Genetic dissection of mutual interference between two consecutive learning tasks in Drosophila
Figures
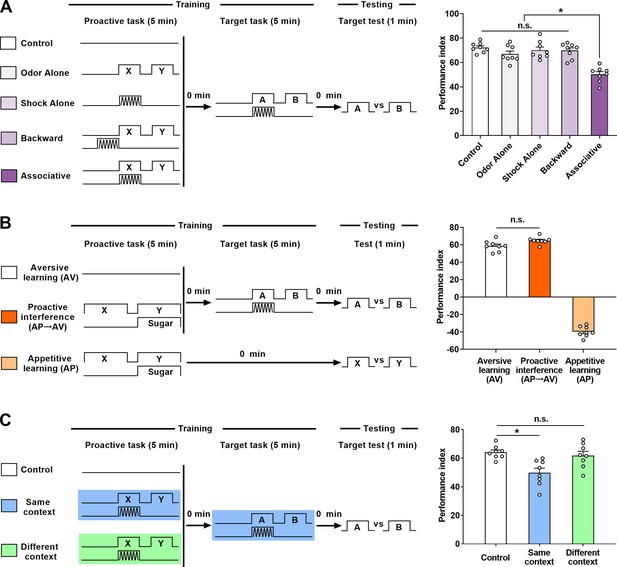
Effects of proactive interference (Pro-I) between two consecutive olfactory learning tasks.
(A) Left: schematic of the experiment. No stimuli (Control), non-associative stimuli (Odor Alone, Shock Alone, and Backward), or associative learning (Associative) were used as the proactive task. Aversive associative learning was used as the target task. Right: Comparison of immediate memory performance of the target task of different groups. Compared with the ‘Control’ group, significant Pro-I was observed in the ‘Associative’ group, but not in non-associative groups. n=8. (B) Left: schematic of the experiment. In the ‘Aversive learning (AV)’ or ‘Appetitive learning (AP)’ group, the immediate performance of a single task was tested; in the ‘Proactive interference (AP→AV)’ group, the proactive task was an appetitive learning and the target task was an aversive learning. Right: Comparison of immediate memory performance of different groups. Compared with the AV group, no significant Pro-I was observed in the ‘Proactive interference (AP→AV)’ group. n=8. (C) Left: schematic of the experiment. In the ‘Control’ group, there was no proactive task; in the ‘Same context’ group, the proactive task and the target task were performed in the same context (blue light); in the ‘Different context’ group, the proactive task was performed in the green light context, while the target task was performed in the blue light context. Right: Comparison of immediate memory performance of the target task of different groups. Compared with the ‘Control’ group, significant Pro-I was observed in the ‘Same context’ group, but not in the ‘Different context’ group. n=8. Statistics: ordinary one-way ANOVA with Dunnett’s multiple comparisons tests. Results with error bars are means ± SEM. *p<0.05. n.s., non-significant. Also see Figure 1—figure supplement 1, Figure 1—source data 1, and Figure 1—figure supplement 1—source data 1 information.
-
Figure 1—source data 1
Raw data of Figure 1.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig1-data1-v2.xlsx
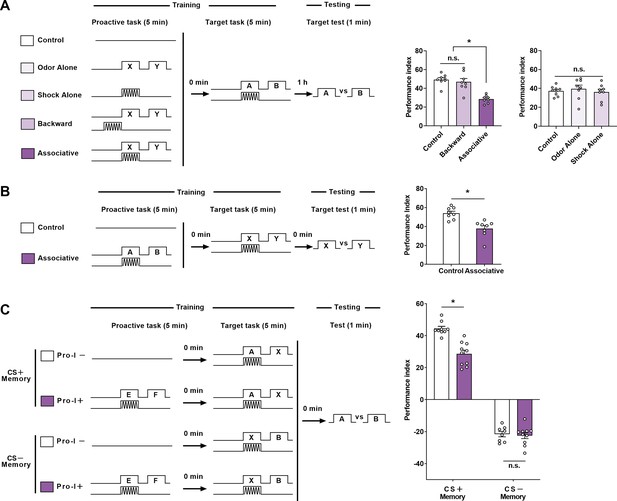
Other effects of proactive interference (Pro-I).
