Elucidating a locus coeruleus-dentate gyrus dopamine pathway for operant reinforcement
Figures
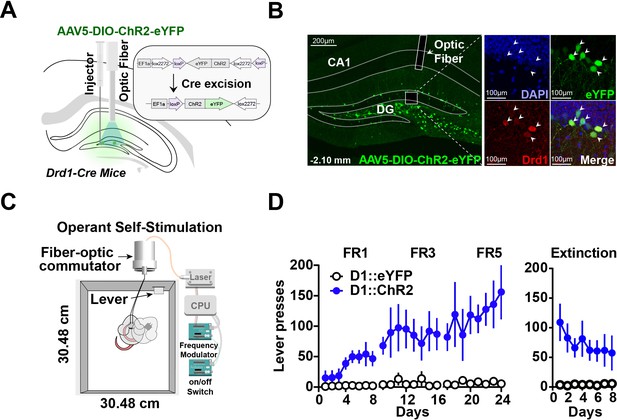
Optogenetic stimulation of D1 + neurons in the dentate gyrus is sufficient for operant self-stimulation.
(A) Schematic of optic fiber placement above virally infected D1 + dentate gyrus (DG) neurons in D1-Cre mice. (B) Left, coronal section showing ChR2 expression in the DG. Right, magnified view of AAV infected DG neurons from inset colocalized with D1 receptors. White arrows indicate cell bodies. (C) Schematic of the operant self-stimulation chamber. (D) Lever pressing rate across three fixed ratios (FR1, FR3, FR5) schedules of reinforcement, and extinction (8 days each) for D1:Chr2-DG animals (n=8) and eYFP (n=8) controls. D1::Chr2-DG mice self-stimulated significantly more than controls (Two-way RM ANOVA, Group [ChR2 or eYFP] × Day, main effect of group F(1,14) = 16.59, p=0.0011, main effect of Day, F(23, 322) = 2.958, p=0.0078, and a significant interaction between day × group F(23,322) = 2.479, p=0.0003). During extinction, there was a significant main effect of group: F(1,112) = 58.87, p<0.0001, no significant effect of Day: F(7, 112) = 0.4571, p=0.8635, and no interaction: F(7, 112) = 0.8243, p=0.8243. Means +/−SEM for all graphs. DG, dentate gyrus; LC, Locus Coeruleus; scp, superior cerebellar peduncle; DAPI, 4′,6-diamidino-2-phenylindole. ****p<0.0001.
-
Figure 1—source data 1
The press rate (presses/min) of D1::ChR2 and D1::eYFP mice across FR1, FR3, and FR5 sessions.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig1-data1-v2.csv
-
Figure 1—source data 2
The press rate (presses/min) of D1::ChR2 and D1::eYFP mice across extinction sessions.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig1-data2-v2.zip
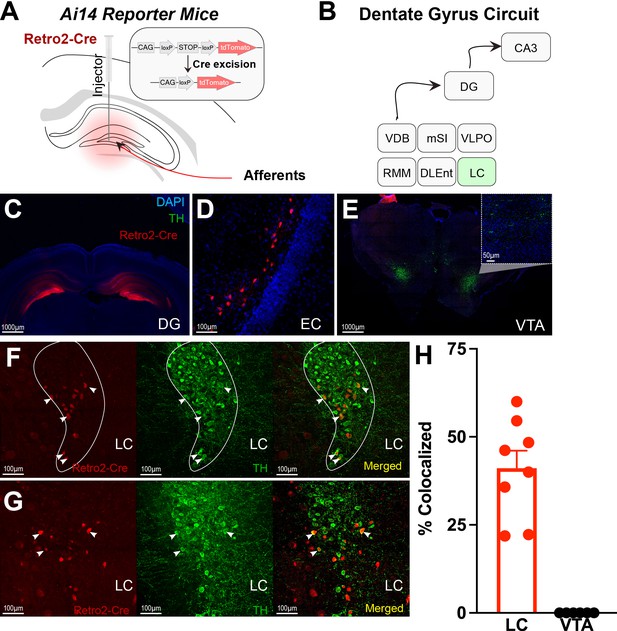
Retro-Cre tracing shows that the main catecholamine input to the dentate gyrus (DG) is the locus coeruleus (LC), not the ventral tegmental area.
