Lonafarnib improves cardiovascular function and survival in a mouse model of Hutchinson-Gilford progeria syndrome
Figures
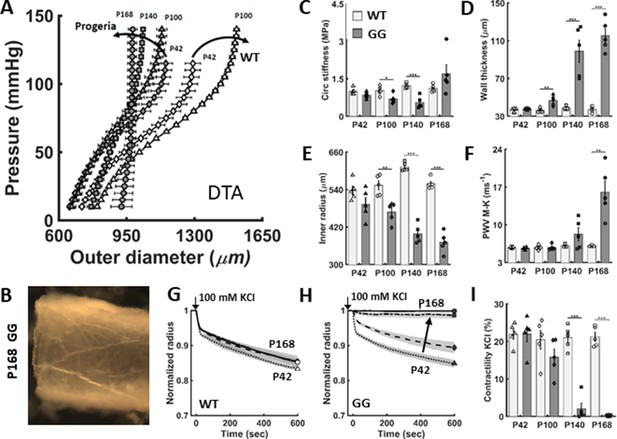
The biomechanical phenotype of the descending thoracic aorta (DTA) worsens progressively in untreated LmnaG609G/G609G progeria (GG) mice relative to age-matched wild-type (WT) littermate mice.
(A) Pressure-diameter responses revealed a progressive structural stiffening in progeria from postnatal day P42 to P100 to P140 to P168 (dark curved arrow). (B) The marked, late-stage aortic stiffening is associated with a translucent appearance and brittle texture. (C–F) Passive geometric and mechanical metrics calculated at a common distending pressure of 100 mmHg for the DTA in untreated progeria mice (darker bars) confirmed marked progressive worsening relative to wild-type (light bars). (G–I) Vasoactive capacity, revealed as diameter reduction in response to high potassium depolarization of the smooth muscle cell membrane (100 mM KCl), first as a function of time and extent of response and second as steady-state response at 10 min, both for different ages. Note the near complete loss of contractility by P140 and its complete loss by P168 days in progeria. Here, n=5 vessels per group, with *, **, and *** denoting statistical significance, between WT and GG at the age indicated, at p<0.05, p<0.01, and p<0.001, respectively. See also Figure 1—figure supplement 1 for additional biomechanical metrics as well as Source data 1 and 2 for all numerical values.
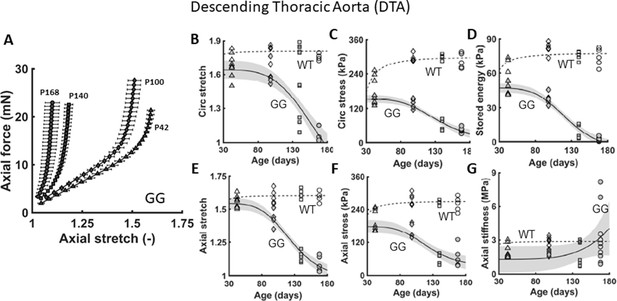
Additional biaxial metrics confirm the progressive worsening of the aortic phenotype.
(A) Similar to Figure 1, except for axial force-stretch responses revealing a progressive structural stiffening (left-ward shift) of the descending thoracic aorta (DTA) in untreated LmnaG609G/G609G progeria (GG) mice as a function of age from postnatal days P42 to P168. (B–G) Multiple geometric and mechanical metrics for the DTA as a function of age, from P42 to P168, for both wild-type (WT – open symbols) and untreated progeria (GG – closed symbols) mice. n=5 per group. See Source data 1.
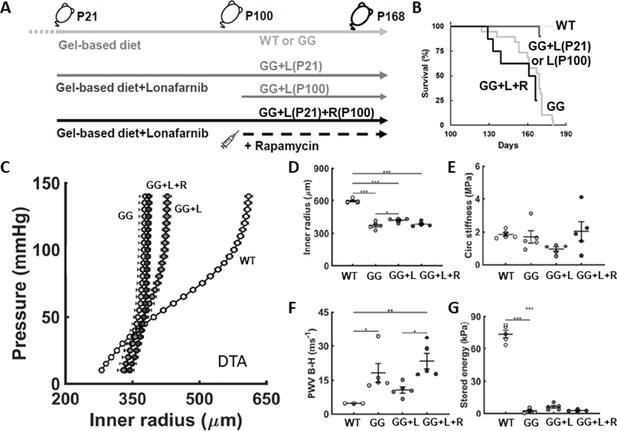
Lonafarnib improves survival and central artery stiffness in progeria mice (GG).
