing the streptavidin hook, SYT1 reporter, and ER-targeted GFP (GFP-KDEL). Live-cell labeling with an anti-pan-neurofascin antibody was used to identify the axon initial segment (AIS; arrow); dendrites were identified by morphology and because they lacked an AIS. SYT1 was labeled with JF549 HaloTag ligand, and kymographs of this reporter, along with GFP-KDEL, were generated from the regions indicated by dashed boxes (20 µm long). Kymographs from a proximal dendrite (E) and proximal axon (F) are shown.
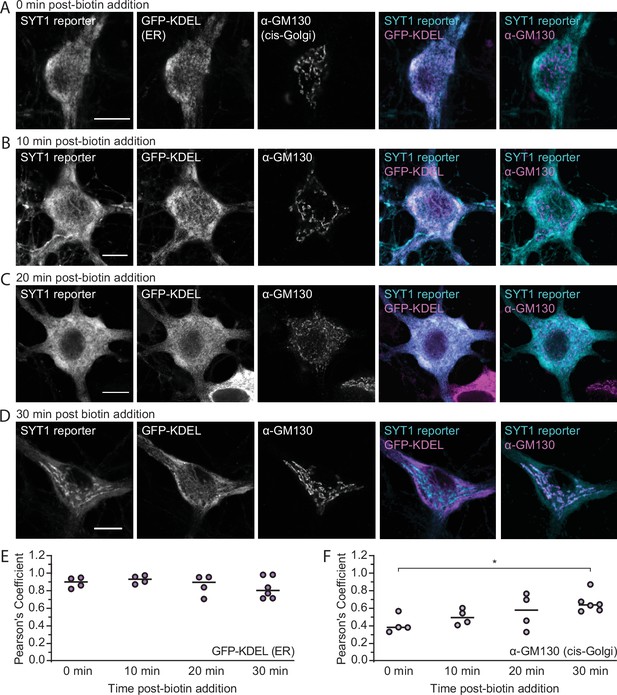
The SYT1 reporter localizes to the early secretory pathway after biotin addition.
(A–D) Super-resolution, fixed-cell optical sections of 15 days in vitro (DIV) rat hippocampal neurons expressing the SYT1 reporter and endoplasmic reticulum (ER)-targeted GFP (GFP-KDEL), detailing the movement of the SYT1 reporter from the ER to the Golgi after biotin addition (0, 10, 20, and 30 min), as indicated. Scale bars represent 10 µm. (E, F) Quantification of overlap between the SYT1 reporter and ER (GFP-KDEL) or cis-Golgi (α-GM130 antibody) markers, respectively, over time; the median is indicated. A two-way ANOVA (p<0.0001) and subsequent Šídák’s multiple comparisons test was run to compare the Pearson’s coefficient between timepoints for the ER: p0 min vs. 10 min=0.99, p0 min vs. 20 min >0.99, p0 min vs. 30 min=0.98, p10 min vs. 20 min=0.97, p10 min vs. 30 min=0.80, p20 min vs. 30 min >0.99, and cis-Golgi: p0 min vs. 10 min=0.90, p0 min vs. 20 min=0.41, p0 min vs. 30 min=0.047, p10 min vs. 20 min=0.97, p10 min vs. 30 min=0.44, p20 min vs. 30 min=0.94.
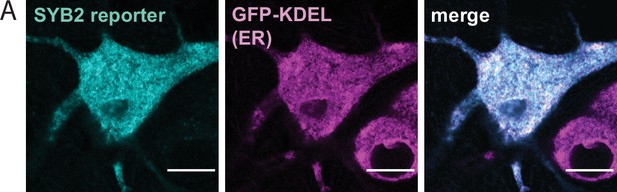
The SYB2 reporter is retained in the endoplasmic reticulum prior to biotin addition.
(A) Super-resolution, fixed-cell optical section of 14 days in vitro (DIV) rat hippocampal neurons expressing the SYB2 reporter and endoplasmic reticulum (ER)-targeted GFP (GFP-KDEL), indicating that the SYB2 reporter is retained in the ER prior to biotin addition. Scale bars represent 10 µm.
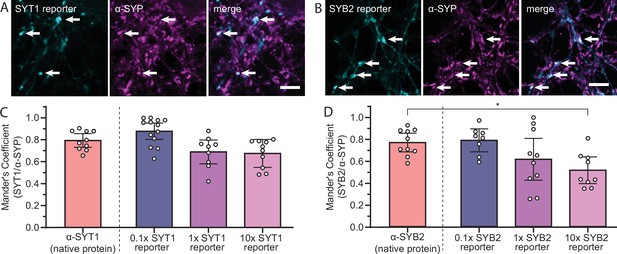
SYT1 and SYB2 reporters are targeted to the presynapse.
