The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice
Figures
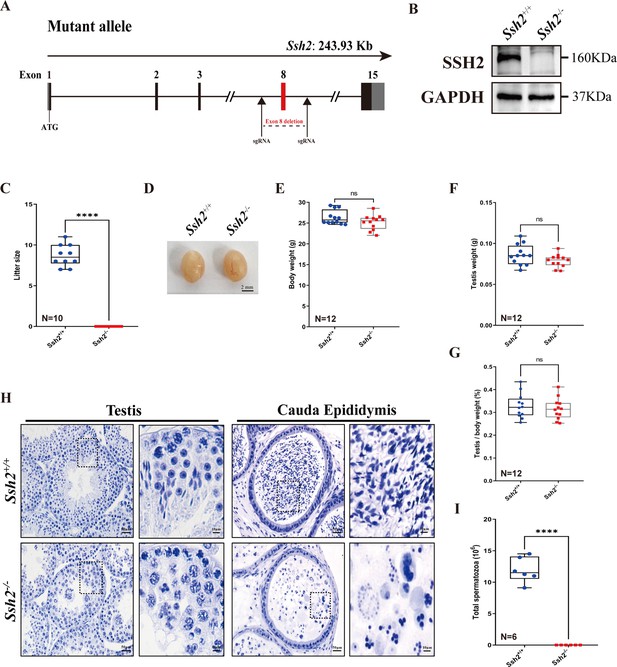
Ssh2 knockout (KO) causes severe reproductive defects and male infertility in mice.
(A) Schematic representation of the generation of Ssh2 KO mice using CRISPR/Cas9. (B) Validation of Ssh2 KO by western blotting in testicular lysates from wild-type (WT) and Ssh2 KO 8-week-old mice (n=3), indicating the absence of SSH2 protein in Ssh2 KO testes. GAPDH was used as the loading control. (C) Number of pups per litter from WT (8.70±0.42) and Ssh2 KO (0.00) male mice (8 weeks of age) after crossing with WT female mice (8–10 weeks of age) for 3 months (n=10). Data are presented as the mean ± SEM; ****p<0.0001, calculated by Student’s t-test. Bars indicate the range of data. (D) The testes from Ssh2 KO mice appeared phenotypically normal when compared to testes of WT mice at 8 weeks of age, n=6. Scale bars: 2 mm. (E–G) Body weights (26.3609±0.4914 for WT; 25.1741±0.5189 for Ssh2 KO), weights of the testes (0.0862±0.0036 for WT; 0.0788±0.0023 for Ssh2 KO), and the testis-to-body weight ratio (0.3281±0.0153 for WT; 0.3154±0.0135 for Ssh2 KO) of adult WT and Ssh2 KO males which aged over 8 weeks (n=12). Data are presented as the mean ± SEM; p>0.05 calculated by Student’s t-test. Bars indicate the range of data. (H) Histology of the testis and cauda epididymis from WT and Ssh2 KO mice. Sections were stained with hematoxylin. No elongating or elongated spermatids were detected in Ssh2 KO testes, and no mature sperm were detected in the Ssh2 KO epididymis. Boxed regions are magnified on the right. Scale bars: 50 μm for the original region (left columns); 10 μm for the magnified region. Images are representative of testes/cauda epididymis extracted from at least six adult male mice per genotype. (I) Total sperm number in the cauda epididymis of WT (11.92×106 ± 0.82×106) and Ssh2 KO (0.00) mice, n=6. Data are presented as the mean ± SEM; ****p<0.0001, calculated by Student’s t-test. Bars indicate the range of data.
-
Figure 1—source data 1
Observational datasets and original blots.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig1-data1-v2.zip
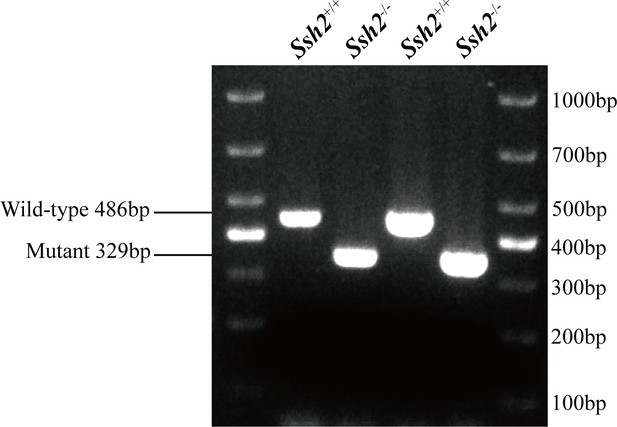
PCR genotyping for wild-type (WT) and Ssh2 knockout (KO) mice.
PCR genotyping demonstrating the absence of the WT band in Ssh2 KO mice.
