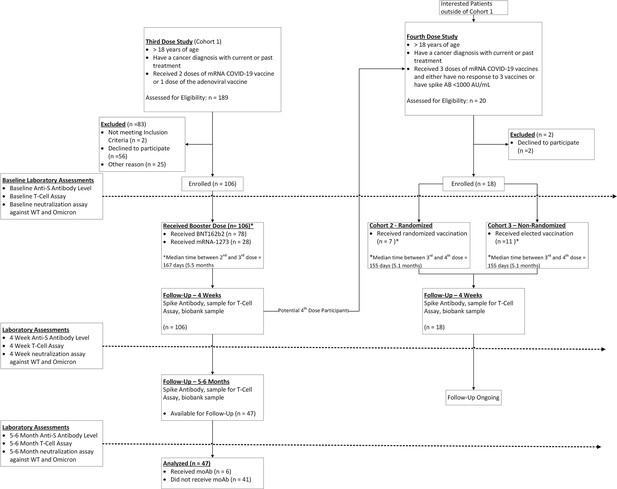
Study of efficacy and longevity of immune response to third and fourth doses of COVID-19 vaccines in patients with cancer: A single arm clinical trial
Figures
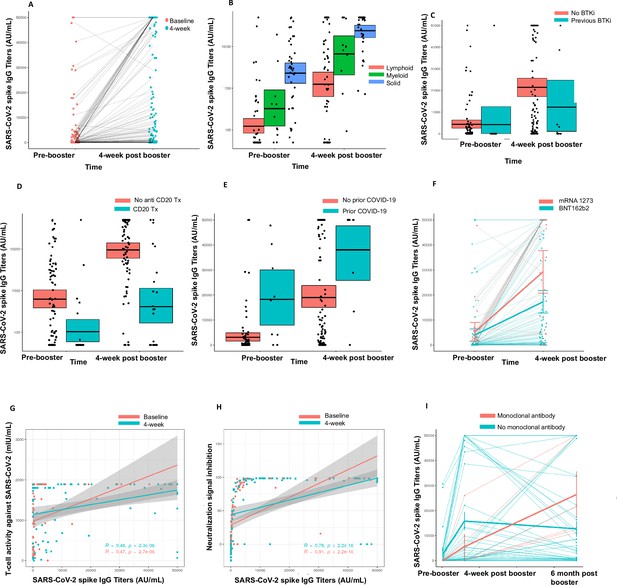
Immunogenicity of third dose of coronavirus disease 2019 (COVID-19) vaccine in seronegative cancer patients.
(A) Figure showing change in anti-SARS-CoV-2 (anti-S) antibody titer at 4 weeks for entire cohort n=106. (B) Figure showing change in anti-S antibody titer at 4 weeks split by cancer type (solid cancer, lymphoid cancer, and myeloid cancer) n=106. (C) Figure showing effect of Bruton’s tyrosine kinase inhibitor (BTKi) therapy on anti-S antibody titer at baseline and 4 weeks of third dose n=12 patients that received BTKi Kruskal-Wallis test. (D) Figure showing effect of anti-CD20 antibody therapy on anti-S antibody titer at baseline and 4 weeks of third dose n=25 patients that received anti-CD20 antibody, Kruskal-Wallis test. (E) Figure showing effect of prior COVID-19 infection on anti-S antibody titer at baseline and 4 weeks of third dose n=9 patients with COVID infection, Kruskal-Wallis test. (F) Figure showing effect of booster type (BNT162b2 vs mRNA 1273) on anti-S antibody titer at baseline and 4 weeks of third dose. (G) Line diagram showing correlation between anti-spike IgG titer and baseline T-cell activity at baseline and 4 weeks n=88 for baseline, n=89 for 4 weeks; Spearman’s test. (H) Line diagram showing correlation between anti-S titer and signal inhibition for neutralization against wild-type (WT) virus at baseline and 4 weeks. n=103 for baseline, n=100 for 4 weeks; Spearman’s test. (I) Anti-spike IgG titers at baseline, 4 weeks, and 6 months after third dose of COVID-19 vaccine in cancer patients. Line shows means with error bars (SD).n=47. All statistical tests performed at a pre-determined threshold of p<0.05 for statistical significance.