(A) Schematic of Retro2-Cre injection into the dentate gyrus in Ai-14 reporter mice. (B) Schematic summarizing all brain regions that project to the DG. Only LC is TH+. Abbreviations – Vertical diagonal band (VDB), Medial septal nucleus (MSN), Ventral lateral preoptic area (VLPO), retro mammillary bodies (RMM), dorsal lateral entorhinal cortex (DLEnt) (C) Injection site of the Retro2 showing the Cre-positive neurons. (D–G) Retrograde labeling of neurons in canonical brain regions that project to the hippocampus. (D) Entorhinal cortex (EC). (E) Limited retrograde labeling of neurons in the VTA, colocalized with tyrosine hydroxylase (TH). (F & G) Retrograde labeling of LC neurons in two out of four mice, colocalized with tyrosine hydroxylase. (H) Percent of colocalized neurons in the LC (n=8; four mice × two hemispheres) and VTA (n=6; three mice × two hemispheres). Unpaired t-test, p<0.0001. Mean and +/−SEM.
-
Figure 2—source data 1
Percent of colocalized (TH+ and tdTomato+) neurons in the DG and VTA.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig2-data1-v2.csv
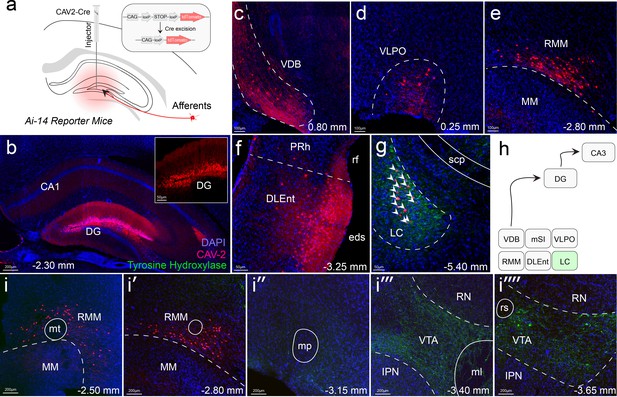
Retrograde tracing shows locus coeruleus (LC) projections, but not ventral tegmental area (VTA) projections to the dorsal dentate gyrus.
(a) Schematic of Cav2-Cre injection into the dentate gyrus of the hippocampus of Ai-14 reporter mice. (b) Injection site of the Cav2 showing the Cre-positive neurons. (c–f) Retrograde labeling of neurons in canonical brain regions that project to the hippocampus. Vertical diagonal band (VDB), Ventral lateral preoptic area (VLPO), retro mammillary bodies (RMM), dorsal lateral entorhinal cortex (DLEnt). (g) Retrograde labeling of neurons in the LC, colocalized with tyrosine hydroxylase (TH). (h) Schematic summarizing all brain regions that project to the dentate gyrus (DG). Only LC is TH+ (i - i’’’’) Anterior to posterior series of brain sections, demonstrating that retrograde labeling ends outside of the VTA.