(A) Left: schematic of the experiment. No stimuli (Control), non-associative stimuli (Odor Alone, Shock Alone, and Backward), or associative learning (Associative) were used as the proactive task. Aversive associative learning was used as the target task. Right: Comparison of 1 hr memory performance of the target task of different groups. Compared with the ‘Control’ group, significant Pro-I was observed in the ‘Associative’ group, but not in non-associative control groups. n=8–9. (B) Left: schematic of the experiment. Odors of A and B were used in the proactive task, while odors of X and Y were used in the target task. Right: Comparison of the immediate memory performance of the target task of different groups. Compared with the ‘Control’ group, significant Pro-I was observed in the ‘Associative’ group. n=8. (C) Left: schematic of the experiment. Odors of E and F were used in the proactive task. Odors of A and X were used in the target task to test CS +memory, and odors of X and B were used in the target task to test CS– memory. Right: Comparison of the immediate memory performance of the target task of different groups. Compared to the ‘Pro-I–’ group, significant Pro-I was observed in the ‘Pro-I+’ group when testing CS + memory but not CS– memory. n=8–10. Statistics: Kruskal-Wallis test with Dunn’s multiple comparisons tests (A-mid panel); ordinary one-way ANOVA with Dunnett’s multiple comparisons tests (A-right panel); Mann-Whitney test (B); two-way ANOVA with Bonferroni’s multiple comparisons tests (C). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant.
-
Figure 1—figure supplement 1—source data 1
Raw data of Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig1-figsupp1-data1-v2.xlsx
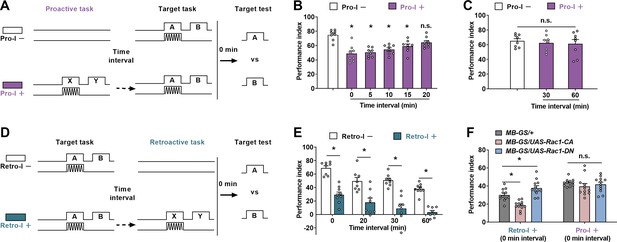
Differences between proactive interference (Pro-I) and retroactive interference (Retro-I).
(A–C) The paradigm (A) and behavioral results (B and C) of Pro-I experiments. The time interval between the proactive task and the target task was changed from 0 to 60 min. Pro-I was significant when the time interval was less than 20 min (0 min, 5 min, 10 min, or 15 min) in wild-type flies. n=8. (D and E) The paradigm (D) and the behavioral result (E) of theRetro-I experiment. Retro-I was significant when the time interval between the target and the retroactive task was 0 min, 20 min, 30 min, and 60 min. n=8–9. (F) The behavioral performance of transgenic flies with retroactive or Pro-I. Compared to the control group (MB-GS/+, RU486+), Rac1-CA-expressing flies (MB-GS/UAS-Rac1-CA, RU486+) showed a significantly lower performance index, while Rac1-DN-expressing flies (MB-GS/UAS-Rac1-DN, RU486+) exhibited a higher memory index in Retro-I. No significant difference was observed in all groups with Pro-I. n=12. Statistics: ordinary one-way ANOVA with Dunnett’s multiple comparisons tests (B and C); two-way ANOVA with Bonferroni’s multiple comparisons tests (E and F). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant. Also see Figure 2—source data 1 for additional information.
-
Figure 2—source data 1
Raw data of Figure 2.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig2-data1-v2.xlsx
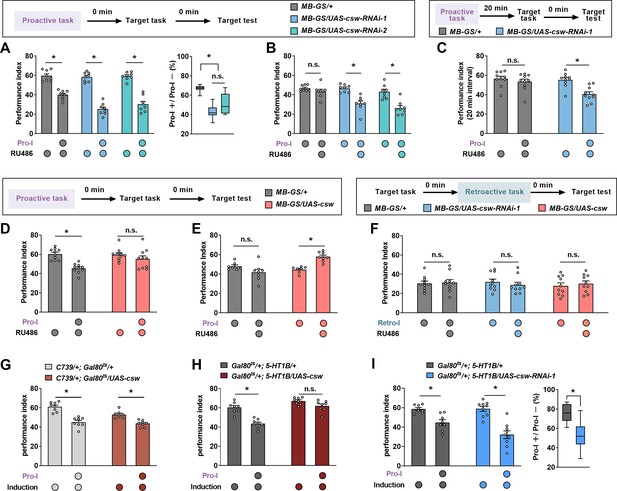
Corkscrew (CSW) bidirectionally regulates proactive but not retroactive interference (Retro-I).