(A) Study design, including untreated progeria mice and progeria mice that were given lonafarnib daily in the soft gel-based chow either from postnatal day P100 to P168, denoted L(P100), or from P21 to P168, denoted L(P21), and finally L(P21) plus rapamycin R(100) from P100 to P168. Soft gel-based chow, without or with the drug, was started at weaning (P21) in all mice. (B) All 10 (100%) of the lonafarnib-treated progeria mice, namely 4/4 of the GG + L(P100) and 6/6 of the GG + L(P21) mice, survived to the intended end-point, P168, while only 10 of the 19 untreated GG mice (~53%) survived to P168; note that the one GG + L death occurred at P169, the day after anesthesia, echocardiography, and recovery while three additional untreated GG mice died by P169. The five untreated wild-types (WT) littermate controls all survived to the study end-point, as expected, whereas lonafarnib plus rapamycin did not improve the survival of the progeria mice. Consistent with the improved survival for lonafarnib treatment from P21, the structural stiffness of the descending thoracic aorta (DTA) improved as revealed by both (C) standard pressure-diameter testing ex vivo and (F) local pulse wave velocity (PWV) calculated using the Bramwell-Hill (B-H) equation. (D,E,G) There was, however, no improvement in intrinsic metrics at physiological conditions. Here, n=4 (lonafarnib from P100) or 5 (all other groups) vessels per biomechanical testing group, with *, **, and *** denoting statistical significance at p<0.05, p<0.01, and p<0.001, respectively. See also Figure 2—figure supplement 1 as well as Source data 1–3 for all numerical values.
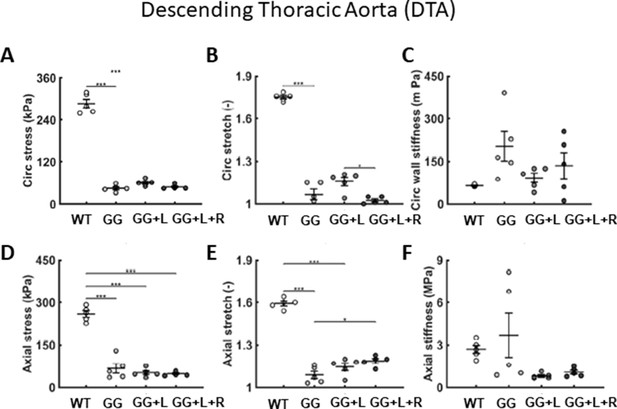
Addtional biaxial findings confirm positive effects of lonafarnib on select metrics.
(A–F) Additional biomechanical metrics for the descending thoracic aorta (DTA) for four groups compared at P168: age-matched wild-type littermate controls (WT), untreated progeria (GG), lonafarnib treated (L, from P21 to P168), and combination therapy (lonafarnib, L, from P21 to P168 plus rapamycin, R, from P100 to P168). The combination therapy (GG + L + R) appeared to be no better than the lonafarnib monotherapy and perhaps worse in terms of a few metrics, circumferential (B) stretch, and (C) stiffness. n=5 per group, as indicated. See Source data 1.
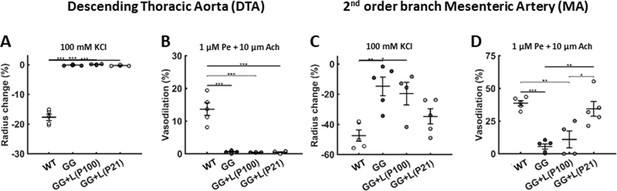
Lonafarnib treatment improves the vasoactive function of muscular but not elastic arteries.
(A–B) The vasoconstrictive capacity of the aorta declined progressively in untreated progeria mice to near nonexistent levels by postnatal day P140, which persisted to P168, and lonafarnib treatment from P21 or P100 did not improve this function when evaluated at P168. (C–D) There was a similar progressive, though a less severe, decline in vasoconstrictive capacity of the second-order branch mesenteric artery in untreated progeria mice, notably at P168. This mesenteric artery function was improved by lonafarnib treatment from P100 and especially from P21 as revealed by improved vasoconstrictive and vasodilatory function at P168. Vasoconstriction was evaluated isobarically in response to 100 mM potassium chloride (KCl); vasodilatation was evaluated isobarically in response to 10 μM acetylcholine (Ach) following attempted pre-constriction with 1 μM phenylephrine (Pe). n=4 (lonafarnib from P100) or 5 (all others) vessels per group, with *, **, and *** denoting statistical significance at p<0.05, p<0.01, and p<0.001, respectively. See Source data 2 for numerical values. Note that we did not assess mesenteric artery function in the lonafarnib plus rapamycin group given the poor survival outcome.