-
Figure 2—figure supplement 1—source data 1
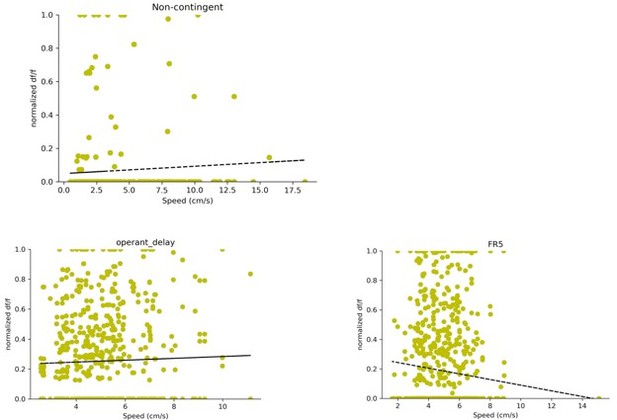
Percent of modulated D1 + DG neurons during different behavioral tasks.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig2-figsupp1-data1-v2.zip
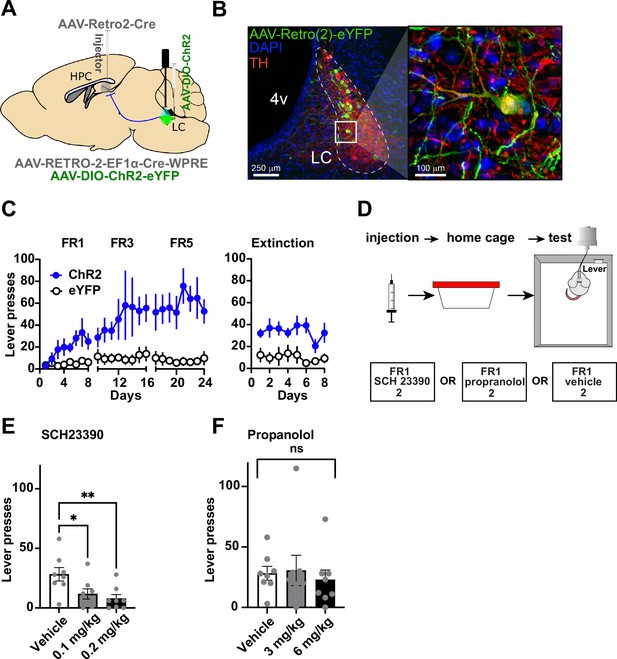
Locus coeruleus (LC) projections to the dentate gyrus (DG) contribute to operant learning.
(A) Schematic showing selective targeting of LC-DG projection. (B) Left: Representative coronal section showing Cre-dependent ChR2 expression in the LC. Right: Magnified view from inset showing eYFP colocalization with tyrosine hydroxylase (TH) LC neurons. (C) Lever presses per session for three fixed ratios (FR1, FR3, FR5) schedules of reinforcement, and extinction (no stimulation following lever pressing) for Retro::ChR2 DG-LC animals (n=8) and eYFP (n=8) controls. Mice with ChR2 expressed in LC self-stimulated significantly more than controls (Two-way RM ANOVA Group [ChR2 or eYFP] × Day, the significant effect of group: F(1,14) = 13.09, p=0.0031), the significant effect of day, F(23, 322) = 3.601, p<0.0001; interaction of day × group: F(23,322) = 2.712, p<0.0001. (D) Left, design of pharmacological experiments. After FR1 training, mice received IP injections of antagonists for either D1 (SCH 23390), or NE-beta receptors (propranolol). (E) D1-antagonist SCH 23390 significantly reduced self-stimulation of DG-projecting LC neurons: F(2, 14) = 6.9, p=0.008. Post hoc analysis (Dunnett’s) shows that both doses reduced lever pressing relative to controls (0.1 mg/kg, p=0.02; 0.2 mg/kg, p=0.007). (F) The NE antagonist propranolol did not have any effect: F(2, 14) = 0.290, p=0.753. Means +/−SEM. HPC, hippocampus; 4 v, fourth ventricle. *p<0.05, **p<0.01.
-
Figure 3—source data 1
The press rate (presses/min) of LC-DG::ChR2 LC-DG::eYFP mice during extinction days.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig3-data1-v2.csv
-
Figure 3—source data 2
The press rate (presses/min) of LC-DG::ChR2 LC-DG::eYFP mice across FR1, FR3, and FR5 sessions.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig3-data2-v2.csv
-
Figure 3—source data 3
Total presses during vehicle and propranolol I.P. administration (3mg/kg and 6mg/kg).
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig3-data3-v2.zip
-
Figure 3—source data 4
Total presses during vehicle and SCH-23390 I.P. administration (0.1mg/kg and 0.2mg/kg).
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig3-data4-v2.zip

Local infusions of D1 but not NE beta antagonist into the DG reduces self-stimulation.