-
Figure 1—figure supplement 1—source data 1
Original gels.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig1-figsupp1-data1-v2.zip
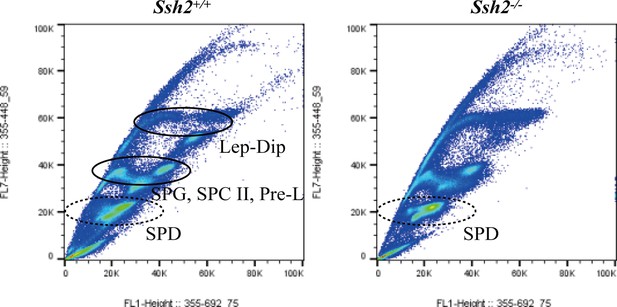
Fluorescence-activated cell sorting (FACS) assessment in spermatogenic cells from wild-type (WT) and Ssh2 knockout (KO) murine testes.
Distribution of spermatogenic cells by FACS. The number of spermatids (round/elongated spermatids in WT testes and round spermatids in Ssh2 KO testes) showed no significant variation in Ssh2 KO testes (as the dotted circles indicate). Lep, leptotene spermatocyte; Dip, diplotene spermatocyte; SPG, spermatogonia; SPC II, secondary spermatocyte, Pre-L, pre-leptotene spermatocyte; SPD, spermatid.
-
Figure 1—figure supplement 2—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig1-figsupp2-data1-v2.zip
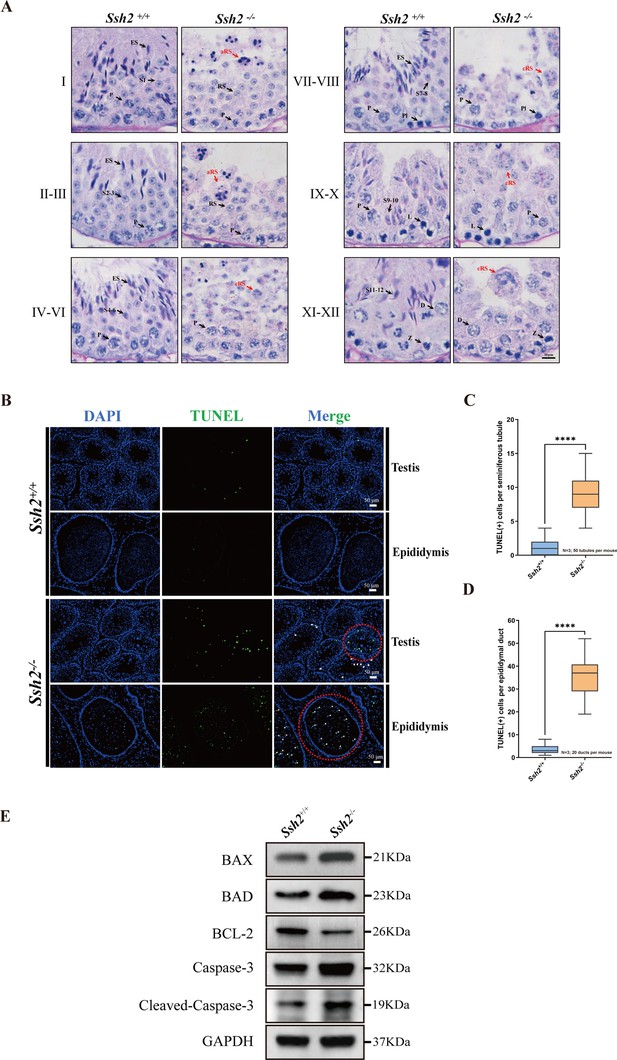
Spermatogenic arrest and enhanced germ cell apoptosis result from Ssh2 knockout (KO).
(A) Periodic acid Schiff (PAS)-hematoxylin-stained sections of seminiferous epithelia from wild-type (WT) mice and Ssh2 KO mice (8 weeks of age, n=3), indicating spermatogenic arrest at steps 2–3 of spermatogenesis in the Ssh2 KO mice. L, leptotene spermatocytes; D, diplotene spermatocytes; Z, zygotene spermatocytes; P, pachytene spermatocytes; RS, round spermatids; ES, elongated spermatids; S1–S12, spermatids at different spermiogenic steps; aRS: apoptotic-like round spermatids; cRS: clustered round spermatids. Scale bar: 10 µm. (B) Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) immunofluorescence staining of the testicular and epididymal sections from WT and Ssh2 KO mice. The TUNEL-positive puncta and signal intensity significantly increased in Ssh2 KO testes and epididymides (as the red circles indicate). Green: TUNEL-positive signal; blue: DAPI; white arrows: TUNEL-positive germ cells. Scale bars: 50 µm. At least three mice (6–8 weeks of age) of each genotype were used in the analysis. (C) Counts of TUNEL-positive cells per seminiferous tubule in adult Ssh2 KO testes (8.92±0.21) compared with control (1.19±0.08). Three mice of each genotype were assessed and 50 tubules were validated for each mouse. Data are shown as mean ± SEM; ****p<0.0001, calculated by Student’s t-test. Bars indicate the range of data. (D) Counts of TUNEL-positive cells per epididymal duct in adult Ssh2 KO testes (35.85±1.06) compared with control (3.57±0.21). Three mice of each genotype were assessed and 20 ducts were validated for each mouse. Data are shown as mean ± SEM; ****p<0.0001, calculated by Student’s t-test. Bars indicate the range of data. (E) Immunoblotting analysis of BCL2-associated X protein (BAX), BCL2-associated agonist of cell death (BAD), B-cell lymphoma-2 (BCL-2), Caspase-3, and cleaved Caspase-3 in testicular lysates from adult WT and Ssh2 KO mice, n=3; GAPDH was used as the loading control.