(A) Super-resolution, fixed-cell optical sections of 15 days in vitro (DIV) rat hippocampal neurons expressing the SYT1 reporter, as visualized by the JF549 HaloTag ligand, and stained for α-synaptophysin (α-SYP) to confirm proper targeting to synapses. (B) Same as panel (A), but for the SYB2 reporter. Note that all neurons were stained for SYP, but only a few cells expressed the SYT1 or SYB2 reporter. Arrows denote colocalization. Scale bars represent 5 µm. (C) A Mander’s coefficient was calculated for native SYT1 (α-SYT1) or transduced SYT1 reporter, as visualized by the JF549 HaloTag ligand, overlapping with native SYP (α-SYP). A Kruskal-Wallis test (p=0.0043) and subsequent Dunn’s multiple comparisons test were conducted to compare the localization of the transduced reporter at various concentrations to that of the native protein (p0.1x SYT1 rep = 0.44, p1x SYT1 rep = 0.53, p10x SYT1 rep = 0.30). (D) Same as panel (C), but for SYB2. A one-way ANOVA (p=0.0083) and subsequent Dunnett’s multiple comparisons post-test were conducted comparing the localization of the transduced reporter to the native protein (p0.1x SYB2 rep = 0.99, p1x SYB2 rep = 0.17, p10x SYB2 rep = 0.013). In all cases, error bars represent the mean with 95% confidence intervals.
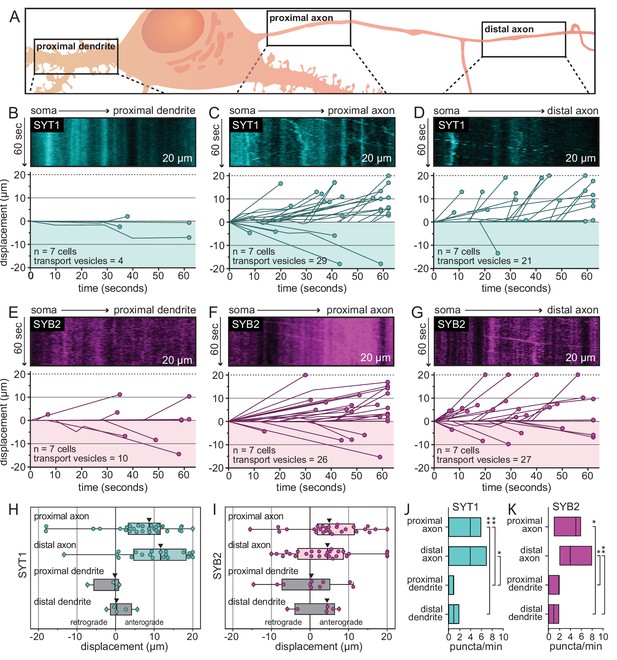
A direct and selective axonal transport pathway for SYT1 and SYB2 in rat hippocampal neurons.
(A) Illustration outlining the proximal and distal regions that were imaged for each neuron. (B) Representative kymographs from the proximal dendrite of 14–16 days in vitro (DIV) rat hippocampal neurons after release of the tethered SYT1 reporter, revealing an absence of SYT1-bearing mobile organelles. For panels (B–G), all data were quantified and plotted immediately below the kymographs; the number of cells and transport vesicles are also indicated. (C, D) Representative kymographs from proximal and distal axons showing robust movement of the released SYT1 reporter, suggesting a direct axonal trafficking pathway. (E–G) Same as for panels (B–D) but using neurons expressing the SYB2 reporter. Displacement of transport vesicles containing the SYT1 (H) or SYB2 reporters (I) is plotted in the anterograde (positive) or retrograde (negative) direction with respect to the soma; arrowheads indicate median values. Both synaptic vesicle (SV) proteins are primarily trafficked in the anterograde direction. Mean values and descriptive statistics are found in Figure 2—source data 1A. (J) Flux of the SYT1-bearing transport vesicles, in the indicated compartments, are plotted as floating bars (min to max), line indicates median value. Data were collected from seven cells. A one-way ANOVA with multiple comparisons was run; p-values were as follows: proximal axon vs. distal axon = 0.56; proximal axon vs. proximal dendrite = 0.0021; proximal axon vs. distal dendrite = 0.0047; distal axon vs. proximal dendrite = 0.046; distal axon vs. distal dendrite = 0.091; proximal dendrite vs. distal dendrite = 0.99. (K) Same as panel (J), but for the SYB2 reporter. Data were collected from seven cells. Statistical tests were run as in panel (J) and p-values were as follows: proximal axon vs. distal axon = 0.998; proximal axon vs. proximal dendrite = 0.058; proximal axon vs. distal dendrite = 0.013; distal axon vs. proximal dendrite = 0.018; distal axon vs. distal dendrite = 0.0088; proximal dendrite vs. distal dendrite = 0.907. Mean values and descriptive statistics are found in Figure 2—source data 1B.