(A) Schematic showing injection strategy for local drug infusions into the DG during self-stimulation of LC neurons that project to the DG (n=8). Cre expression was induced in LC neurons projecting to the DG by first injecting AAV-Retro-2 into the DG. An injection of a Cre-dependent virus (AAV5-DIO-ChR2-eYFP) was then made in the LC before optic fiber implantation. Canulae were used to inject DA and NE antagonists into the DG. (B) Top, representative coronal section showing ChR2 expression in the LC. Bottom, ChR2 terminals in DG and cannula tracks. (C) Acquisition of lever pressing Retro::ChR2 DG-LC mice or Retro::eYFP DG-LC (controls). Experimental animals self-stimulated more than controls (Two-way ANOVA [Day × Group], effect of Day F(7, 84) = 5.222, p<0.0001; effect of group, F(1,12) = 37.98, p<0.0001; interaction F(7,84) = 4.932, p<0.0001). (D) One-way RM ANOVA showed D1 antagonist SCH-23390 significantly reduced lever pressing. There is a significant drug effect: F(2,14) = 39.16, p<0.0001. Dunnett’s multiple comparisons show both doses produced significant suppression of lever pressing (1.8 mM, p<0.0001; 3.6 mM, p<0.0001). (E) NE antagonist propranolol had no significant effect on self-stimulation. F(2,14) = 0.1912, p=0.8281. Dunnett’s multiple comparisons show no significant differences 10.5 mM, p=0.7829; 21 nM, p=0.8624. (F) Using DeepLabCut we tracked the distance traveled by the each animal and found no significant differences in the movement for the vehicle, SCH23390 (3.6 mM) or propranolol (21 nM) RM one-way ANOVA no effect of group, F(1, 4) = 3, p=0.1516. Means +/−SEM for all graphs. DG, dentate gyrus; LC, locus coeruleus; 4 v, fourth ventricle.
-
Figure 4—source data 1
Total presses during vehicle and propranolol administration through cranial cannula (21mM and 10.5mM).
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig4-data1-v2.zip
-
Figure 4—source data 2
Total presses during vehicle and SCH-23390 administration through cranial cannula (3.6mM and 1.8mM).
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig4-data2-v2.zip
-
Figure 4—source data 3
The press rate (presses/min) of LC-DG::ChR2 + DG cannula and LC-DG::eYFP + DG cannula mice across FR1 sessions.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig4-data3-v2.csv
-
Figure 4—source data 4
Total head movement (meters) during SCH-23390(3.6mM) and propranolol(21mM) administration through cranial cannula.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig4-data4-v2.zip
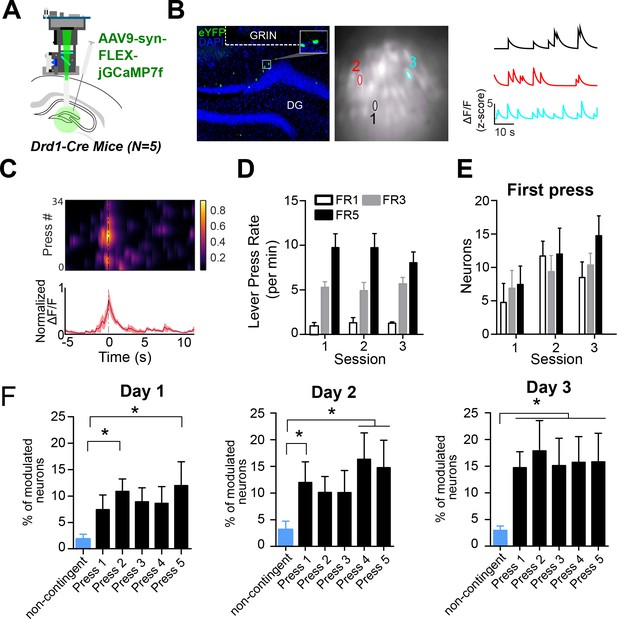
D1 + dentate gyrus neurons are significantly modulated by lever pressing during operant conditioning.