-
Figure 2—source data 1
Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL)-related observational datasets and original images/blots.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig2-data1-v2.zip
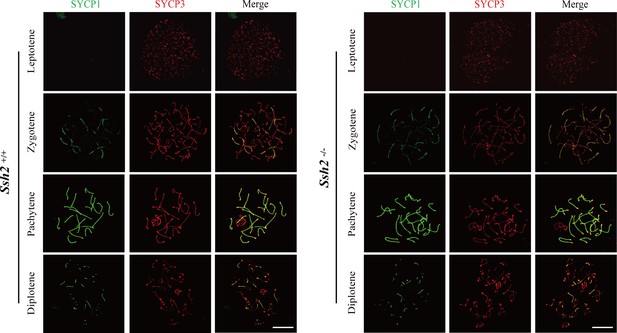
SSH2 is not required for meiotic progression in mouse spermatogenesis.
Testes from nearly 3-week-old wild-type (WT) mice (left panels) and Ssh2 knockout (KO) mice (right panels) were sampled for preparing chromosome spreads immunostained for SYCP1 and SYCP3. Spermatocytes from Ssh2 KO mice at leptotene, zygotene, pachytene, and diplotene of prophase I show no obvious abnormalities. Images are representative of three mice per genotype. Scale bars: 10 µm.
-
Figure 2—figure supplement 1—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig2-figsupp1-data1-v2.zip
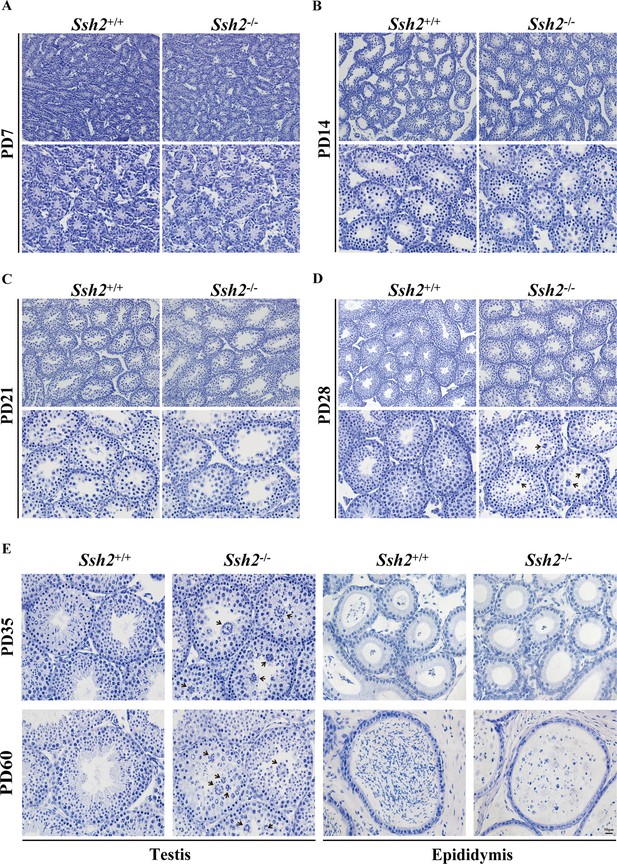
Comparison of spermatogenesis in wild-type (WT) and Ssh2 knockout (KO) mice.
Comparison of spermatogenesis in WT and Ssh2 KO mice assessed in testicular sections stained for hematoxylin at postnatal day (PD)7, PD14, PD21, PD28, PD35, and PD60 accompanied by epididymal sections at PD35 and PD60. Black arrows: round spermatid clusters. Scale bar: 50 µm.