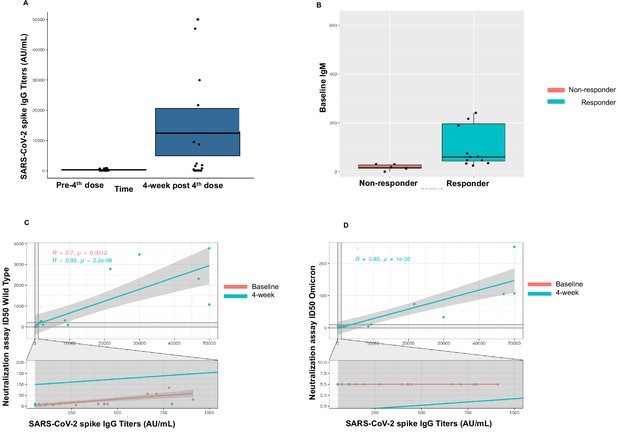
Immunogenicity of the fourth dose of coronavirus disease 2019 (COVID-19) vaccine in cancer patients with seronegativity after three doses.
(A) Anti-spike IgG levels after the fourth dose of COVID-19 vaccine for the entire cohort n=18. (B) Correlation of baseline IgM levels with response to fourth dose of vaccine, n=18 Kruskal-Wallis test. (C) Line diagram showing correlation between anti-SARS-CoV-2 (anti-S) titer and neutralization activity for wild-type (WT) virus at baseline and 4 weeks, n=18, Spearman’s test. (D) Line diagram showing correlation between titer and neutralization activity for Omicron strain at baseline and 4 weeks n=18, Spearman’s test. All statistical tests performed at a pre-determined threshold of p<0.05 for statistical significance.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Software, algorithm | RStudio, v3.6.2 | posit | RRID:SCR_000432 | |
| Commercial assay, kit | AdviseDx Abbott SARS-CoV-2 anti-S antibody assay | Abbott | I1000SR instrument | |
| Other | cPass SARS-CoV-2 Neutralization Antibody Detection Kit | GenScript | L00847 | EUA by FDA; https://www.genscript.com/covid-19-detection-cpass.html |
| Other | Quan-T-Cell SARS-CoV-2 and Quan-T-Cell ELISA | EUROIMMUN | ET 2606 and EQ 6841 | CE-marked and for Research Use Only in the United States https://www.coronavirus-diagnostics.com/immune-response-test-systems-for-covid-19.html IFN-γ ELISA: plasma diluted 1:5 |
| Other | mAb 1 C7C7 anti-SARS nucleoprotein antibody | Center for Therapeutic Antibody Development at the ISMMS (Same clone as Sigma Millipore) | ZMS1075 | Working dilution 1 μg/ml |
| Other | (H&L) Antibody Peroxidase ConjugatedGoat Polyclonal | Rockland | 610–1302 | 1:3000 dilution |
| Other | SIGMAFAST OPD (o-Phenylenediamine dihydrochloride) | Sigma-Aldrich | Cat# P9187 | |
| Other | 3-molar hydrochloric acid | Thermo Fisher Scientific | Cat# S25856 |
Baseline characteristics for third dose cohort.
| Baseline characteristics | n=106 |
|---|---|
| Age (median, IQR) | 68 (63.25–76.5) |
| Sex | |
| Male | 48 (45%) |
| Female | 58 (55%) |
| Race | |
| Caucasian | 36 (34%) |
| African-American | 33 (31%) |
| Hispanic | 27 (25%) |
| Asian | 9 (8%) |
| Other | 1 (1%) |
| Previous vaccine given | |
| BNT162b2 | 72 (68%) |
| mRNA-1273 | 28 (26%) |
| Ad26.CoV2.S | 6 (6%) |
| Type of booster vaccine | |
| BNT162b2 | 78 (74%) |
| mRNA-1273 | 28 (26%) |
| Malignancy category | |
| Hematologic malignancy | 66 (62%) |
| Solid Malignancy | 40 (38%) |
| Lymphoid/myeloid/solid | |
| Lymphoid | 55 (52%) |
| Myeloid | 11 (10%) |
| Solid | 40 (38%) |
| Cancer status | |
| Active | 69 (65%) |
| Progressive | 3 (3%) |
| Recurrent | 3 (3%) |
| Relapse | 7 (7%) |
| Remission | 24 (23%) |
| On treatment at the time of booster | |
| Yes | 80 (75%) |
| No | 26 (25%) |
Results for third dose of vaccine.
