Using multi-modal neuroimaging to characterise social brain specialisation in infants
Figures
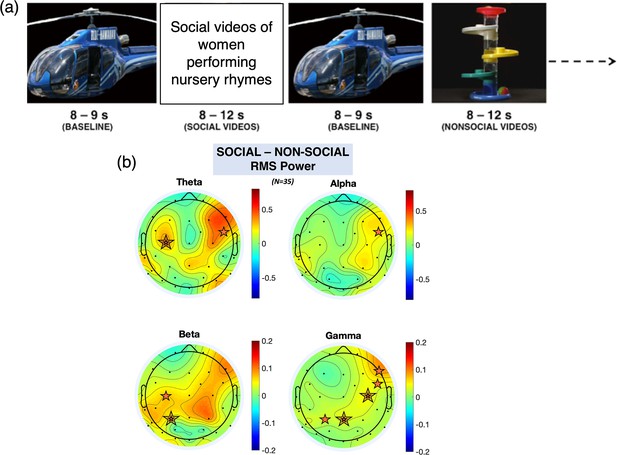
Order of stimulus presentation and EEG scalp topography.
(a) Illustration of the paradigm. (b) Scalp topographies of the grand average root mean square (RMS) power for theta, alpha, beta, and gamma frequency bands (averaged across participants, averaged across the stimulus period) for the social minus non-social condition.
The orange stars indicate statistically significant electroencephalography (EEG) electrodes where an increase in activity was observed (e.g. increase in response to the social condition compared to the non-social condition); a double line indicates significance after false discovery rate (FDR) correction.
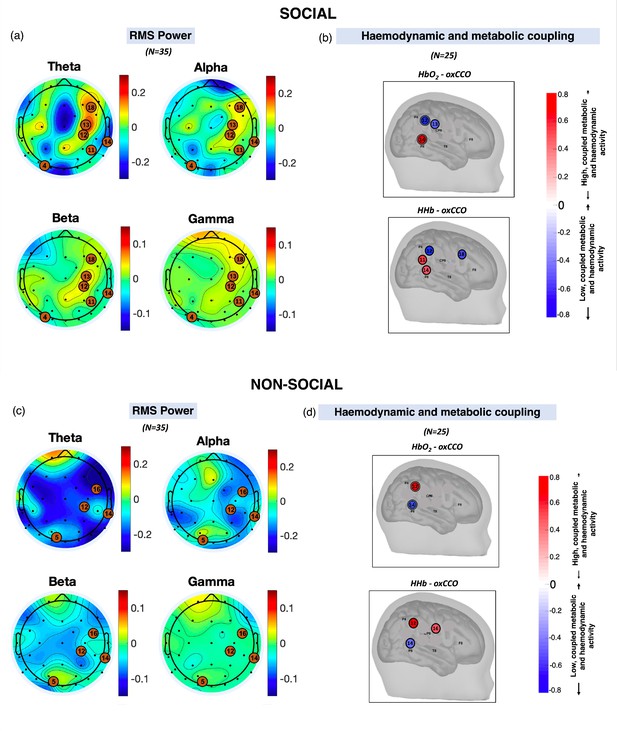
Scalp topographies of the grand average root mean square (RMS) power for theta, alpha, beta, and gamma frequency bands averaged across participants, averaged across the stimulus period for (a) social and (c) non-social conditions.
The black dots show the locations of the electroencephalography (EEG) electrodes while the orange circles represent the broadband near-infrared spectroscopy (bNIRS) channels. Locations of high haemodynamic and metabolic coupling for (b) social and (d) non-social condition. (b and d) are reproduced from Figure 7 in Siddiqui et al., 2021.
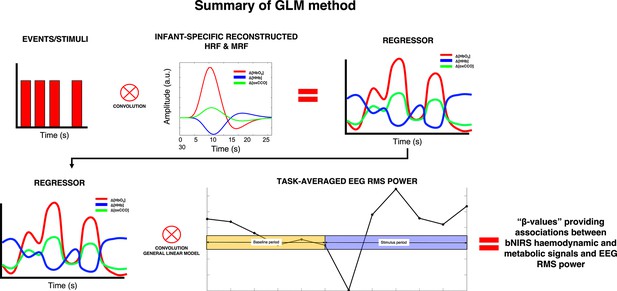
Summary of the procedure for obtaining the associations between broadband near-infrared spectroscopy (bNIRS) signals and electroencephalography (EEG) root mean square (RMS) power at each bNIRS combination, for each frequency band.