-
Figure 2—figure supplement 2—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig2-figsupp2-data1-v2.zip
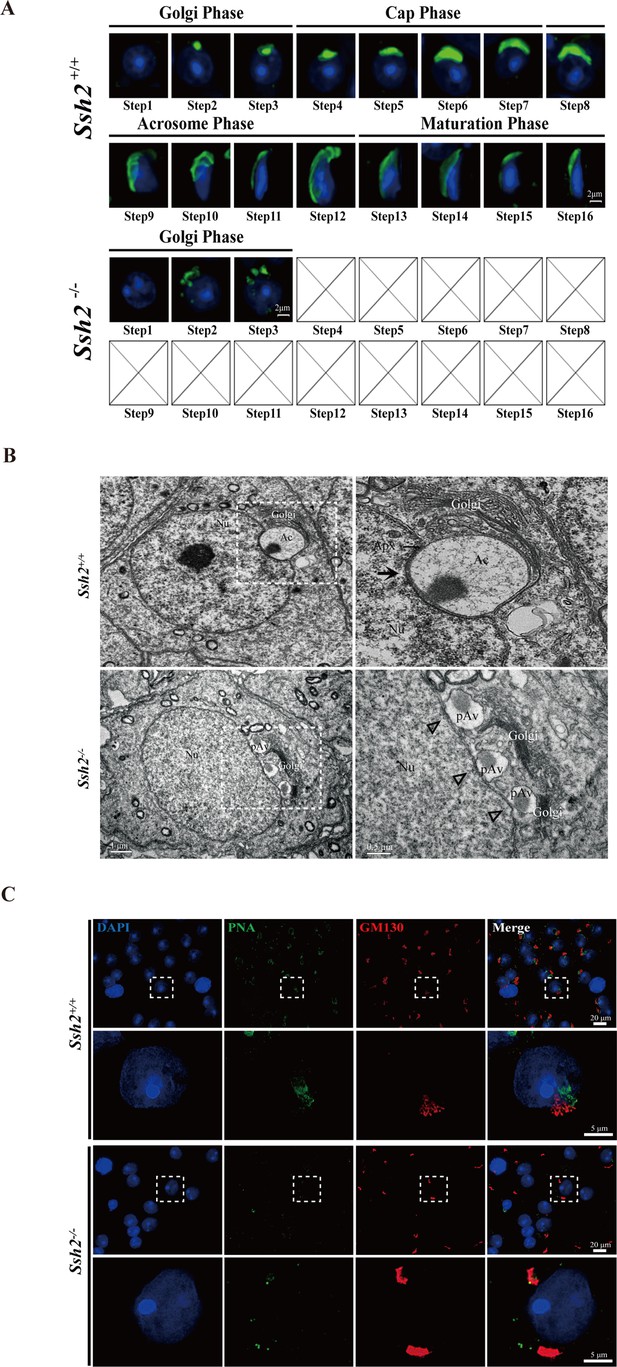
Acrosome biogenesis is disrupted during spermiogenesis in Ssh2 knockout (KO) mice.
(A) Analysis of spermiogenesis and acrosome biogenesis in wild-type (WT) and Ssh2 KO mice (8 weeks of age, n=3) by fluorescence imaging of spermatids labeled with peanut agglutinin (PNA) lectin (green). Nuclei were stained with DAPI (blue). The phases of acrosome biogenesis and corresponding spermiogenesis steps are the Golgi phase (steps 1–3), cap phase (steps 4–7), acrosome phase (steps 8–12), and maturation phase (steps 13–16). Scale bars: 2 µm. (B) Ultrastructural analysis of WT and Ssh2 KO spermatids. Intact acrosomes (black arrow) were observed in WT mice. The hollow triangles indicate proacrosomal vesicles that failed to fuse in Ssh2 KO mice. The regions outlined by white boxes are shown at higher magnification to the right. Nu, nucleus; Ac, acrosome; Golgi, Golgi apparatus; Apx, acroplaxome; Pav, proacrosomal vesicle. Scale bars: left panel, 1 µm; right panel, 0.5 µm. (C) Immunofluorescence analysis of the vesicular fusion-related Golgi-specific protein GM130 (red) in WT and Ssh2 KO round spermatids in testicular sections co-stained with PNA lectin (green). Nuclei were stained with DAPI (blue). Scale bars: original images, 20 µm; magnified images, 5 µm.
-
Figure 3—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig3-data1-v2.zip
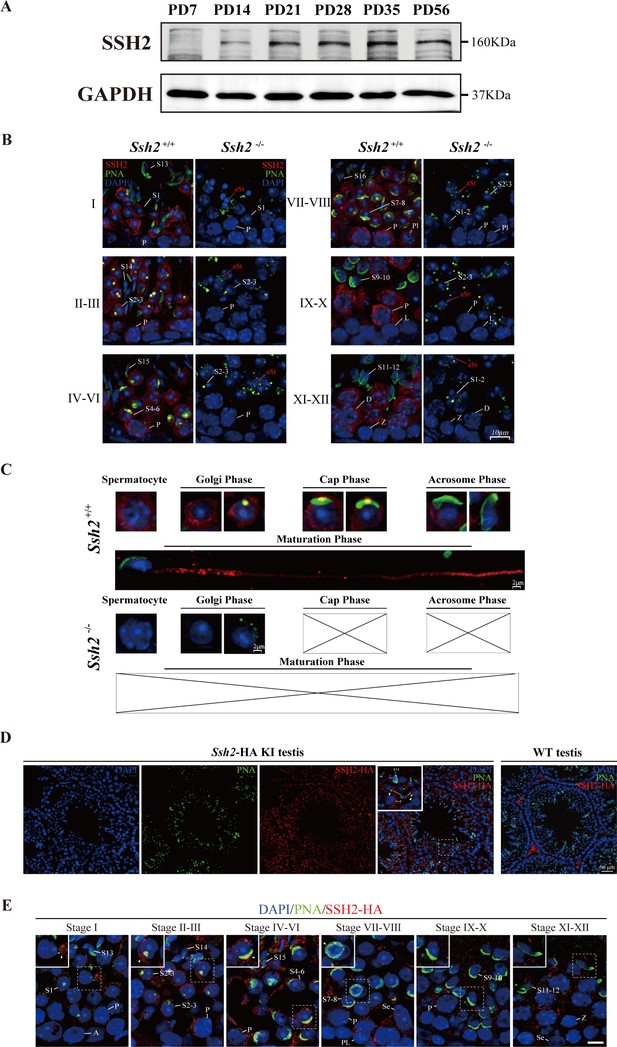
SSH2 accumulates at the acrosomal region of spermatids.