-
Figure 2—source data 1
Descriptive statistics corresponding to Figure 2.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig2-data1-v1.docx
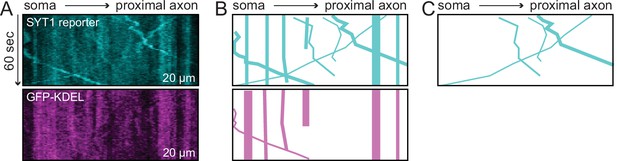
Kymograph analysis.
(A) A representative kymograph of the SYT1 reporter in proximal axons. The SYT1 channel, visualized by HaloTag and the JF549 ligand, and the endoplasmic reticulum (ER)-targeted GFP (GFP-KDEL) channel, are shown. (B) Tracks from the SYT1 and GFP-KDEL kymographs in panel (A) are shown. (C) The movement of vesicles harboring the SYT1 reporter were analyzed; vesicles that also carried GFP-KDEL were excluded.
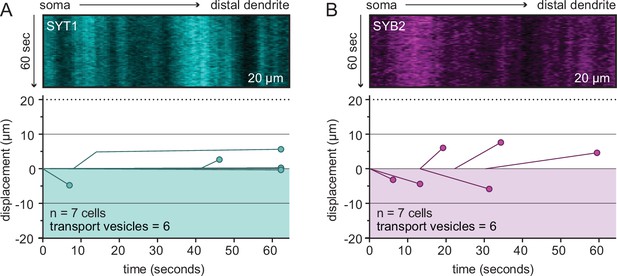
Representative kymographs from the distal dendrite of rat hippocampal neurons at 14–16 days in vitro (DIV) expressing the SYT1 (A) or SYB2 (B) reporter.
Few vesicles were observed in distal dendrites, despite the fact that the total number of cells, time, and distance observed was the same as for axons, where robust transport activity was detected.
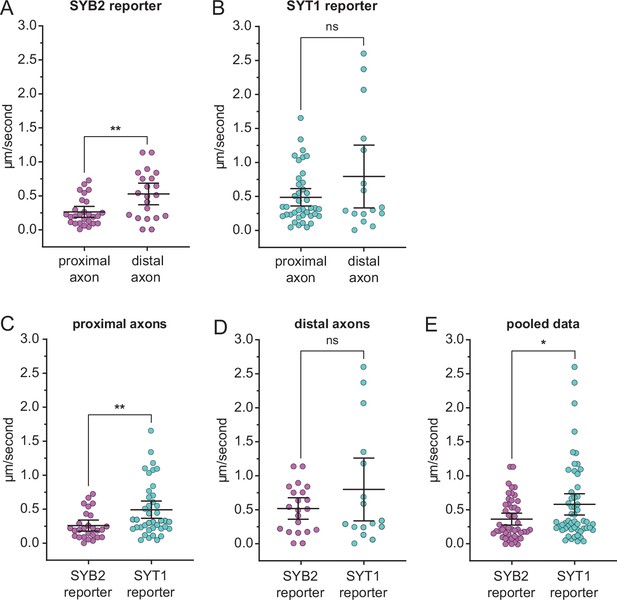
SYB2 transport is slowed in proximal axons.
The speed of anterograde-moving transport vesicles carrying the SYB2 (A) or SYT1 (B) reporters in both proximal and distal axons, where the proximal axon includes the axon initial segment. SYB2 transport was significantly slower in the proximal (0.26±0.2 µm/s) as compared to the distal axon (0.52±0.3 µm/s) (p=0.0091); SYT1 transport speed was the same in both regions (0.49±0.4 µm/s vs. 0.85±0.9 µm/s; p=0.28). (C) Within the proximal axon, SYB2-carrying transport vesicles moved more slowly (p=0.0041) than SYT1 vesicles. No differences in speed were observed between the two reporters in distal axons (p=0.77) (D). When transport speeds in proximal and distal axons were pooled for each reporter, SYB2 vesicles were transported more slowly than SYT1 vesicles (p=0.032). (E). All data sets were analyzed using the Mann-Whitney test and plotted as the mean with 95% confidence intervals.
