GABABR silencing of nerve terminals
Figures
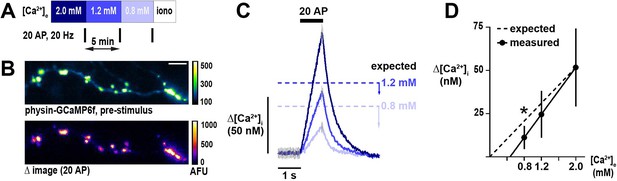
Unexpected sub-proportionality in dependence of presynaptic calcium (Ca2+) influx on [Ca2+]e.
(A) Schematic of the experimental protocol. Neurons are stimulated with a brief action potential (AP) train (20 AP in 1 s) at three different [Ca2+]e centered on the physiologic set point of 1.2 mM. Ionomycin, a Ca2+ ionophore, is administered at the end of the experiment to convert fluorescence into absolute [Ca2+]i. (B) An axon from a neuron expressing GCaMP6f localized to nerve terminals by synaptophysin (physin-GCaMP) at rest (top) and a difference image showing the response to 20 AP (bottom). The difference image is the mean peak (five frames) subtracted by the mean baseline (49 frames) in [Ca2+]e 2 mM. For display, a representative subset of terminals was highlighted. Scale bar is 10 µm. (C) Traces of responses to 20 AP from the neuron shown in B, with [Ca2+]e color-coded as in A. Expected Δ[Ca2+]i at 0.8 and 1.2 mM is represented by dashed lines and calculated as a change in influx relative to 2.0 mM proportional to the ratios of [Ca2+]e. Arrow bars show the difference between expected and measured Δ[Ca2+]i. Traces are mean, with error (SEM) represented by gray lines in only pre-stimulus and peak frames for clarity (n=211 nerve terminals). (D) Summary of Δ[Ca2+]i as a function of [Ca2+]e (mean ± 95%CI, n=9 neurons). The x-axis intercept for measured changes in Δ[Ca2+]i is 0.47 mM. *p<0.05, one-sample t-test compared to the expected value.
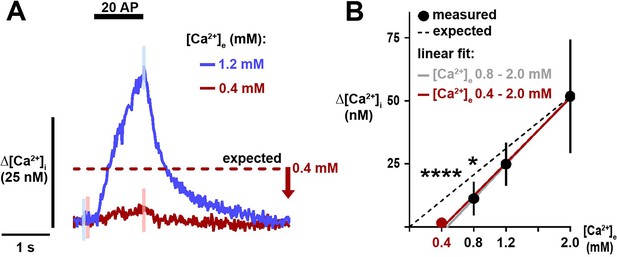
Ca2+ entry into nerve terminals is nearly completely abolished at a non-zero threshold.
(A) Traces of a neuron expressing physin-GCaMP stimulated with 20 AP in [Ca2+]e 1.2 mM and 0.4 mM. The dotted line represents the expected peak Δ[Ca2+]i in [Ca2+]e 0.4 mM relative to 1.2 mM in a model in which Ca2+ influx is proportional to [Ca2+]e, and the arrow shows the difference to the measured peak. Traces are mean and error bars are SEM, showing a subset of error bars for clarity, n=216 terminals. (B) Summary of Δ[Ca2+]i as a function of [Ca2+]e (mean ± 95% CI, n=9 neurons for measurements in [Ca2+]e 0.8–2.0 mM and n=5 for [Ca2+]e 0.4 and 1.2 mM). Data for [Ca2+]e 0.8–2.0 mM are from Figure 1. Expected Δ[Ca2+]i is represented by the dashed line and calculated as a change in influx relative to 2.0 mM proportional to the ratios of [Ca2+]e. The linear fit with [Ca2+]e 0.4 mM includes all [Ca2+]e and the x-axis intercept is [Ca2+]e 0.45 mM. *p<0.05, ****p<0.0001, one-sample t-test compared to the expected value.