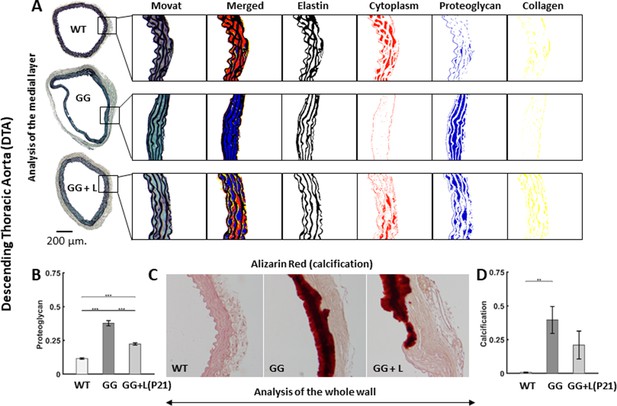
Lonafarnib treatment improves histological features in the descending thoracic aorta (DTA), with the drug given from P21 to P168.
(A) Representative Movat-stained cross-sections with computer-based automatic separation of elastic fibers (black), cell cytoplasm (red), proteoglycans/glycosaminoglycans (blue), and collagen (yellow) in the medial layer. Note the marked decrease in cytoplasm and increase in proteoglycans/glycosaminoglycan in untreated progeria (GG) relative to wild-type controls (WT), which is partially prevented by lonafarnib (GG + L) treatment started at P21. (B) Quantification of whole wall proteoglycan/glycosaminoglycan area fraction without or with lonafarnib treatment from P21 reveals a significant improvement with the drug. (C) Representative Alizarin Red-stained cross-sections with marked medial calcification were revealed in progeria mice by the dark red color, less with lonafarnib treatment from P21 to P168 (GG + L) relative to untreated progeria. Finally, (D) quantitation of calcification, with a trend toward a reduction with lonafarnib treatment. n=5 per group (with three technical replicate sections per specimen), with *, **, and *** denoting statistical significance at p<0.05, p<0.01, and p<0.001, respectively. See also Figure 4—figure supplement 1 and Source data 4.
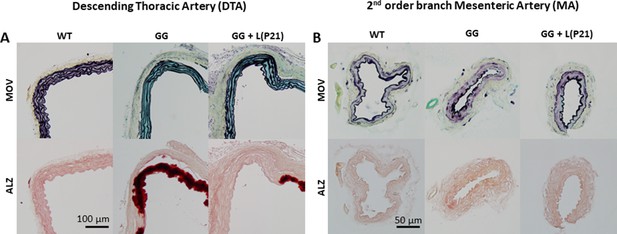
Similar to Figure 4 in the main text, but showing additional representative Movat pentachrome (MOV) and Alizarin Red (ALZ) stained sections.
(A) Sections of descending thoracic aortas (DTAs) showed marked proteoglycan staining (top row, blue) in progeria vessels (GG) at P168 wherein the elastic laminae were also straightened; note, too, the modest reduction in proteoglycan staining following treatment (GG + L at P168 with lonafarnib administered from P21 to P168) with more undulated elastic laminae. Also shown is the marked calcification (bottom row, dark red) in progeria mice (GG) at P168 wherein the stiffened media separated from the adventitia during sectioning; note, again, a reduction following treatment (GG + L at P168 with lonafarnib administered from P21 to P168). B. Similar sections for the second-order branch mesenteric artery (MA), which reveal few overt differences in progeria (GG) at P168 relative to wild-type (WT) at P168, with little change with treatment (GG + L with lonafarnib administered from P21 to P168). The scale bars indicate 100 microns for the DTA and 50 microns for the MA. Overall n=4–5 vessels per group with three technical replicates per vessel. See Source data 4.
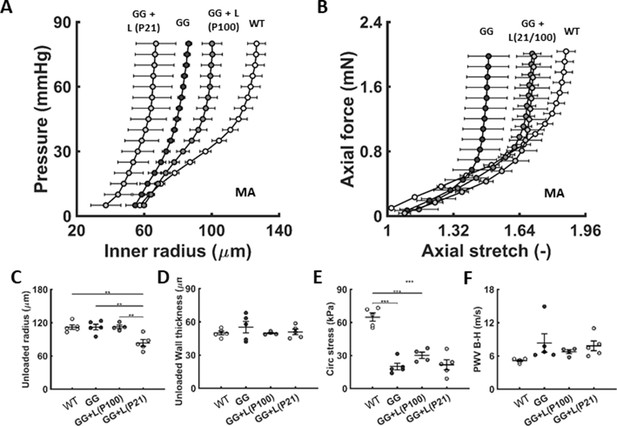
Similar to Figure 2, except for the second-order branch mesenteric artery in age-matched littermate wild-type (WT) control mice and untreated (GG) and treated (GG + L) progeria mice for lonafarnib administration from either P21 or P100 to P168.n=4–5 per group.
(A-F) Lonafarnib did not improve passive properties. See also Figure 5—figure supplement 1 as well as Source data 1. Note that we did not assess mesenteric artery properties in the lonafarnib plus rapamycin group given the poor survival outcome.