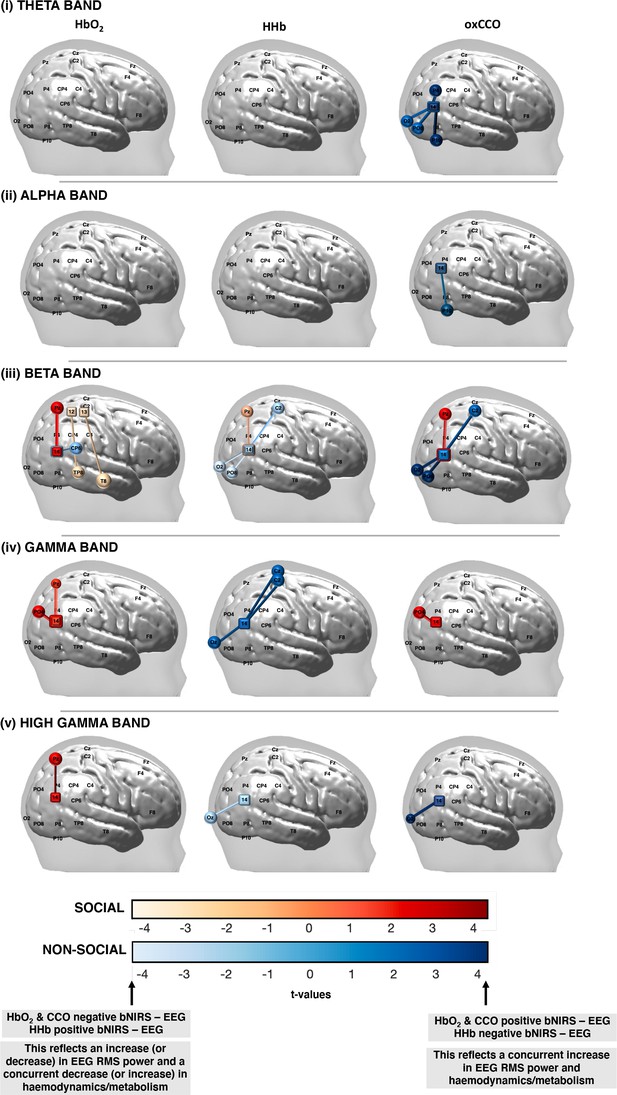
False discovery rate (FDR)-corrected significant connections between broadband near-infrared spectroscopy (bNIRS) channels (squares) and electroencephalography (EEG) electrodes (circles) for the (i) theta, (ii) alpha, (iii) beta, (iv) gamma, and (v) high gamma bands for the social condition (red colour bar) and the non-social condition (blue colour bar) for HbO2, HHb, and oxCCO.
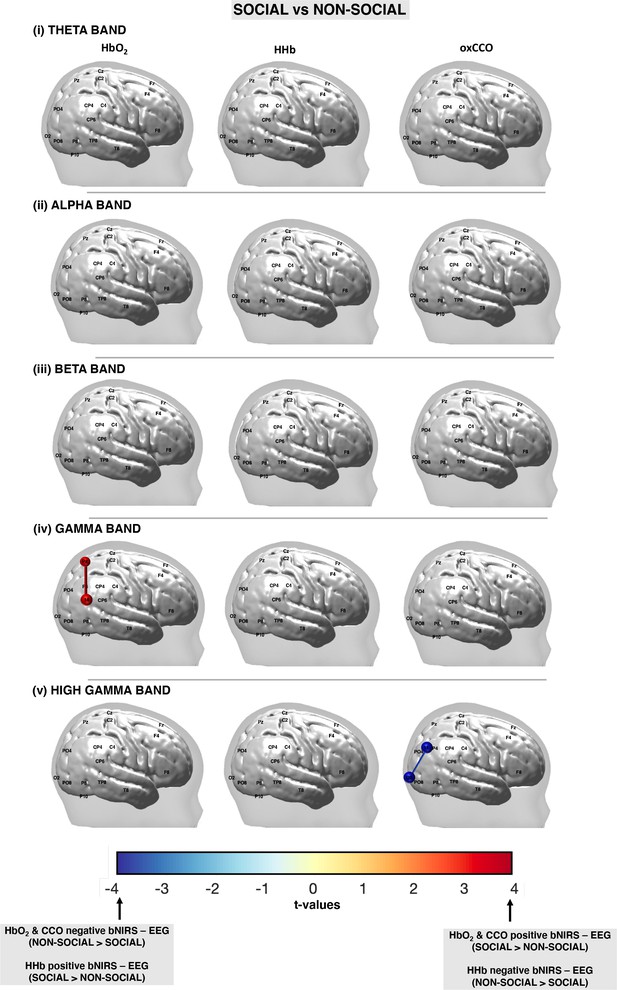
False discovery rate (FDR)-corrected significant connections between broadband near-infrared spectroscopy (bNIRS) channels and electroencephalography (EEG) electrodes for the (i) theta, (ii) alpha, (iii) beta, (iv) gamma, and (v) high gamma bands for the social condition versus the non-social condition for HbO2, HHb, and oxCCO.