(A) Immunoblotting against SSH2 in wild-type murine testes sampled at the indicated postnatal days (PD). The expression of SSH2 at PD14, PD21, PD28, PD35, and PD56 was measured. GAPDH was used as the loading control. (B) Co-immunofluorescence staining of Alexa Fluor 488-conjugated peanut agglutinin (PNA) lectin (green) and SSH2 (red) on testicular sections from wild-type (WT) (left lane) and Ssh2 knockout (KO) (right lane) mice. Nuclei were stained with DAPI (blue). The epithelial spermatogenic cycle is routinely divided into 12 stages on the basis of changes in the morphology of the acrosome and nucleus in spermatids, and this was determined using the combination of PNA lectin and DAPI staining according to the established criteria. Cytoplasmic SSH2 localization in spermatocytes and spermatids of WT mice was observed, while fractured acrosomes were observed at all stages of spermatogenesis in Ssh2 KO mice. P, pachytene spermatocytes; Pl, preleptotene spermatocytes; L, leptotene spermatocytes; Z, zygotene spermatocytes; D, diplotene spermatocytes; S1–16, step 1–16 spermatids. aRS: apoptotic-like spermatids. Scale bar: 10 µm. (C) Analysis of acrosomal morphogenesis in spermatogenic cells by co-staining of PNA lectin (green) and SSH2 (red) in WT (upper panel) and Ssh2 KO (lower panel) murine testes. Nuclei were stained with DAPI (blue). Acrosomal morphology during acrosome biogenesis (Golgi, cap, acrosome, and maturation phase) is shown. No cap, acrosome, or maturation-phase spermatids were observed in Ssh2 KO mice. Scale bar: 2 µm. (D) Immunofluorescence co-staining hemagglutinin (HA)-tag (red) with Alexa Fluor 488-conjugated PNA lectin (green) showing the predominant cytoplasmic SSH2 localization in spermatocytes and spermatids with an accumulation of SSH2 in acrosomal region in round spermatids on testicular sections of HA-tagged Ssh2 KI mice while no obvious HA-tag fluorescence signal was detected in WT testes. Nuclei were stained with DAPI (blue). The framed regions are magnified. White triangles indicate the SSH2 acrosomal localization. P, pachytene spermatocytes; S2–3, step 2–3 spermatids; S14, step 14 spermatids. Scale bar: 50 μm. (E) Immunostaining of HA-tagged SSH2 (red) and PNA lectin (green) in testes of HA-tagged Ssh2 knock-in (KI) mice indicating the dynamic subcellular localization of SSH2 in spermatids during the process of acrosome biogenesis. Nuclei were stained with DAPI (blue). Framed region containing representative spermatids at different phases of acrosome biogenesis are magnified. White triangles indicate the SSH2 acrosomal localization. A, type A spermatogonia; P, pachytene spermatocytes; Pl, preleptotene spermatocytes; Z, zygotene spermatocytes; S1–16, step 1–16 spermatids; Se, Sertoli cells. Scale bar: 10 µm.