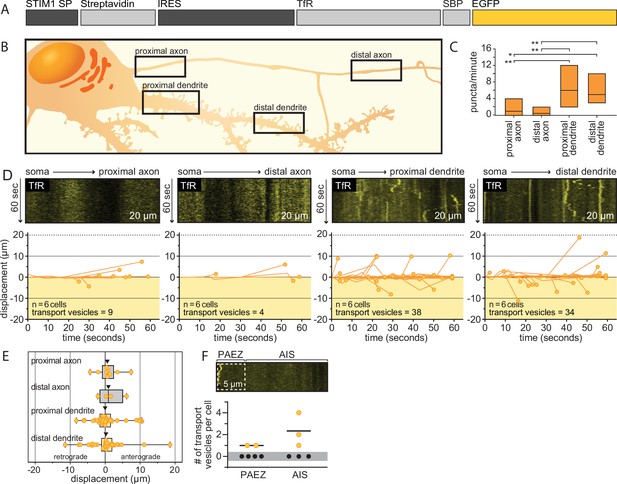
Dendritic cargo is delivered to dendrites without passing through axons.
(A) A schematic of the bicistronic pIRES vector that was used to express the transferrin receptor (TfR) reporter and endoplasmic reticulum (ER)-targeted streptavidin hook in the same cell: STIM1 SP, signal peptide from STIM1 to translocate the streptavidin into the ER; IRES, an internal ribosome entry site to allow cap-independent translation of the hook and reporter. EGFP was used to visualize TfR. (B) An illustration outlining proximal and distal axons, and proximal and distal dendrites that were imaged for analysis. These proximal and distal regions follow the same definitions described for Figure 2. (C) The number of TfR-bearing transport vesicles observed in a cell per minute in each region was plotted as floating bars (min to max; line indicates median value) for proximal axons (1.50±1.6 puncta), distal axons (0.667±0.82 puncta), proximal dendrites (6.33±3.5 puncta), and distal dendrites (5.67±2.3 puncta). Data were collected from six cells from five litters. A one-way ANOVA with multiple comparisons was run, and p-values were as follows: proximal axon vs. distal axon = 0.79; proximal axon vs. proximal dendrite = 0.0064; proximal axon vs. distal dendrite = 0.015; distal axon vs. proximal dendrite = 0.0022; distal axon vs. distal dendrite = 0.0060; proximal dendrite vs. distal dendrite = 0.79. All kymographs were 20 µm in length. (D) Representative kymographs from rat hippocampal neurons (14–16 days in vitro [DIV]), after release of the tethered TfR reporter, for each compartment are shown, with these data quantified and plotted immediately below. The number of cells and transport vesicles are also indicated. Kymographs, and the corresponding displacement graphs, from proximal and distal dendrites demonstrate transport of the TfR reporter; in contrast, axons lacked TfR transport. (E) Displacement of transport vesicles containing the TfR reporter were plotted in the anterograde (positive) or retrograde (negative) direction with respect to the soma for each neurite; arrowheads indicate median values. (F) A kymograph of the proximal axon where a white dashed box indicates the pre-axonal exclusion zone (PAEZ). Below, the total number of transport vesicles that remained in the PAEZ (1.00±0 puncta) or passed into the AIS (2.33±1.5 puncta) are plotted for each cell; means are indicated. Cells that lack of transport vesicles within the given region are represented by closed black circles within the gray box at 0 on the y-axis.
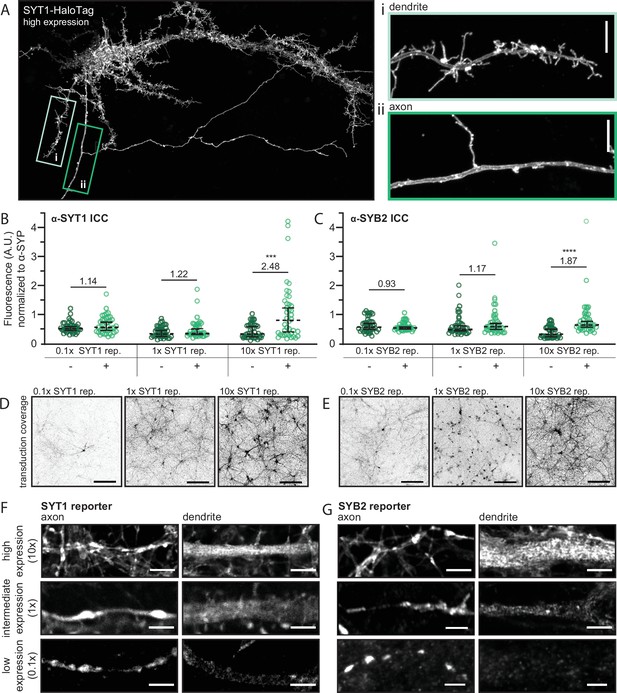
The direct and selective transport of synaptic vesicle (SV) proteins is obscured by overexpression.