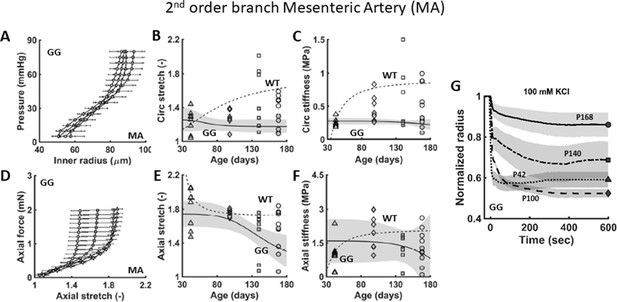
Similar to Figure 1, except for the second-order branch mesenteric artery (MA) from age-matched littermate wild-type (WT) control mice and untreated progeria (GG) mice at postnatal days P42, P100, P140, and P168.
Note the progressive decline in both (A–F) passive and (G) active properties in progeria, though neither as dramatic as those for the descending thoracic aorta. n=4–5 per group. See Source data 1 and 2.
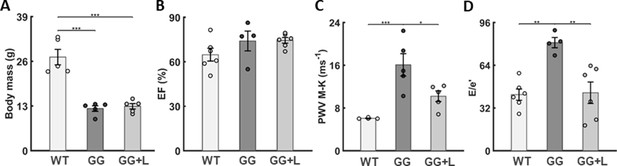
Lonafarnib improves pulse wave velocity (PWV) and left-ventricular diastolic function but not somatic growth.
Focusing on progeria mice (GG) treated with lonafarnib (GG + L) from P21, (A) there was no improvement in body mass measured at P168 despite 100% survival to 168 of the six mice so treated (noting that 1 of the treated mice was found dead on P169 following anesthesia, echocardiology, and successful recovery on P168). (B) There was no decline in left ventricular ejection fraction (EF) in the untreated progeria mice and no improvement with lonafarnib. (C) Conversely, lonafarnib significantly improved pulse wave velocity, as computed using the Moens-Korteweg equation (PWV M-K), and (D) similarly improved left-ventricular diastolic function, as computed by . There was similarly an improvement in cardiac output ( as detailed in the text). See Source data 5 for all in vivo cardiovascular measurements (n=4–6 per group), with *, **, and *** denoting statistical significance at p<0.05, p<0.01, and p<0.001, respectively. See also Figure 6—figure supplement 1A and Source data 1–5.
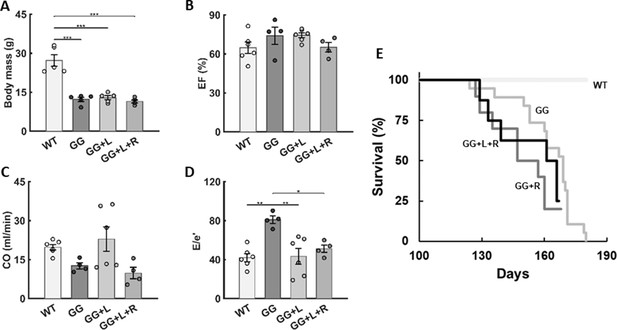
Additional findings for rapamycin both alone and in combination with lonafarnib.
Similar to Figure 6 in the main text, except with an emphasis on combination lonafarnib + rapamycin (GG + L + R) treatment of progeria mice (GG) for four different metrics evaluated at postnatal day P168, including (A) body mass, (B) left ventricular ejection fraction (EF), (C) cardiac output (CO), and (D) a measure of left ventricular diastolic function (E/e’). Again, there was no improvement with the combination therapy (lonafarnib, L, from P21 to P168, plus rapamycin, R, from P100 to P168) relative to monotherapy with lonafarnib (GG + L, from P21 to P168), data for which are repeated from Figure 5 in the main text for convenience of comparison. n=4–6 per group, as indicated. See Source data 5. Finally, (E) we show the survival curve for rapamycin treatment alone, which did not confer any survival benefit.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/82728/elife-82728-mdarchecklist1-v1.docx
-
Source data 1
Passive mechanics.
- https://cdn.elifesciences.org/articles/82728/elife-82728-data1-v1.xlsx
-
Source data 2
Active mechanics.
- https://cdn.elifesciences.org/articles/82728/elife-82728-data2-v1.xlsx
-
Source data 3
Survival information.
- https://cdn.elifesciences.org/articles/82728/elife-82728-data3-v1.xlsx
-
Source data 4
Histological information.
- https://cdn.elifesciences.org/articles/82728/elife-82728-data4-v1.xlsx
-
Source data 5
Echo data.
- https://cdn.elifesciences.org/articles/82728/elife-82728-data5-v1.xlsx