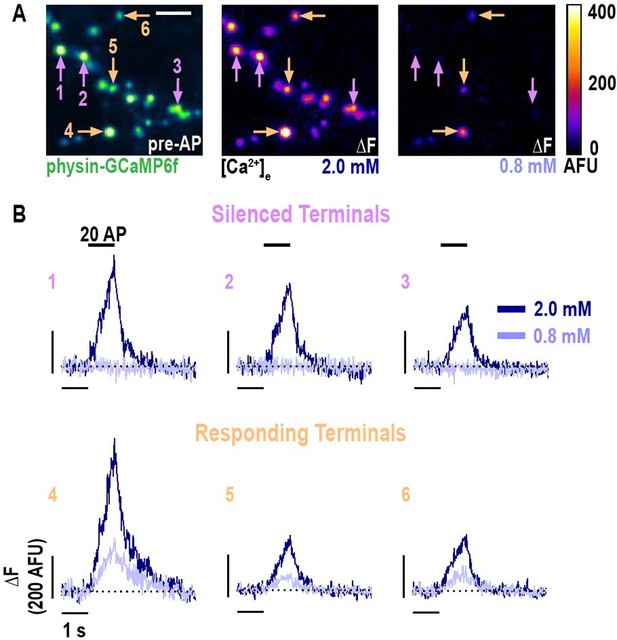
Selective nerve terminal silencing is caused by changing [Ca2+]e around the physiologic set point.
(A) Pre-stimulus average (left) and difference images (right) of AP stimulation (20 APs in 1 s) of a neuron expressing physin-GCaMP. The difference image is the mean peak (five frames) subtracted by the mean baseline (49 frames) at [Ca2+]e 2.0 and 0.8 mM. For display, a subset of representative terminals was selected and a Gaussian convolution with the radius of 1 pixel was applied. (B) Traces of the terminals indicated in A, shown as ΔF, demonstrate selective silencing (upper row compared to lower row) of a subset of terminals. Dotted line is the pre-stimulus mean fluorescence for each terminal.
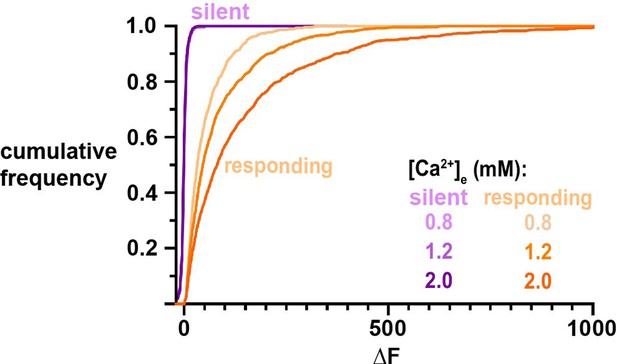
Distinctly different ΔF distributions of silent and responding nerve terminals, demonstrating [Ca2+]e invariance of silent terminals.
Comparison of cumulative frequencies of ΔF for each [Ca2+]e of terminals separated into silent or responding populations.
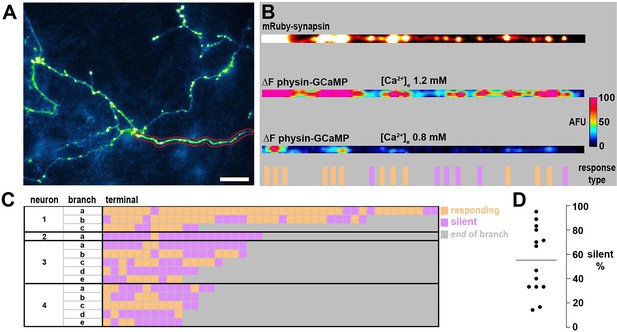
Neuronal branches contain both silent and responding nerve terminals.