(A) A super-resolution MAX-projection image of 15 days in vitro (DIV) rat neurons expressing SYT1-HaloTag at high levels, as evidenced by the localization of SYT1-HaloTag in both axons and dendrites, along with the formation of filopodia-like structures in the somatodendritic compartment. The construct is visualized with JF549 HaloTag ligand. The boxed regions were expanded to show that the overexpressed protein accumulates on both the dendritic (i) and axonal (ii) plasma membrane (PM). Scale bar is 5 µm. (B) Graphs comparing the fluorescence intensity of the α-SYT1 antibody at synapses with or without tagged SYT1 reporter at low, intermediate, and high expression levels. We note that here, “x” represents the titer of virus (see Figure 3—figure supplement 1 for comparison of wild type (WT) vs. transduced protein) where 1x is slightly less than endogenous levels; average relative expression levels are shown in panels (B) and (C). Data were plotted as median with 95% confidence intervals and Mann-Whitney tests were run comparing native protein to native and tagged protein for each virus dose (p-value0.1x = 0.36, p-value1x = 0.20, p-value10x = 0.0004). Average relative expression levels are included on the graph. (C) The same as panel (B) but comparing the fluorescence intensity of SYB2 with and without expression of the SYB2 reporter. Data were analyzed as in panel (B) (p-value0.1x = 0.79, p-value1x = 0.17, p-value10x = <0.0001). Data from panels (B) and (C) represent 40 synapses per condition, collected from four total fields of view from two different litters. Mean values and descriptive statistics are found in Figure 3—source data 1. (D) MAX-projection images of 14–16 DIV rat neurons expressing the SYT1 reporter showing the transduction coverage achieved with each viral dose. Scale bar represents 150 µm. Images were adjusted individually, with linear brightness and contrast, to the brightest area of the image to aid in visualization. (E) The same as panel (D), but for the SYB2 reporter. Super-resolution optical sections of the SYT1 (F) and SYB2 (G) reporters at low, intermediate, and high expression levels, in axons and dendrites, demonstrate that as expression levels increase, SV proteins spillover into dendrites. Scale bar represents 2.5 µm. Corresponding axon and dendrite images, at each expression level, were adjusted with the same linear brightness and contrast settings.
-
Figure 3—source data 1
Descriptive statistics corresponding to Figure 3.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig3-data1-v1.docx
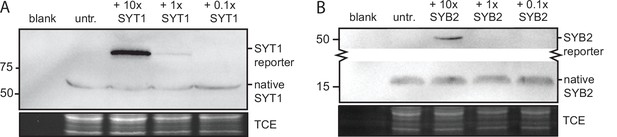
Expression levels of the SYT1 and SYB2 reporters as compared to native protein.
(A) An immunoblot of 15 days in vitro (DIV) rat neurons expressing the SYT1 reporter at low, intermediate, and high levels using virus. Probing with an α-SYT1 antibody reveals that at the 10x viral dose, the exogenously expressed SYT1 reporter is present at much higher levels than the endogenous protein, confirming high expression. (B) Likewise, an immunoblot of rat neurons expressing the SYB2 reporter at various levels was probed with an α-SYB2 antibody to reveal the degree of overexpression compared to the native protein. Lower expression levels of SYT1 and SYB2 escaped detection due to the sparse transduction.
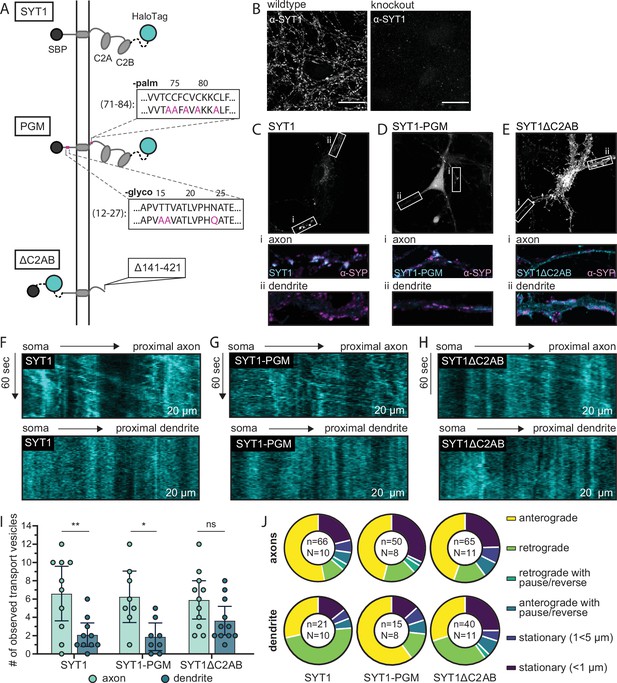
Molecular determinants that underlie the polarized transport of SYT1 to axons in mouse hippocampal neurons.