The colour bar represents the t-values from the general linear model (GLM) analysis with a positive t-value representing a significant, positive connection between the broadband near-infrared spectroscopy (bNIRS) channel and EEG electrode while a negative t-value represents a negative connection.
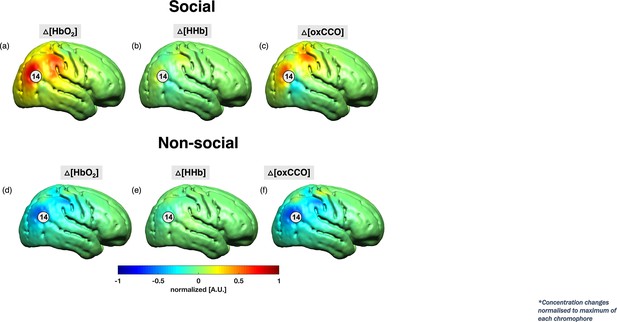
Grand average image reconstruction at 18 s post-stimulus onset for the social condition (a–c) and the non-social condition (d–f) at a single time point of 18 s post-stimulus onset.
The concentration changes for HbO2 and HHb were normalised to the maximum concentration change of HbO2 while ΔoxCCO was normalised to its own maximum change in concentration. Channel 14 has been indicated.
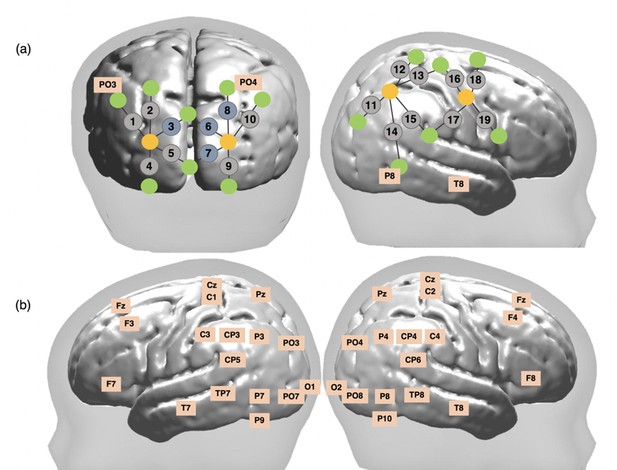
Schematic representation of broadband near-infrared spectroscopy (bNIRS) and electroencephalography (EEG) channel locations.
(a) Locations of bNIRS channels (grey circles) over the occipital cortex and the right hemisphere and locations of the bNIRS sources (orange circles) and detectors (green circles) relative to EEG 10/20 locations. Channels shown in blue (3, 6, 8, and 10) were excluded from the analysis (b) Locations of the 32 EEG electrodes.
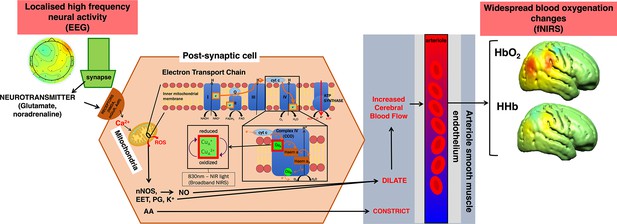
Simplified summary of the signalling pathways that mediate neurovascular coupling.