(A) Cytosolic GCaMP6f expression in a neuron co-transferred with mRuby-synapsin to label nerve terminals. Scale bar is 20 um. Image is a subset to highlight branches of interest. (B) Linearized fluorescence of mRuby-synapsin (top) and difference image of physin-GCaMP following 20 AP (bottom two) in [Ca2+]e 1.2 mm and 0.8 mM from branch highlighted by red outline in A. Orange and magenta bars at the bottom summarize the response type in [Ca2+]e 0.8 mM. (C) Representation of responses in [Ca2+]e 0.8 mM of individual nerve terminals from 14 branches across four neurons in which orange is a responding terminal and magenta is a silent terminal. Selections for analysis were limited to unambiguous branches with at least 10 nerve terminals to provide a robust quantification. (D) Summary of the percentage of silent nerve terminals from the branches shown in C, which agrees well with the proportion of silencing from entire neurons in [Ca2+]e 0.8 mM.
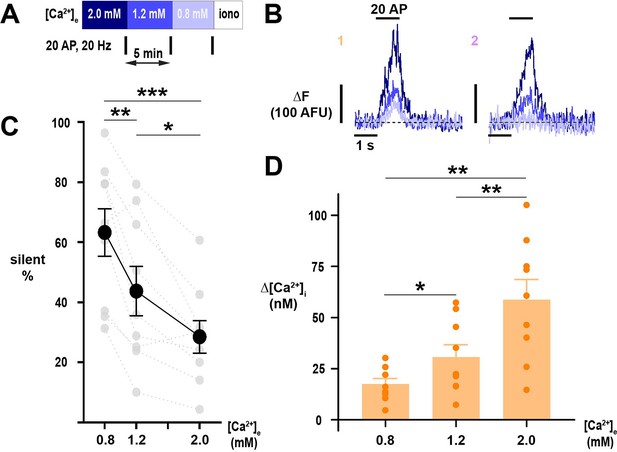
Silencing of Ca2+ influx into nerve terminals is potently modulated by changes in [Ca2+]e about the physiologic set point.
(A) Diagram of the experimental protocol. (B) Example traces of single nerve terminals expressing physin-GCaMP, demonstrating persistent responsiveness (1) or silencing (2) with lowering of Ca2+ below physiologic extracellular levels. Dotted line is pre-stimulus mean fluorescence (ΔF=0). (C) The percentage of silent nerve terminals. Black dots are mean, error bars are SEM, and gray dots are individual cells. (D) Summary of Δ[Ca2+]i of responding terminals. Dots are individual cells, bars are mean, and error bars are SEM. (C) and (D) were analyzed with one-way ANOVA and Tukey’s post-test for multiple comparisons, *p<0.05, **p<0.01, ***p<0.001, n=9.
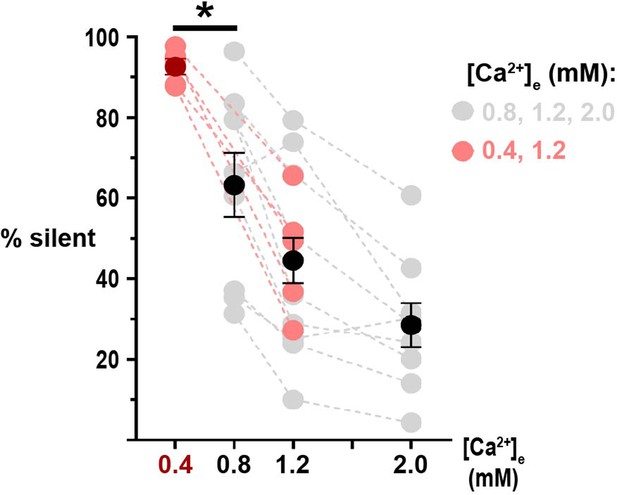
Quantification of nerve terminal silencing in [Ca2+]e 0.4 mM.