(A) Illustration of retention using selective hooks (RUSH) reporters used for these experiments: wild type (WT) SYT1 reporter, the SYT1 palmitoylation and glycosylation mutant (SYT1-PGM), and SYT1 truncated after position 140 (SYT1ΔC2AB). Each construct has a HaloTag for visualization. (B) ICC confirms the knockout of endogenous SYT1. For WT and knockout conditions, identical laser and gain settings were used. Scale bar represents 20 µm. (C–E) The endpoint localization of WT SYT1, SYT1-PGM, and SYT1ΔC2AB was visualized by labeling the appended HaloTag with JF549. The boxed regions were expanded and are shown below each panel to better reveal the localization of each construct in axons (i) and dendrites (ii), as compared to the α-SYP ICC signals. Note that all neurons were immunostained for SYP, but only a handful of cells expressed each SYT1 construct. ICC images were adjusted to the brightest area of the image to aid in visualization. All settings were kept consistent between corresponding axon/dendrite insets for a given cell and condition, and all images (B–E) were adjusted with linear brightness and contrast. Representative kymographs from proximal axons showing robust anterograde movement of the released SYT1 (F), SYT1-PGM (G), and SYT1ΔC2AB (H) reporters as compared to dendrites, demonstrating selective trafficking of WT and SYT1-PGM, but not SYT1∆C2AB, to axons. (I) The number of transport vesicles was plotted for each construct as the mean with 95% CI. A one-way ANOVA was run (p=0.0008), and a Šídák’s multiple comparisons test was used to compare transport in axons and dendrites of all three RUSH reporters. Significant differences in axonal vs. dendritic transport were observed for WT SYT1 (p=0.0097) and SYT-PGM (p=0.036), indicating polarized trafficking. In contrast, the transport of SYT1∆C2AB was not significantly polarized (p=0.49). A complete list of multiple comparisons results can be found in Figure 4—source data 1. Data were collected for 10 cells (SYT1), 8 cells (SYT1-PGM), or 11 cells (SYT1ΔC2AB), from four litters. Mean values and descriptive statistics are found in Figure 4—source data 2. (J) The movement of each transport vesicle categorized as anterograde, retrograde, retrograde with pause/reverse, anterograde with pause/reverse, stationary (1<5 µm), or stationary (<1 µm) and plotted as a fraction of the total number of transport vesicles observed for each compartment, for each construct. The total number of (n) transport vesicles from (N) cells are indicated. Exact fractions can be found in Figure 4—source data 3.
-
Figure 4—source data 1
Šídák’s multiple comparisons test results corresponding to Figure 4I.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig4-data1-v1.docx
-
Figure 4—source data 2
Descriptive statistics corresponding to Figure 4I.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig4-data2-v1.docx
-
Figure 4—source data 3
Transport vesicle movement analysis (fraction of a whole) related to Figure 4J.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig4-data3-v1.docx
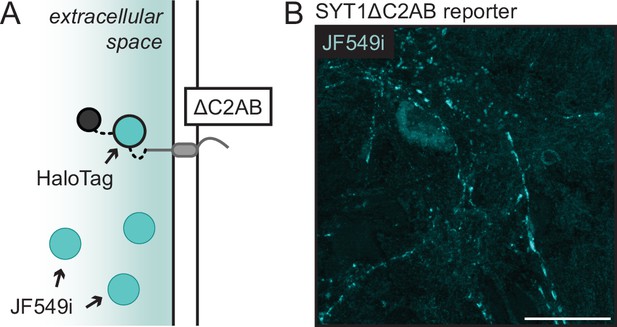
The SYT1ΔC2AB reporter is present on the plasma membrane.
(A) Illustration of the SYT1ΔC2AB reporter (ΔC2AB), inserted into the plasma membrane (PM), with its HaloTag exposed to the extracellular space. The reporter construct is incubated with JF549i non-permeant HaloTag ligand, shown in cyan, to selectively label tagged protein at the PM. (B) Representative live-cell MAX projection of 16 days in vitro (DIV) mouse hippocampal neurons expressing the SYT1 ΔC2AB reporter, as visualized by JF549i HaloTag ligand, confirmed that the truncated protein is present on the PM. Scale bar represents 20 µm.