High-frequency neural activity causes the release of neurotransmitters such as glutamate and noradrenaline which bind to either N-methyl-D-aspartate (NMDA) receptors in interneurons or metabotropic glutamate receptors (mGluR) or adrenaline receptors in astrocytes. In both cases, this causes an influx of calcium (Ca2+) which in turn leads to an increase in adenosine triphosphate (ATP) production through the mitochondrial electron transport chain (ETC). As a by-product, in interneurons, nitric oxide (NO) is produced in the interneurons which dilates arterioles to increase blood flow leading to increased oxygen delivery in surrounding brain regions. Alternatively, in astrocytes derivates of arachidonic acid (AA) include prostaglandins (PG) and epoxyeicosatrienoic acids (EET) which cause vasodilation. This figure has been adapted from Harris et al., 2011.
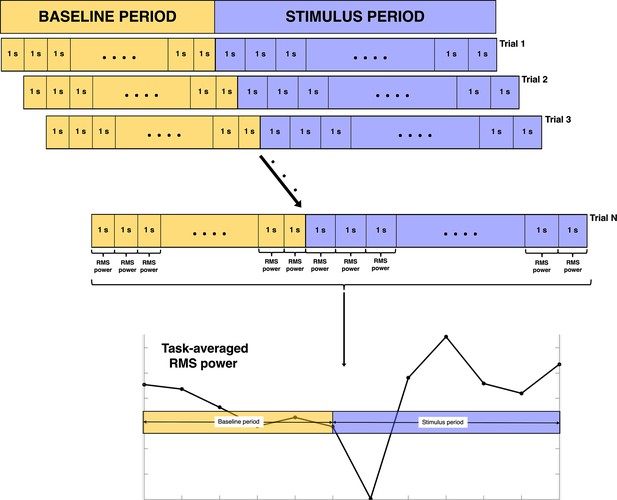
Procedure for deriving the electroencephalography (EEG) root mean square (RMS) power from the pre-processed EEG data.
Each 1 s segment is made up of 200 ms of the previous segment and 800 ms of the current segment. The task-averaged RMS power shown here is average theta power across all infants from a single channel for the purposes of outlining the procedure.
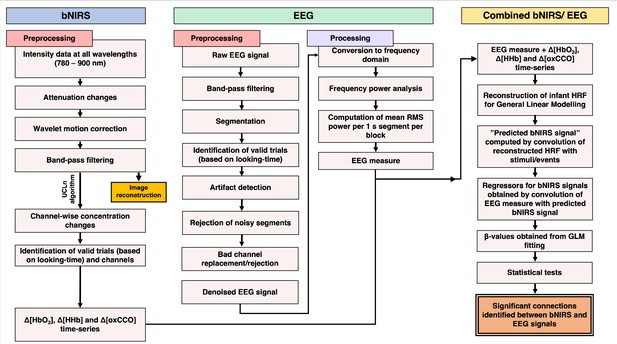
Flowchart for the data analysis pipelines for broadband near-infrared spectroscopy (bNIRS) (left), electroencephalography (EEG) (middle), and combined bNIRS-EEG (right).
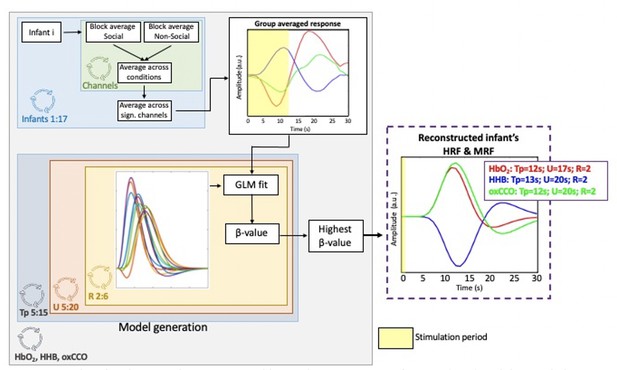
Procedure for obtaining the reconstructed haemodynamic response function (HRF) and the metabolic response function (MRF).
The panel on the right shows the estimated HRF and MRF with the corresponding basis function parameters giving the best fit with the group-averaged HbO2, HHb, oxCCO responses. The yellow shaded areas represent the stimulation periods.