Percentage of silent terminals in neurons expressing physin-GCaMP and stimulated with 20 AP. Red dots represent neurons stimulated in [Ca2+]e 0.4 and 1.2 mM only, while gray dots are data from Figure 3. Dark red dots and black dots are mean and error bars are SEM. Data in [Ca2+]e 0.4 mM and 0.8 mM are analyzed with unpaired t-test, *p<0.05, n=5 and 9 neurons, respectively.
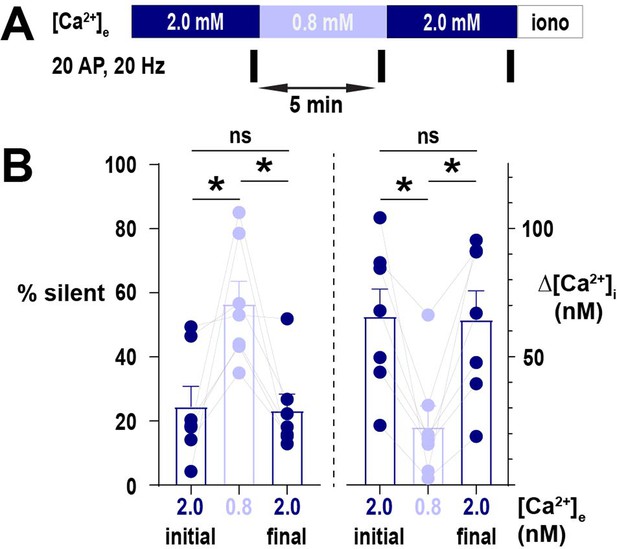
Silencing of nerve terminals by lowering [Ca2+]e is reversible.
(A) Diagram of the experimental protocol applied to neurons expressing physin-GCaMP. (B) Comparison of the percentage of silent terminals and mean Δ[Ca2+]i. Dots are individual cells, bars are mean and error bars are SEM. Data were analyzed with one-way ANOVA and Tukey’s post-test for multiple comparisons, *p<0.05, ns p>0.05, n=7.
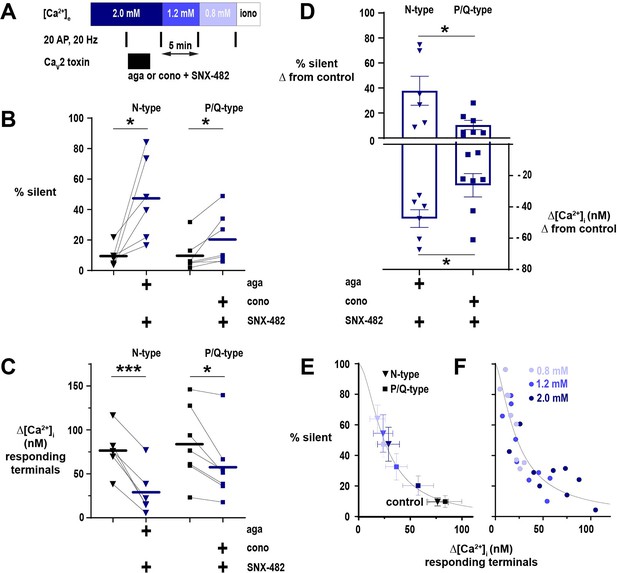
The proportion of nerve terminal silencing in neurons is related to absolute calcium (Ca2+) influx irrespective of CaV2 subtype.
(A) Diagram of the experimental protocol applied to neurons expressing physin-GCaMP. (B) The percentage of silent nerve terminals before and after toxin application to isolate a CaV2 subtype at [Ca2+]e 2 mM. (C) Summary of Δ[Ca2+]i in responding terminals after toxicologic isolation of N-type or P/Q-type channels. (D) Comparison of the effects of toxin application to isolate CaV2 subtypes on silencing (upper graph, shown on the left axis) and Ca2+ influx (lower graph, shown on the right axis). For B-D, symbols are the mean of individual neurons, lines and bars are the mean of all cells, and error bars are SEM. (B) and (C) were analyzed with a paired t-test, and D was analyzed with unpaired t-test. *p<0.05, ***p<0.001, n=6 for N-type and n=7 for P/Q-type. (E) The percentage of silent terminals plotted against Δ[Ca2+]i in responding terminals in control (black symbols) and following toxin application at varying [Ca2+]e. Symbols are mean and error bars are SEM. Data are fit with a Hill model, with the maximum at Δ[Ca2+]i = 0 constrained to 100%. (F) Relationship of the percentage of silent terminals to Δ[Ca2+]i in responding terminals with varying [Ca2+]e in neurons not treated with CaV2 toxins. Dots are the mean of individual cells from neurons presented in Figure 2. Data are fit as in E.