(A) A schematic of a UCLA miniscope and GRIN lens implanted over jGCamp7f-infected D1 + cells in the dentate gyrus (DG) of D1-Cre mice for in vivo imaging (n=5). (B) Left: Representative coronal section showing GCaMP7f expression in D1 neurons of the DG. GRIN lens, marked in white. Middle: Imaging field of view with contours of identified neurons. Right: For example, calcium traces are significantly modulated by lever presses. (C) An example neuron showing increased calcium transient during lever pressing. Top: Heat map shows normalized calcium activity aligned to a single press as a function of time during fixed-ratio (FR) 1 trial. Bottom: Averaged calcium activity across all presses. (D) The press rates for FR1, FR3, and FR5 for each day of testing. The press rates increase across the FR schedule. (E) The percent of neurons modulated by the first press in each FR schedule across days. Two-way RM ANOVA FR schedule × Day, no effect of FR schedule F(2,4) = 0.6702, p=0.5298; effect of day, F(2,4) = 4.536, p=0.0213; no effect of interaction F(2,4) = 0.6403, p=0.6389 (F) The percent of neurons modulated by a non-contingent reward task, or by each press in an FR5 task. Day 1, One-way RM ANOVA F(4,5) = 3.389, p=0.0222. Day 2, One-way RM ANOVA F(4,5) = 16.19, p=0.0046. Day 3, One-way RM ANOVA F(4,5) = 4.807, p=0.0048. Dunnett’s multiple comparison tests was used to compare the percent of press-modulated neurons to the non-contingent modulated neurons. Significance values are marked (*) for p<0.05.
-
Figure 5—source data 1
Lever press rate (presses/min) during FR1, FR3 and FR5 sessions, number of modulated neurons during the first press, percent of modulated neurons during each press across days.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig5-data1-v2.xls
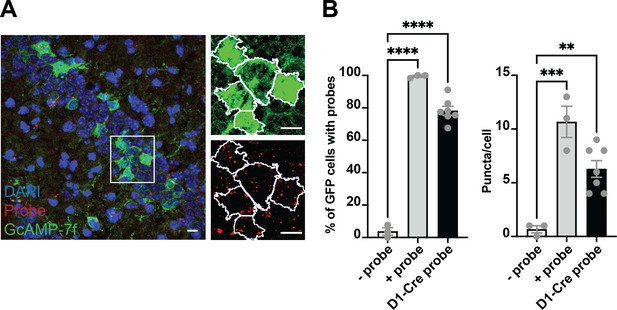
Colocalization of GcAMP-7f with D1 RNA scope probes.
(A) Left, the representative coronal section from a Drd1a-cre mouse injected with GCaMP-7f. Right, zoomed-in view of green (GCaMP7f) and red (probe) channels, showing the outlines (white) of GFP + cells identified in FIJI. (B) Left, the percent of GFP cells colocalized with the negative control probe (n=3 sections; one brain, nine images), positive control probe (n=3 sections; one brain, five images), and Drd1a probe (n=7 sections; three brains, 38 images). One-way ANOVA showed a significant group difference (F = 229.2, p<0.0001). About 80% of GFP neurons colocalized with the Drd1a, much higher than the background negative control probe (Tukey‘s, p<0.0001). Right, there was also a significant group difference in puncta per cell (ANOVA, F = 19.51, p=0.0004) between Drd1a probe and negative control probe (Tukey‘s, mean diff = –5.619, adjusted p<0.0052) and positive control probe (Tukey‘s, mean diff = 4.381, adjusted p=0.0226). Thus the SIO/DIO constructs are only being expressed in D1 + neurons. Scale bars represent 10 um.
-
Figure 6—source data 1
Percent of GFP cells colocalized with negative control, positive control and Drd1a probes.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig6-data1-v2.xls
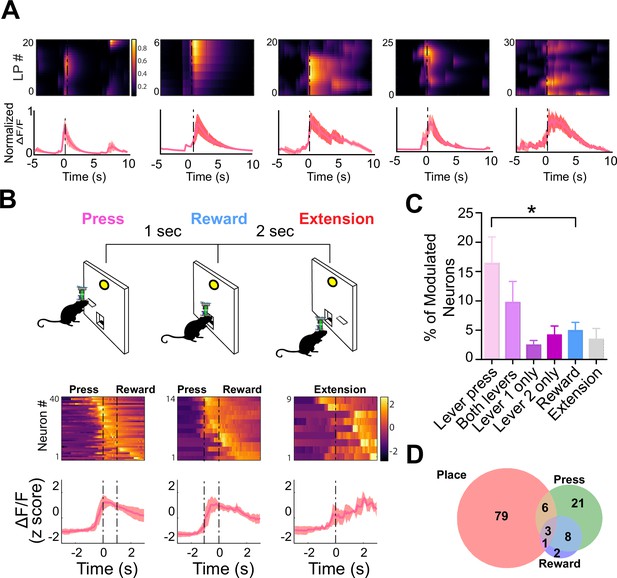
D1 + neurons in dentate gyrus (DG) are significantly modulated by lever pressing, but not by passive reward delivery.