-
Figure 4—source data 1
Original images/blots.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig4-data1-v2.zip
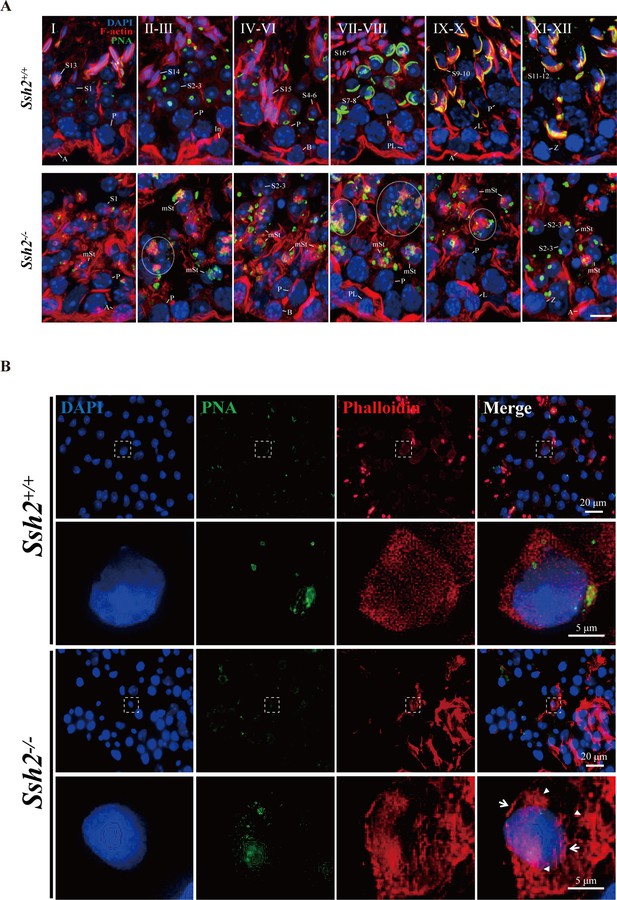
Ssh2 knockout (KO) results in disturbed filamentous actin (F-actin) remodeling in spermatids.
(A) Immunofluorescence detection of F-actin (red) and peanut agglutinin (PNA) lectin (green) during spermatogenesis in testicular sections from wild-type (WT) (top lane) and Ssh2 KO (bottom lane) mice at postnatal day (PD)85. Blocky actin filaments (as indicated by the circles) were observed in spermatids with malformed acrosomes. A, type A spermatogonia; In, intermediate spermatogonia; B, type B spermatogonia; P, pachytene spermatocytes; Pl, preleptotene spermatocytes; L, leptotene spermatocytes; Z, zygotene spermatocytes; D, diplotene spermatocytes; S1–16, step 1–16 spermatids; mSt, spermatids with malformed acrosomes. Scale bar: 10 µm. (B) Fluorescence analysis of phalloidin (red) and PNA lectin (green) on tubule squashes from adult WT and Ssh2 KO mice. Nuclei were stained with DAPI (blue). Diminutive actin filaments exhibited a uniform cytoplasmic distribution in WT spermatids, whereas the actin remodeling was disrupted as thick actin filaments (indicated by white arrows) and lumpy actin aggregates (indicated by white triangles) were formed in Ssh2 KO spermatids indicated by phalloidin staining. The framed regions are magnified beneath. Scale bars: original images, 20 µm; magnified images, 5 µm.
-
Figure 5—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig5-data1-v2.zip
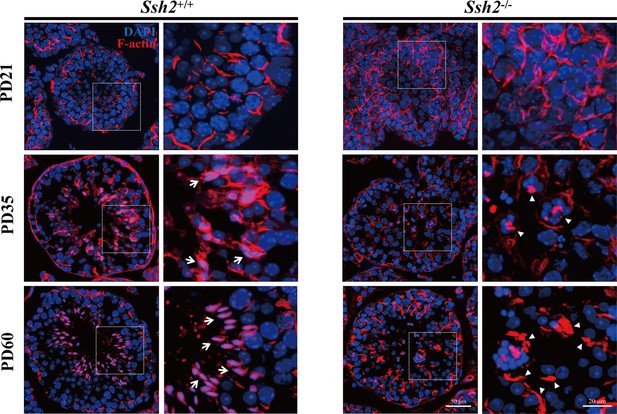
Disordered filamentous actin (F-actin) in Ssh2 knockout (KO) testes.
Fluorescence imaging of phalloidin-labeled F-actin (red)-stained seminiferous tubules from wild-type (WT) (left panels) and Ssh2 KO (right panels) murine testes at postnatal day (PD)21, PD35, and PD60. Nuclei were stained with DAPI (blue). Representative images show the sharp sickle head of WT spermatids ‘hooped’ by F-actin bundles (indicated with arrows), whereas actin filaments aggregated to form several lumps in Ssh2 KO spermatids (triangles). The framed regions are shown at higher magnification to the right. Scale bars: original images, 50 µm; magnified images, 20 µm.