| Spike antibody results | n=106 | |||
|---|---|---|---|---|
| Four-week negative | Four-week positive | Seroconversion rate | p value | |
| Baseline negative | 15 | 20 | 57% | <0.001* |
| Baseline positive | 0 | 71 | ||
| Total | 15 | 91 | ||
| Rise in spike antibody titers overall (AU/mL) | Median | IQR | ||
| Titer at baseline | 212.1 | 50–2873 | ||
| Titer at 4 weeks | 9997 | 880.7–47,063 | ||
| Rise in spike antibody titers (AU/mL) | Median | IQR | ||
| Hematologic malignancy | 2167 | 0–10,131 | <0.001* | |
| Solid malignancy | 31,010 | 9531–44,464 | ||
| Rise in spike antibody titers by solid/lymphoid/myeloid (AU/mL) | ||||
| Lymphoid cancers | 1169 | 0–8661 | <0.001* | |
| Myeloid cancers | 9424 | 4381–20,444 | ||
| Solid cancers | 31,010 | 9531–44,464 | ||
| Association with certain cancer-directed therapies | ||||
| Bruton’s tyrosine kinase inhibitors | ||||
| Change in spike antibody titers (AU/mL) | Median | IQR | ||
| Patients on BTKi (n=12) | 0 | 0–3393 | <0.001* | |
| Patients not on BTKi | 9355 | 877.3–34,410 | ||
| Anti-CD20 antibody treatment | ||||
| Change in spike antibody titers (AU/mL) | Median | IQR | ||
| Patients on CD20 (n=25) | 0 | 0–910.5 | 0.0133* | |
| Patients not on CD20 | 12735 | 2842–38,863 | ||
| Anti-CD20 antibody treatment within 6 months | Median | IQR | ||
| Yes | 0 | 0–0 | 0.05482 | |
| No | 587 | 0–4314 | ||
| Change in spike antibody titer by prior COVID infection | Median | IQR | ||
| Yes (n=9) | 19,350 | 9286–32,151 | 0.3051 | |
| No (n=96) | 6706 | 444.1–33,831 | ||
| Change in spike antibody titer by type of booster given | Median | IQR | ||
| BNT162b2 | 5534 | 433.8–18,074 | 0.09014 | |
| mRNA-1273 | 31451 | 515.5–45,057 | ||
| Change in spike antibody titer by age | Median | IQR | ||
| Age <65 years | 27451 | 2641–50,000 | 0.03438* | |
| Age ≥65 years | 6152 | 558.9–41,765 | ||
| T-cell activity | ||||
| Baseline | n=88 | % | ||
| Positive | 65 | 74% | ||
| Negative | 23 | 26% | ||
| Four-week | n=89 | |||
| Positive | 76 | 85% | ||
| Negative | 13 | 15% | ||
| Baseline neutralization activity assay (all evaluable patients, WT virus) | ||||
| Anti-S antibody negative | Anti-S antibody positive | Total | p value | |
| Neutralizing antibodies detected | 0 | 47 | 47 | <0.001 |
| Neutralizing antibodies not detected | 35 | 21 | 56 | |
| Total | 35 | 68 | 103 | |
| Four-week neutralization activity assay (all evaluable patients, WT virus) | ||||
| Anti-S antibody negative | Anti-S antibody positive | Total | p value | |
| Neutralizing antibodies detected | 0 | 77 | 77 | <0.001 |
| Neutralizing antibodies not detected | 15 | 8 | 23 | |
| Total | 15 | 85 | 100 | |
| Four-week neutralization assay (seronegative cohort 4 weeks) | n=35 | |||
| Wild type | ||||
| Negative | 19 | 54% | ||
| Positive | 16 | 46% | ||
| Omicron | ||||
| Negative | 29 | 83% | ||
| Positive | 6 | 17% |
-
*
Statistically significant.