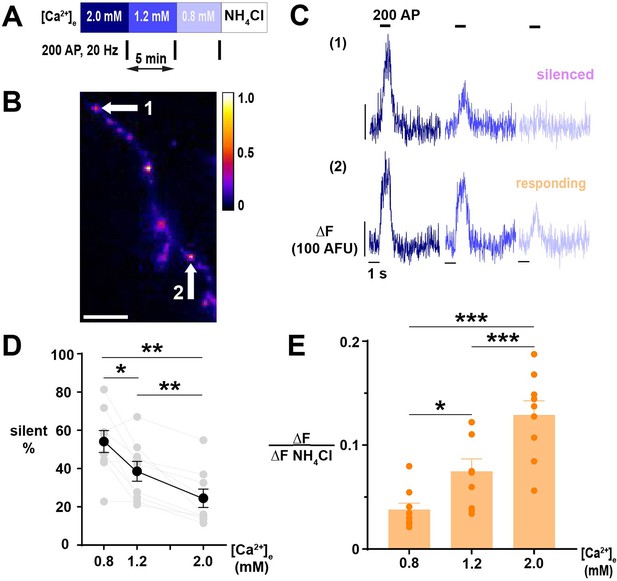
Exocytosis of SVs exhibits [Ca2+]e-driven silencing.
(A) Diagram of the experimental protocol. (B) Segment of an axon expressing vGpH, showing a difference in fluorescence in nerve terminals revealed by the application of NH4Cl 50 mM. Scale bar is 10 µm, and the calibration is maximum-normalized fluorescence intensity. (C) SV recycling of individual nerve terminals indicated in B demonstrating selective silencing at [Ca2+]e 0.8 mM in (1) compared to the persistent response in (2). Traces of different [Ca2+]e are color-coded as in A. (D) Percentage of silent nerve terminals. Black dots are mean, error bars are SEM, and gray dots are individual cells. (E) SV exocytosis of responding terminals in different [Ca2+]e. Dots are individual neurons, bars are the mean, and error bars are SEM. (D) and (E) were analyzed with one-way ANOVA and Tukey’s post-test for multiple comparisons, *p<0.05, **p<0.01, ***p<0.01, n=9.
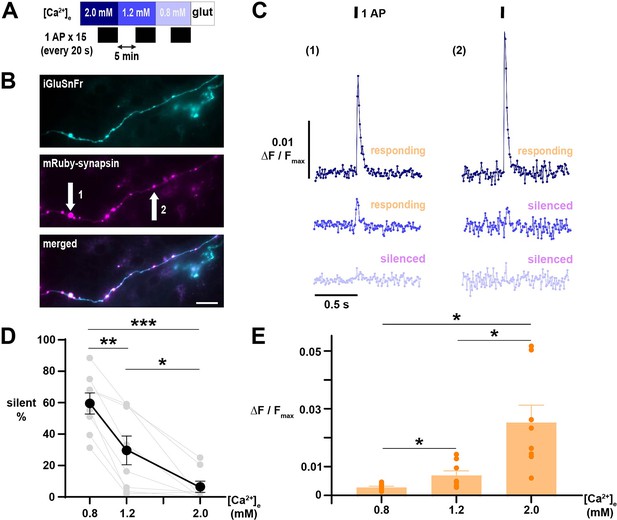
Glutamate release elicited by 1 AP demonstrates [Ca2+]e-driven silencing at neurotransmitter release sites.