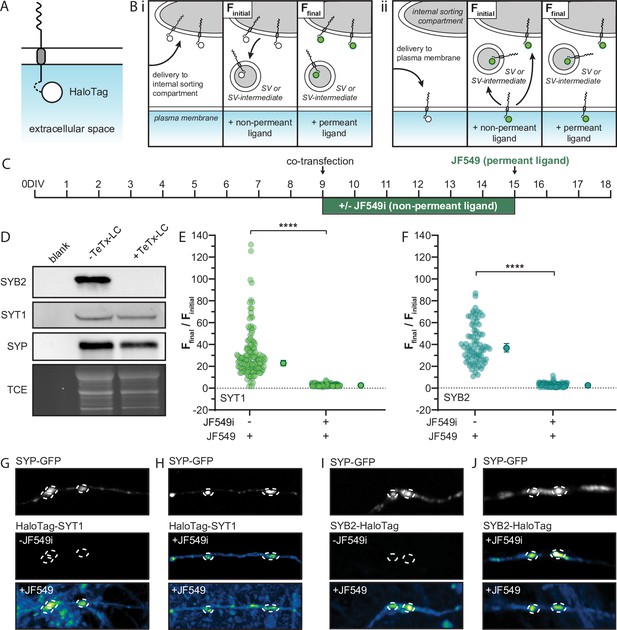
Transport vesicles deliver synaptic vesicle (SV) proteins to the presynaptic plasma membrane (PM) in rat hippocampal neurons, creating a depot for SV biogenesis.
(A) Illustration of a generic integral membrane protein (representing SYT1 and SYB2) with a luminal HaloTag to allow for selective labeling at the PM. (B) Schematic of the HaloTag ligand (HTL) labeling protocol, shown within a nerve terminal, with the non-permeant ligand incubation step to label surface protein. If SYT1 and SYB2 are first delivered to an internal sorting compartment (i), rather than the PM (ii) prior to SV or SV-intermediate formation, they will not pass through the PM and so will not be labeled by the non-permeant ligand (green). Incubation with permeant ligand labels the remaining tagged protein and the resulting change in fluorescence denotes the efficiency of PM delivery. The signal from labeling with the non-permeant ligand was referred to as Finitial, where the unlabeled control coverslips still yielded a small background signal, producing a reproducible non-zero value that allowed us to calculate ratios. The subsequent signal after labeling with permeant ligand was called Ffinal. This labeling step included unbound ligand which, while weak and diffuse, results in a slight increase in the background signal. To counteract this, ROIs were drawn to include only the fluorescence intensity within the synapse. (C) Timeline for the transfection and labeling protocols. Briefly, cultured rat hippocampal neurons were transduced with TeTx-LC virus on 5 days in vitro (DIV) and then co-transfected on 9 DIV with HaloTag-SYT1 or SYB2-HaloTag, and SYP-GFP; the GFP construct was included to mark synapses and ‘dilute’ the HaloTag plasmid to achieve lower expression levels. Half of the coverslips were incubated in non-permeant HTL (JF549i) immediately after co-transfection to label any tagged protein that was delivered to the PM. Six days later (15 DIV) neurons were rinsed, imaged, and incubated with permeant ligand (JF549), to label any remaining tagged protein, and imaged again. (D) Immunoblot of cells transduced with a virus expressing TeTx-LC, resulting in the cleavage of endogenous SYB2 and the inhibition of SV recycling. We note that the SYB2 fusion protein used in these experiments harbored two point mutations to render it resistant to TeTx-LC (see Methods). Blots were probed for endogenous SYB2, SYT1, and SYP, with a TCE loading control. The normalized (Ffinal/Finitial) change in fluorescence intensity of the SYT1 (E) and SYB2 (F) fusion proteins upon adding permeant fluorescent ligand to cells grown with or without non-permeant ligand for 6 days; mean values with 95% CI are plotted to the right of each scatter plot. Data were analyzed with unpaired t-tests for both proteins; p-values = <0.0001. Panel (E) contains data from 156 synapses cultured in the presence of JF549i, and 136 synapses grown in the absence of this HTL. Data for both groups were from 8 fields of view from 4 different litters. Panel (F) contains data from 107 synapses cultured in the presence of JF549i, and 79 synapses grown in the absence of this HTL. Data for both groups were from five fields of view from three different litters. Mean values and descriptive statistics for SYT1 and SYB2 can be found in Figure 5—source data 1. Panels (G, H) are representative images of SYP-GFP to mark synapses (dashed circles), and the corresponding HaloTag-SYT1 signals under the indicated conditions; in the bottom panels the JF549 ligand was not washed away, resulting in a higher background. For all conditions, identical laser and gain settings were used. Any linear brightness and contrast adjustments were applied to all conditions. (I, J) Same as panels (G) and (H), but for SYB2-HaloTag.