-
Figure 5—figure supplement 1—source data 1
Original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig5-figsupp1-data1-v2.zip
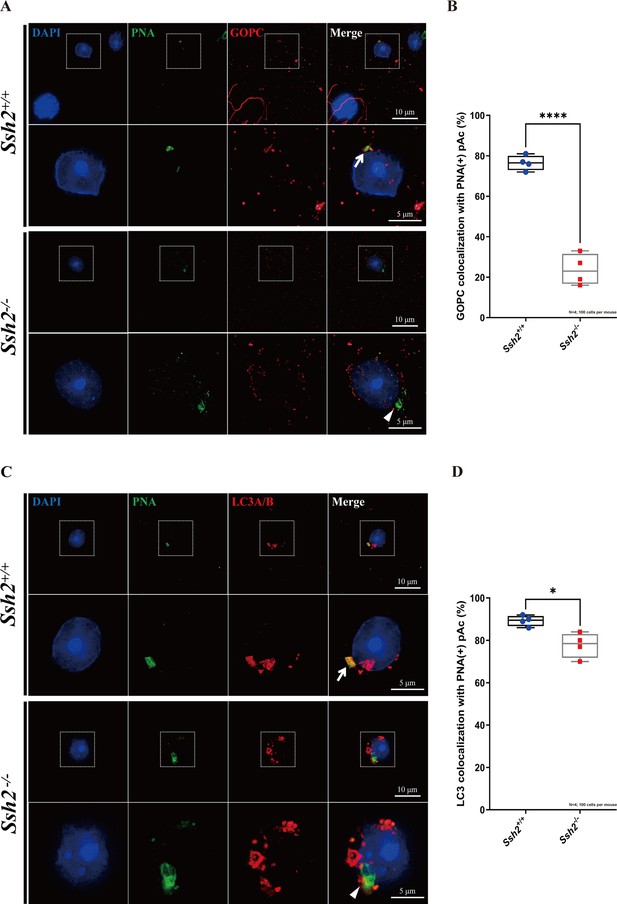
Ssh2 knockout (KO) spermatids exhibit defects in proacrosomal vesicle transport.
(A) Immunofluorescence staining of the vesicular trafficking-related Golgi-specific protein GOPC (red) in wild-type (WT) and Ssh2 KO round spermatids in testicular sections co-stained with peanut agglutinin (PNA) lectin (green). Nuclei were stained with DAPI (blue). Acrosomal debris was observed in Ssh2 KO round spermatids. GOPC colocalized with PNA lectin in WT spermatids (upper panels, arrow indicates the colocalization) but not in those of Ssh2 KO mice (lower panels, triangle indicates the absence of colocalization), as shown in the representative images. Framed areas are magnified beneath. Scale bars: original images, 10 µm; magnified images, 5 µm. (B) Quantitative analysis of GOPC and PNA lectin colocalization in WT mice, 76.50 ± 3.70%; Ssh2 KO mice, 23.75 ± 7.72% (n=4; 400 cells). Data are shown as mean ± SEM, ***p<0.001 by Student’s t-test. Bars indicate the range of data. (C) Immunofluorescence staining of the autophagosome-related protein LC3A/B (red) in WT and Ssh2 KO round spermatids on the testicular sections co-stained with PNA lectin (green). Nuclei were stained with DAPI (blue). LC3A/B colocalized with PNA lectin in WT spermatids (upper panels, arrow indicates the colocalization) but not in those of Ssh2 KO mice (lower panels, triangle indicates the absence of colocalization) as representative images showed. Framed areas are magnified beneath. Scale bars: original images, 10 µm; magnified images, 5 µm. (D) Quantitative analysis of LC3A/B and PNA lectin colocalization in WT mice, 89.45 ± 2.50%; Ssh2 KO mice, 77.75 ± 5.91% (n=4; 400 cells). Data are shown as the mean ± SEM, ***p<0.05 by Student’s t-test. Bars indicate the range of data.
-
Figure 6—source data 1
Observational datasets and original images.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig6-data1-v2.zip
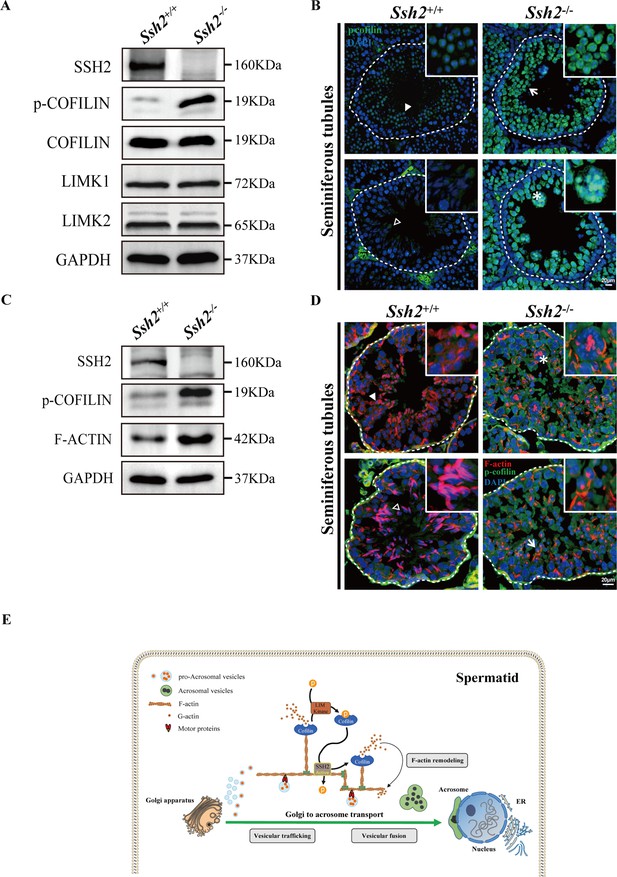
Ssh2 knockout (KO) spermatids display impaired COFILIN phospho-regulation that disrupts filamentous actin (F-actin) remodeling.
