Mechanism of Ca2+ transport by ferroportin
Peer review process
This article was accepted for publication as part of eLife's original publishing model.
History
- Version of Record published
- Accepted Manuscript published
- Accepted
- Received
- Preprint posted
Decision letter
-
David DrewReviewing Editor; Stockholm University, Sweden
-
Kenton J SwartzSenior Editor; National Institute of Neurological Disorders and Stroke, National Institutes of Health, United States
-
David DrewReviewer; Stockholm University, Sweden
-
Aashish ManglikReviewer; University of California, San Francisco, United States
Our editorial process produces two outputs: (i) public reviews designed to be posted alongside the preprint for the benefit of readers; (ii) feedback on the manuscript for the authors, including requests for revisions, shown below. We also include an acceptance summary that explains what the editors found interesting or important about the work.
Decision letter after peer review:
Thank you for submitting your article "Mechanism of Ca2+ transport by ferroportin" for consideration by eLife. Your article has been reviewed by 3 peer reviewers, including David Drew as the Reviewing Editor and Reviewer #1, and the evaluation has been overseen by Kenton Swartz as the Senior Editor. The following individual involved in review of your submission has agreed to reveal their identity: Aashish Manglik (Reviewer #3).
The reviewers have discussed their reviews with one another, and the Reviewing Editor has drafted this to help you prepare a revised submission.
Major concerns:
You have been able to robustly demonstrate Ca2+ binding. What is unclear is if Ca2+ is actually transported to a significant level? Is the Fpn protein just capable of one turnover in proteoliposomes? We highly recommend kinetics using 45CaCl2 (eg. PNAS E5354-E5362) to calculate actual Ca2+ turnover (kcat).
Reviewer #1 (Recommendations for the authors):
The authors have been able to robustly demonstrate Ca2+ binding. What is unclear to me is how much Ca2+ is actually transported? Is the Fpn protein just capable of one turnover in proteoliposomes? The "cleanest" experiment would be to measure Ca2+ uptake using 45CaCl2 (eg. PNAS E5354-E5362) so one can calculate Vmax and kcat. From my understanding, metalloproteins do not necessarily use the optimum metal for catalysis, but the metal available in the appropriate concentration in where they function. I wonder if the Ca2+ site is just not selected against, because its not needed too, i.e., Ca2+ doesn't compete for Fe2+ uptake and the actual "turnover" for Ca2+ is very low?
Reviewer #2 (Recommendations for the authors):
The data reported here appear to be of high quality and are convincing; the experimental design is excellent and the necessary controls are appropriately employed. A couple of issues need clarification in the text. FPN is clearly an iron efflux pump and these studies make clear that Fpn can also import Ca2+, although it does not appear to function as an Fe2+-Ca2+ antiporter. What is less clear is whether Fpn will transport calcium bi-directionally. There are technical issues that would make this a bit hard to study, but if the authors think the answer is clear from their data, they should be explicit in explaining this. A further question that needs explaining is why bind and transport calcium? Cells have a high capacity for calcium flux independent of Fpn. Is there a physiological importance to this activity?
https://doi.org/10.7554/eLife.82947.sa1Author response
Major concerns:
You have been able to robustly demonstrate Ca2+ binding. What is unclear is if Ca2+ is actually transported to a significant level? Is the Fpn protein just capable of one turnover in proteoliposomes? We highly recommend kinetics using 45CaCl2 (eg. PNAS E5354-E5362) to calculate actual Ca2+ turnover (kcat).
Uptake of Ca2+ is measured by changes of Fluo-4 fluorescence. The fluorescence intensity increases progressively as we increase the concentrations of the Ca2+ and reaches up to five-fold higher than the initial value. As shown in Author response 1, a five-fold increase of fluorescence intensity requires >20 µM of Ca2+. If we assume that a lipid headgroup has an area of ~60 Å2 and that liposomes have an average diameter of ~400 nm, we arrive at a rough estimate of >100 turnovers for each Fpn under 500 µM external Ca2+ as shown in Figure 1—figure supplement 4a.
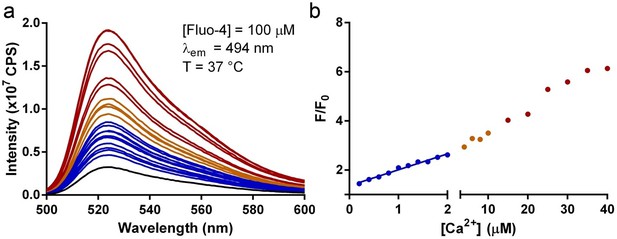
Titration of Ca2+ to Fluo-4 used in liposome transport assays.
(a) Emission spectra of 100 µM Fluo-4 in the inside buffer at 37 °C titrated with increasing [Ca2+] (from blue to red). (b) Correlation of F/F0 to [Ca2+]. The blue line is the linear fit to data in the region of [Ca2+] within 2 µM.
Reviewer #1 (Recommendations for the authors):
The authors have been able to robustly demonstrate Ca2+ binding. What is unclear to me is how much Ca2+ is actually transported? Is the Fpn protein just capable of one turnover in proteoliposomes? The "cleanest" experiment would be to measure Ca2+ uptake using 45CaCl2 (eg. PNAS E5354-E5362) so one can calculate Vmax and kcat. From my understanding, metalloproteins do not necessarily use the optimum metal for catalysis, but the metal available in the appropriate concentration in where they function. I wonder if the Ca2+ site is just not selected against, because its not needed too, i.e., Ca2+ doesn't compete for Fe2+ uptake and the actual "turnover" for Ca2+ is very low?
See response to Major concerns.
Reviewer #2 (Recommendations for the authors):
The data reported here appear to be of high quality and are convincing; the experimental design is excellent and the necessary controls are appropriately employed. A couple of issues need clarification in the text. FPN is clearly an iron efflux pump and these studies make clear that Fpn can also import Ca2+, although it does not appear to function as an Fe2+-Ca2+ antiporter. What is less clear is whether Fpn will transport calcium bi-directionally. There are technical issues that would make this a bit hard to study, but if the authors think the answer is clear from their data, they should be explicit in explaining this. A further question that needs explaining is why bind and transport calcium? Cells have a high capacity for calcium flux independent of Fpn. Is there a physiological importance to this activity?
We added an explanation that the Fpn in liposomes is randomly oriented and made a schematic drawing (Figure 1—figure supplement 1c) to illustrate this point. We also measured Ca2+ transport when the Fab was added only to the external side, and we found that the rate of transport is higher than the rate when the Fab was added to both sides and lower than the rate when no Fab was added (Figure 1—figure supplement 1f–g). We take this result as further indication that Fpn is reconstituted in both orientations.
We are also intrigued by the calcium transport activity of Fpn, and more study is required to address the questions on biology Ca2+ uptake by Fpn. We speculate that loss of cellular Fe2+ will lead to Ca2+ influx through Fpn, which may trigger further cellular responses.
Although our study arrived at a different conclusion from that of Deshpande et al. 2018 with regard to Ca2+ transport, both studies share common grounds on the calcium binding site and transport of Fe2+ in the presence of Ca2+. We made a comment on this in Discussion.
https://doi.org/10.7554/eLife.82947.sa2