-
Figure 5—source data 1
Descriptive statistics corresponding to Figure 5.
- https://cdn.elifesciences.org/articles/82568/elife-82568-fig5-data1-v1.docx
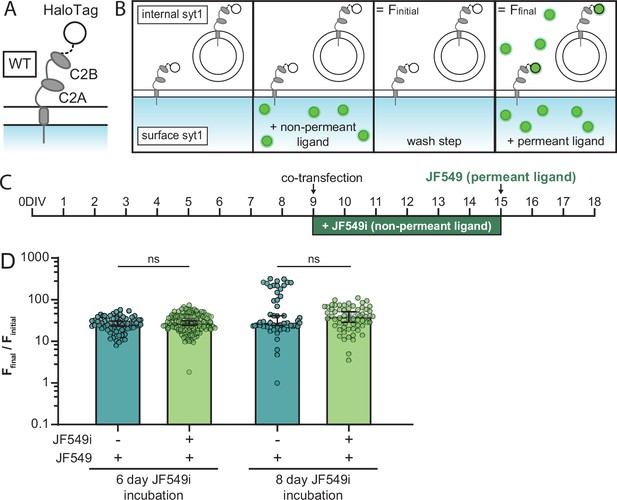
JF549i HaloTag ligand is not cell-permeant after six days.
(A) Illustration of SYT1 with a C-terminal HaloTag and (B) the time course of ligand addition during the pulse-chase assay. By appending the HaloTag to the cytoplasmic domain, the tag is not exposed to the extracellular milieu, and should not be labeled with non-permeant JF549i ligand. (C) Timeline for transfecting and labeling neurons. This scheme is the same as the experiment conducted in Figure 5, but with the HaloTag oriented inside the cell when SYT1 is on the plasma membrane (PM). (D) Plots of the change in fluorescence (Ffinal/Finitial) upon adding a permeant fluorescent ligand for cells grown with or without non-permeant ligand for 6 or 8 days. Median values, with 95% CI, are shown. These values were: 6 days with (27.87, [24.37, 30.79]) and without JF549i (27.11, [23.42, 30.37]), or 8 days with (39.40, [28.87, 50.64]) and without JF549i (28.27, [24.41, 40.95]). A Mann-Whitney test was run for both 6- (p=0.26) and 8-day (p=0.89) incubation conditions. No difference in Ffinal/Finitial between cultures grown with and without the non-permeant ligand was observed. Thus, incubation with the JF549i ligand did not result in any significant labeling of the cytoplasmic HaloTag and is non-permeant under these experimental conditions. Data were collected as follows, with synapse, fields of view, and number of litters listed in order: 6-day incubation with JF549i: 125, 3, 1; 6-day incubation without JF549i: 68, 4, 1; 8-day incubation with JF549i: 60, 3, 1; 8-day incubation without JF549i: 51, 3, 1.
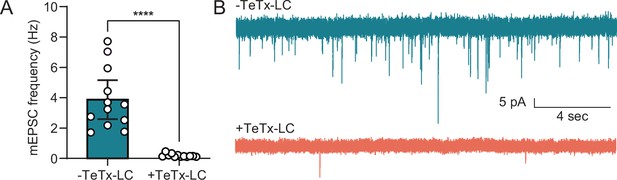
Expression of TeTx-LC disrupts synaptic activity.
(A) Miniature EPSC frequency in 14–16 days in vitro (DIV) cultured mouse hippocampal neurons with (0.19±0.11 Hz) and without (3.9±2.0 Hz) virus that expresses TeTx-LC. Spontaneous release was disrupted in cultures expressing TeTx-LC (p<0.0001), consistent with the SYB2 KO (Schoch et al., 2001). Data were analyzed with a Mann-Whitney test and plotted as median with 95% CI. Data were recorded from one litter and 12 cells per condition. (B) Representative traces from wild type (WT) cells (top trace) and cells expressing TeTx-LC (bottom trace).
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/82568/elife-82568-mdarchecklist1-v1.pdf
-
Source data 1
Source data for Figure 5 and Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/82568/elife-82568-data1-v1.zip