(A and B) The behavioral performance of transgenic flies with proactive interference (Pro-I) (0 min interval). Acute knockdown of CSW in mushroom body neurons (MB-GS/UAS-csw-RNAi-1 or MB-GS/UAS-csw-RNAi-2; RU486+) led to more severe Pro-I relative to the genetic control group (MB-GS/+, RU486+) (A) and uninduced controls (RU486–) (B). n=8. (C) The behavioral performance of transgenic flies with Pro-I (20 min interval). csw-RNAi-expressing flies (MB-GS/UAS-csw-RNAi-1, RU486+) but not control flies (MB-GS/+, RU486+) showed significant Pro-I. n=10. (D and E) The behavioral performance of transgenic flies with Pro-I (0 min interval). Acute overexpression of CSW in mushroom body neurons (MB-GS/UAS-csw; RU486+) prevented Pro-I (D) and was more resistant to Pro-I than uninduced control (RU486–) (E). n=7–8. (F) The behavioral performance of transgenic flies with Retro-I (0 min interval). Acute knockdown (MB-GS/UAS-csw-RNAi-1, RU486+) or overexpression (MB-GS/UAS-csw, RU486+) of CSW in mushroom body neurons did not affect Retro-I compared with uninduced controls. n=10. (G) Significant Pro-I was found in flies with acute overexpression of CSW in MB α/β neurons (C739/+; Gal80ts/UAS-csw) and genetic control flies (C739/+; Gal80ts/+). n=8. (H) Significant Pro-I was found in genetic control flies (Gal80ts/+; 5-HT1B/+) but not flies with acute overexpression of CSW in MB γ neurons (Gal80ts/+; 5-HT1B/UAS-csw). n=8. (I) Acute knockdown of CSW in MB γ neurons (Gal80ts/+; 5-HT1B/UAS-csw-RNAi-1) increased Pro-I relative to the genetic control group (Gal80ts/+; 5-HT1B/+). n=8–9. Statistics: two-way ANOVA with Bonferroni’s multiple comparisons tests (A-left panel, B, D-H, and I-left panel); Kruskal-Wallis test with Dunn’s multiple comparisons tests (A-right panel);. Mann-Whitney test (C); unpaired t-test (I-right panel). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant. Also see Figure 3—figure supplement 1, Figure 3—source data 1, and Figure 3—figure supplement 1—source data 1 for additional information.
-
Figure 3—source data 1
Raw data of Figure 3.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig3-data1-v2.xlsx
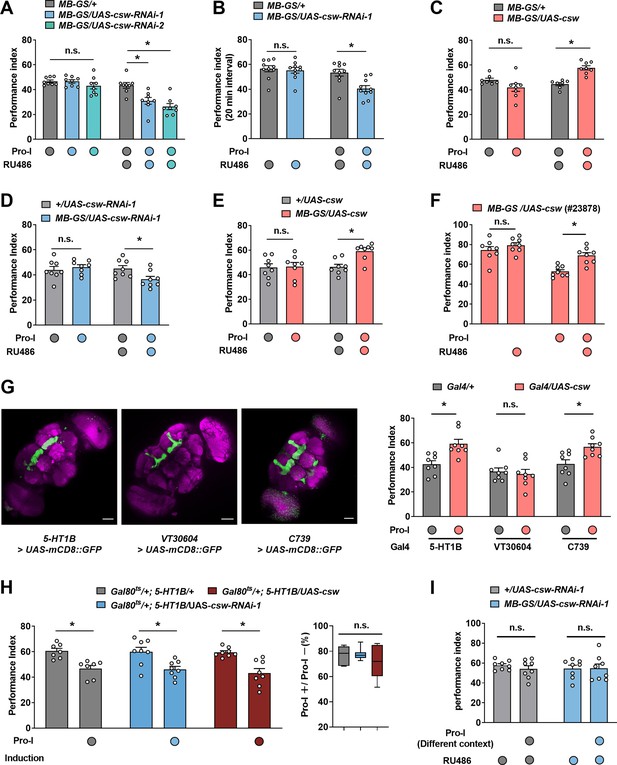
Additional control experiments of Figure 3.
The immediate memory performance with or without proactive interference (Pro-I) was tested in different transgenic flies (A–I). The inter-task interval (ITI) was 0 min (A, C, and D-I) or 20 min (B). (A) An alternative representation of the data in Figure 3B. n=8. (B) An alternative representation of the data in Figure 3C. n=10. (C) An alternative representation of the data in Figure 3E. n=7–8. (D) Acute knockdown of Corkscrew (CSW) in mushroom body neurons (MB-GS/UAS-csw-RNAi-1, RU486+) led to more severe Pro-I relative to the genetic control group (+/UAS-csw-RNAi-1, RU486+). n=8. (E) Acute overexpression of CSW in mushroom body neurons (MB-GS/UAS-csw; RU486+) reduced Pro-I compared with the genetic control group (+/UAS-csw, RU486+). n=8. (F) Acutely overexpressing CSW in mushroom body (MB) neurons (MB-GS/UAS-csw (23878), RU486+) reduced the Pro-I compared with its uninduced control group. n=8. (G) Left: expression patterns of different drivers. Scale bar, 50 μm. Right: flies with overexpression of CSW in MB γ neurons (5-HT1B>UAS csw) and α/β neurons (C739 >UAS csw) showed higher memory performance than their genetic control group. n=8. (H) Effects of Pro-I without transgene induction. n=7–8. (I) Acutely knocking down CSW in MB neurons (MB-GS/UAS-csw-RNAi-1, RU486+) did not significantly affect Pro-I when the proactive task (green light) was in a different context with the target task (blue light). n=8. Statistics: two-way ANOVA with Bonferroni’s multiple comparisons tests (A, C, D, F, and I); Mann-Whitney test (B, E, G, and H). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant.