(A) One example neuron from each of the five calcium imaging animals, showed increased calcium transient during lever pressing. Top: Heat map shows normalized calcium activity aligned to a single press as a function of time during fixed-ratio (FR) 1 trial. Bottom: Averaged calcium activity across all presses. For the five different animals, we recorded n=64 (Animal 1), n=62 (Animal 2), n=22 (Animal 3), n=59 (Animal 4), and n=100 (Animal 5) (B) Top: Schematic of FR1 paradigm. Animals press one of two levers that are randomly presented, which then retracts followed by pellet delivery 1 s later. After 2 s, one of the two levers extends again at random. Bottom: Peri-event heat maps and average traces of calcium activity aligned to either all lever presses, reward, or lever extension. Only neurons that are significantly modulated around each event are shown. (C) Percentages of modulated neurons by each event. More neurons are modulated by lever pressing than reward delivery (One-way ANOVA, F(5,24) = 4.1077, p=0.0078; Dunnett’s multiple comparisons: Lever press vs reward p<0.05). (D) Venn diagram displaying the number of neurons in a session modulated by spatial location (place), lever pressing, or reward delivery.
-
Figure 7—source data 1
Percent of modulated neurons during lever press, both levers, left lever only, right lever only, reward, and extension.
- https://cdn.elifesciences.org/articles/83600/elife-83600-fig7-data1-v2.zip
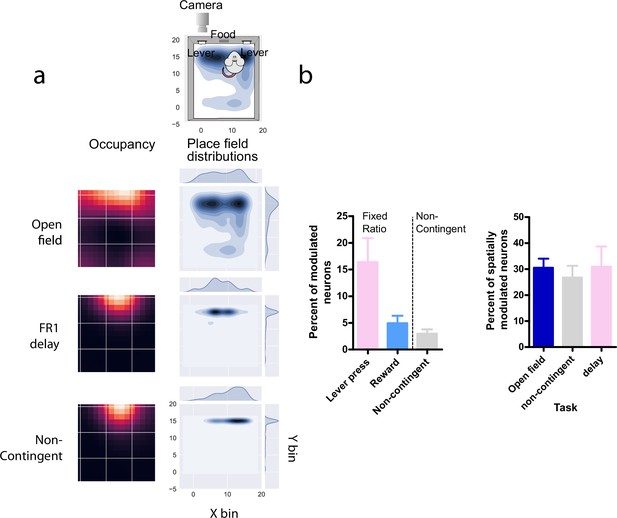
Comparison of neural activity in D1 + DG neurons during different behavioral tasks (a) Top-down views of the arena.
Left column, occupancy of the animal during the task. Right column, kernel density estimation plots, and corresponding distributions show the center of mass of spatially modulated cells during different tasks. The occupancy and place field distribution largely overlap. (b) Left, percent of modulated neurons for specific events during either an FR1 delay task, or a non-contingent reward task. We found a higher number of neurons active during lever pressing compared to reward delivery in the same task, or reward delivery in a non-contingent task. Right, The proportion of spatially modulated neurons during an open-field baseline session, non-contingent reward task, or an FR1-delay task.