(A) Immunoblotting against SSH2, p-COFILIN, COFILIN, LIMK1, and LIMK2 in wild-type (WT) and Ssh2 KO testes sampled on postnatal day (PD)82. GAPDH was used as the loading control. (B) Testicular sections of WT and Ssh2 KO mice stained with p-COFILIN (green) and DAPI (blue), n=3. The solid and hollow triangles point to the round spermatids and elongated spermatids of low p-COFILIN expression in seminiferous tubules of WT mice. The white arrow points to the round spermatids of strong p-COFILIN expression in seminiferous tubules of Ssh2 KO mice. The asterisk indicates a germ cell cluster. Scale bars: 20 μm. (C) Western blot analysis of SSH2, p-COFILIN, and F-ACTIN in lysates from 8-week-old WT and Ssh2 KO testes, n=3; GAPDH was used as the loading control. (D) Testicular sections of 8-week-old WT and Ssh2 KO mice stained for p-COFILIN (green) and F-actin (red), n=3. The solid and hollow triangles point to the round spermatids and elongated spermatids with intact F-actin organization in seminiferous tubules of WT mice. The white arrow points to the round spermatids of strong p-COFILIN expression with disorganized F-actin in seminiferous tubules of Ssh2 KO mice. The asterisk indicates a germ cell cluster. Scale bars: 20 µm. (E) Proposed model for the functional role of the SSH2-COFILIN pathway in acrosome biogenesis. SSH2 participates in F-actin remodeling by regulating the phosphorylation of COFILIN. Intact and organized F-actin dynamics enables the transport of proacrosomal vesicles from the Golgi apparatus to the apical pole of the nucleus of the spermatid. F-actin also participates in the fusion of Golgi-derived vesicles and extra-Golgi vesicles.
-
Figure 7—source data 1
Original images/blots.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig7-data1-v2.zip
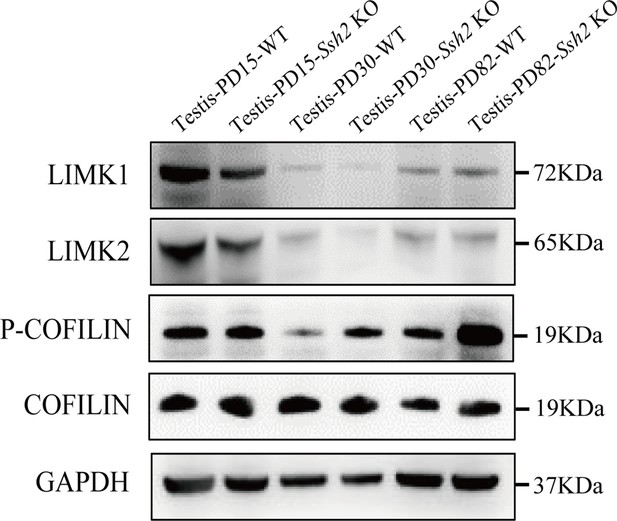
COFILIN-associated protein expression level in wild-type (WT) and Ssh2 knockout (KO) testes.
Protein extracts from testes isolated from WT and Ssh2 KO mice at postnatal day (PD)15, PD30, and PD82 were used for western blot analysis for LIMK1, LIMK2, p-COFILIN, and COFILIN. GAPDH was used as the loading control.
-
Figure 7—figure supplement 1—source data 1
Original blots.
- https://cdn.elifesciences.org/articles/83129/elife-83129-fig7-figsupp1-data1-v2.zip
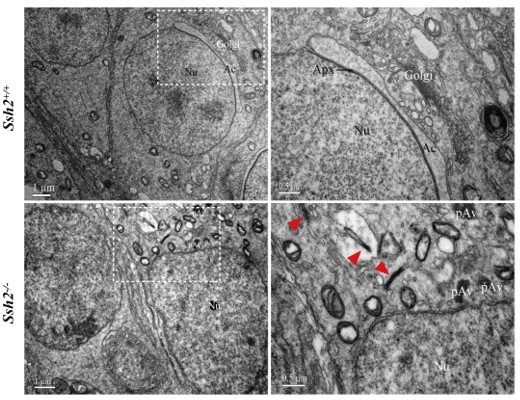
Representative images of ultrastructural analysis of actin organization in WT and Ssh2 KO spermatids.
No intact F-actin structure was observed in WT round spermatids with few aggregated actin bundles (as red arrowheads indicated) detected in Ssh2 KO spermatids under TEM. The regions outlined by white boxes are shown at higher magnification to the right. Nu, nucleus; Ac, acrosome; Golgi, Golgi apparatus; Apx, acroplaxome; Pav, proacrosomal vesicle. Scale bars: left panel, 1 µm; right panel, 0.5 µm.