-
Figure 3—figure supplement 1—source data 1
Raw data of Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig3-figsupp1-data1-v2.xlsx
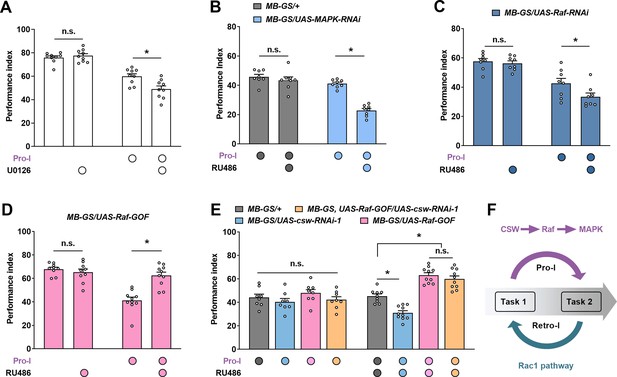
Corkscrew (CSW) regulates proactive interference (Pro-I) through Raf/MAPK pathway.
The immediate memory performance with or without Pro-I (Pro-I, 0 min interval) was tested in wild-type and different transgenic flies (A–E). (A) Pharmacological inhibition of MAPK by feeding U0126 inhibitor aggravated the Pro-I in wild-type flies. n=9. (B) Flies with acute knockdown of MAPK in mushroom body (MB) neurons (MB-GS/UAS-MAPK-RNAi, RU486+) exhibited more severe Pro-I compared with uninduced control flies. n=8. (C) Acute knockdown of Raf in MB neurons (MB-GS/UAS-Raf-RNAi, RU486+) aggravated the Pro-I relative to the uninduced control. n=8. (D) Acutely overexpressing Raf-GOF in MB neurons (MB-GS/UAS-Raf-GOF, RU486+) reduced the Pro-I compared with its uninduced control group. n=9–10. (E) Acute overexpression of Raf-GOF (MB-GS/UAS-Raf-GOF, RU486+) dominated the effect of CSW knockdown (MB-GS/UAS-csw-RNAi, RU486+) on Pro-I. No significant difference was found between uninduced groups without RU486 feeding. n=8–10. (F) Model of molecular mechanisms underlying proactive and retroactive interference. Statistics: Mann-Whitney test (A and D); two-way ANOVA with Bonferroni’s multiple comparisons tests (B, C, and E). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant. Also see Figure 4—figure supplement 1, Figure 4—source data 1, and Figure 4—figure supplement 1—source data 1 for additional information.
-
Figure 4—source data 1
Raw data of Figure 4.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig4-data1-v2.xlsx
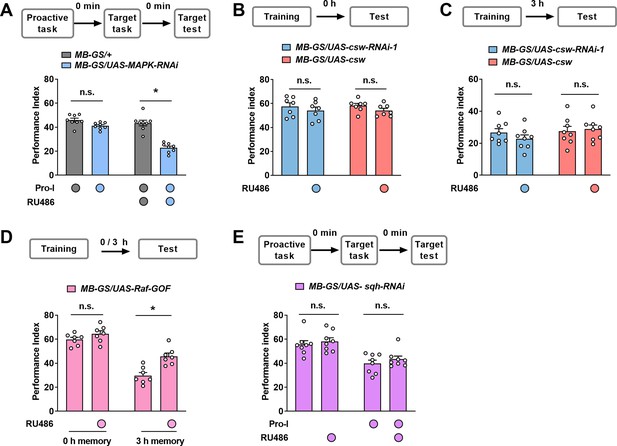
Additional behavioral data of a single learning task and proactive interference (Pro-I).
(A) An alternative representation of the data in Figure 4B. n=8. (B) Learning performance. Acute knockdown or overexpression of Corkscrew (CSW) in mushroom body (MB) neurons did not affect the learning of a single task. n=7. (C) 3 hr memory performance. Acute knockdown or overexpression of CSW in MB neurons did not affect the 3 hr memory performance of a single task. n=8. (D) Learning and 3 hr memory performance. Acutely overexpressing Raf-GOF increased 3 hr memory performance without affecting learning. n=7. (E) Acute knockdown of Sqh in MB neurons (MB-GS/UAS-sqh-RNAi, RU486+) did not significantly affect Pro-I. n=8. Statistics: two-way ANOVA with Bonferroni’s multiple comparisons tests (A–C); Mann-Whitney test (D). Results with error bars are means ± SEM. *p<0.05. n.s., non-significant.
-
Figure 4—figure supplement 1—source data 1
Raw data of Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/83516/elife-83516-fig4-figsupp1-data1-v2.xlsx