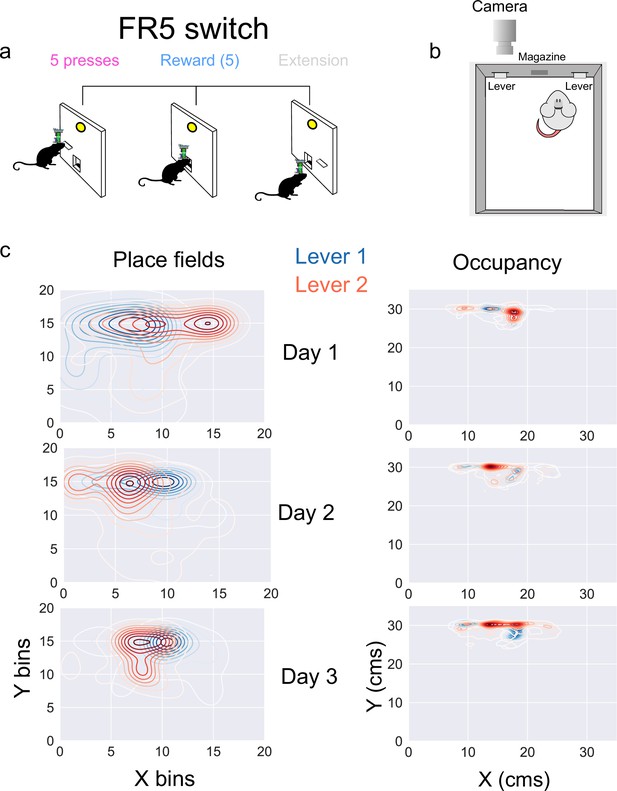
Comparison of spatially modulated D1 + DG neurons while pressing two different levers.
(a) A schematic of the task, where five presses result in a food reward delivery. Once animals receive five rewards (25 presses) the lever is retracted and a second lever is extended. Animals were tested for 3 days, and data were pooled across days here. (b) Top-down schematic of the experimental setup with two levers. (c) Left, place field centers for lever 1 (blue) and lever 2 (red), where the data is binned into 2 cm bins, and kernel density estimations are plotted (paired samples t-test: [Day1, L1 vs L2] N-51, p- 3.44e-0.7, [Day2, L1 vs L2] N-72, p- 0.00186, [Day3, L1 vs L2] N-73, p- 0.000304. Holm-Bonferroni correction for multiple comparisons = 0.0166). Right, occupancy when lever 1 or lever 2 is extended.
Tables
Colocalization of tyrosine hydroxylase with retrograde dentate gyrus (DG) labeling in the locus coeruleus (LC).
Colocalization of tyrosine hydroxylase (TH) and Retro-cre labeling (n=8; four mice, two hemispheres), showing that at least some of the LC-DG neurons are TH positive. In contrast, no colocalization was found with retro-Cre and TH labeling in the VTA (n=6; three mice, two hemispheres), and ventral tegmental area (VTA) slices were not taken from one animal.
| Animal | Retro-cre label VTA (from DG) | Retro-Cre label LC (from DG) | LC colocalized with TH |
|---|---|---|---|
| Mouse 1 (LH) | 0 | 18 | 4 |
| Mouse 1 (RH) | 0 | 32 | 7 |
| Mouse 2 (LH) | 0 | 5 | 2 |
| Mouse 2 (RH) | 0 | 13 | 6 |
| Mouse 3 (LH) | 0 | 20 | 12 |
| Mouse 3 (RH) | 0 | 31 | 15 |
| Mouse 4 (LH) | n/a | 22 | 12 |
| Mouse 4 (RH) | n/a | 14 | 5 |
Calcium imaging neuron counts for each task.
| Task | Animal 1 | Animal 2 | Animal 3 | Animal 4 | Animal 5 |
|---|---|---|---|---|---|
| Delay Task | 64 | 62 | 22 | 59 | 100 |
| FR1 Day 1 | 40 | 53 | 31 | 82 | 85 |
| FR1 Day 2 | 42 | 48 | 26 | 99 | 45 |
| FR1 Day 3 | 26 | 48 | 20 | 94 | 46 |
| FR3 Day 1 | 26 | 54 | 23 | 90 | 32 |
| FR3 Day 2 | 27 | 71 | 23 | 75 | 33 |
| FR3 Day 3 | 27 | 75 | 27 | 80 | 33 |
| FR5 Day 1 | 32 | 91 | 25 | 98 | 43 |
| FR5 Day 2 | 28 | 106 | 27 | 100 | 57 |
| FR5 Day 3 | 33 | 106 | 28 | 75 | 57 |
| FR5 switch Day 1 | 99 | 65 | 18 | 56 | 63 |
| FR5 switch Day 2 | 100 | 64 | 20 | 49 | 63 |
| FR5 switch Day 3 | 96 | 66 | 21 | 54 | 62 |
| Non-contingent | 123 | 94 | 23 | 78 | 96 |