Baseline characteristics of the fourth dose cohort.
| N (%) | |
|---|---|
| Baseline seronegative | 7 (39%) |
| Baseline low positive (spike ab <1000 AU/mL) | 11 (61%) |
| Cancer diagnosis | |
| CLL | 7 (39%) |
| Waldenstrom’s macroglobulinemia | 3 (17%) |
| DLBCL | 2 (11%) |
| Multiple myeloma | 2 (11%) |
| Mantle cell Lymphoma | 1 (6%) |
| Marginal zone lymphoma | 1 (6%) |
| Hodgkins lymphoma | 1 (6%) |
| MDS | 1 (6%) |
| Fourth dose vaccine type | |
| BNT162b2 | 15 (83%) |
| Ad26.CoV2.S | 3 (17%) |
Correlation of fourth dose vaccine response with baseline characteristics.
| Non-responder (n=6) | Responder (n=12) | p value | |
|---|---|---|---|
| Age | 79.5 | 67.5 | 0.01293* |
| Baseline WBC | 4.95 | 5.15 | 0.45 |
| Baseline ANC | 2.6 | 3.5 | 0.26 |
| Baseline ALC | 1.2 | 1.3 | 0.57 |
| Baseline AMC | 0.5 | 0.65 | 0.73 |
| Baseline absolute CD3 | 773 | 835.5 | 0.57 |
| Baseline absolute CD4 | 406.5 | 407.5 | 0.71 |
| Baseline absolute CD8 | 310 | 247 | 0.40 |
| Baseline absolute CD19 | 1 | 113.5 | 0.04874* |
| Baseline absolute CD16/56 | 243.5 | 200 | 0.57 |
| Baseline IgG | 777 | 757 | 0.51 |
| Baseline IgA | 90.5 | 118 | 0.57 |
| Baseline IgM | 17 | 60.5 | 0.001442† |
| 4-Week WBC | 5.1 | 5.8 | 0.40 |
| 4-Week ANC | 2.7 | 3.45 | 0.57 |
| 4-Week ALC | 1.1 | 1.4 | 0.60 |
| 4-Week AMC | 0.55 | 0.65 | 0.60 |
| 4-Week absolute CD3 | 754 | 983 | 0.40 |
| 4-Week absolute CD4 | 461.5 | 369.5 | 0.93 |
| 4-Week absolute CD8 | 297.5 | 269 | 0.40 |
| 4-Week absolute CD19 | 2.5 | 105 | 0.07 |
| 4-Week absolute CD16/56 | 232.5 | 219 | 0.93 |
| 4-Week IgG† | 741.5 | 832 | 0.62 |
| 4-Week IgA† | 86 | 112 | 0.69 |
| 4-Week IgM† | 15 | 62 | 0.003561† |
-
*
Statistically significant.
-
†
n=11.
Results for fourth dose study.
| Overall response | 18 | ||
|---|---|---|---|
| Responder | 12 | 67% | |
| Non-responder | 6 | 33% | |
| Median age | IQR | ||
| Responder | 67.5 | 63.75–70.75 | 0.01293* |
| Non-responder | 79.5 | 72.75–81.75 | |
| Median baseline IgM | |||
| Responder | 60.5 | 0.001442 * | |
| Non-responder | 17 | ||
| Median spike antibody at baseline (AU/mL) | 131.1 | <50–432.9 | |
| Median spike antibody at 4 weeks (AU/mL) | 1700 | 64.3–18627 | |
| T-cell activity at baseline | n=14 | ||
| Positive | 11 | 79% | |
| Negative | 3 | 21% | |
| T-cell activity at 4 weeks | n=18 | ||
| Positive | 17 | 94% | |
| Negative | 1 | 6% | |
| Baseline | |||
| Neutralization assay baseline | Negative | Positive | |
| WT | 6 (33%) | 12 (67%) | |
| Omicron | 18 (100%) | 0 (0%) | |
| Neutralization assay 4 week | Negative | Positive | |
| WT | 5 (28%) | 13 (72%) | |
| Omicron | 12 (67%) | 6 (33%) |
-
*
Statistically significant.