(A) Diagram of the experimental protocol. Responses of iGluSnFR-expressing neurons were averaged over 15 single AP trials delivered in ~20 s intervals. (B) Difference image (peak minus baseline fluorescence) of an axon expressing iGluSnFR (cyan, upper) stimulated with 1 AP in [Ca2+]e 1.2 mM, with terminals marked by mRuby-synapsin (magenta, middle; merge lower). Scale bar 20 µm. (B) Traces of iGluSnFR measured at nerve terminals indicated in B, illustrating differential silencing as [Ca2+]e is lowered. Traces of different [Ca2+]e are color-coded as in A. (D) Percentage of silent nerve terminals. Black dots are mean, error bars are SEM, and gray dots are individual cells. (E) Glutamate release, quantified as ΔF/Fmax, in responding terminals as [Ca2+]e is decreased. Dots are the mean of individual cells, bars are mean, and error bars ± SEM. (D) and (E) were analyzed with one-way ANOVA and Tukey’s post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, n=8.
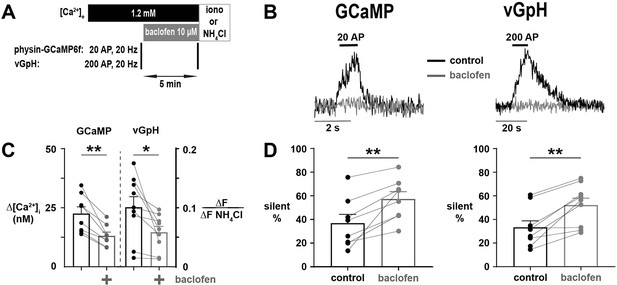
Agonism of GABABR with baclofen leads to silencing of calcium (Ca2+) influx and SV exocytosis.
(A) Diagram of the experimental protocol. (B) Representative traces of silencing of Ca2+ influx and SV exocytosis resulting from agonism of GABABR with baclofen in single nerve terminals expressing physin-GCaMP or vGpH, respectively. (C) Δ[Ca2+]i and ΔF/ΔFNH4Cl before and following the application of baclofen in responding terminals. (D) The percentage of silent terminals before and following treatment with baclofen was measured in physin-GCaMP and vGpH-expressing neurons (see labels above). For (C) and (D), dots are individual neurons, bars are mean of all neurons, and error bars are SEM. (C) and (D) were analyzed with a paired t-test, *p<0.05, **p<0.01, n=8 for physin-GCaMP, and n=9 for vGpH.
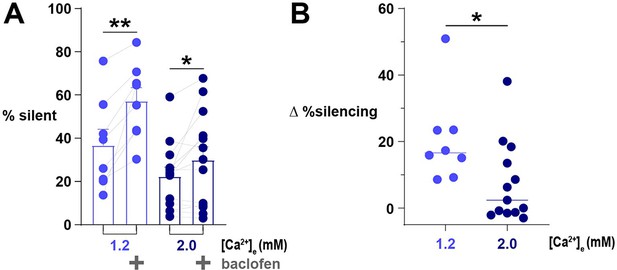
Agonism of GABABR with baclofen causes fewer terminals to become silent in [Ca2+]e 2.0 mM compared to 1.2 mM.
(A) The percentage of silent terminals in neurons expressing physin-GCaMP stimulated with 20 AP before or during the administration of baclofen in [Ca2+]e 1.2 mM or 2.0 mM. Dots are individual cells, bars are mean, and error bars are SEM. Data are analyzed with paired t-test (B) The change in the percentage of silencing caused by the administration of baclofen. Data are analyzed with the Mann-Whitney U test because of the non-normal distribution. For A and B, *p<0.05, **p<0.01, n=8 and 13 for 1.2 mM and 2.0 mM, respectively. Data for % silent in [Ca2+]e 1.2 mM are the same as in Figure 7.





