An herbal drug combination identified by knowledge graph alleviates the clinical symptoms of plasma cell mastitis patients: A nonrandomized controlled trial
Figures
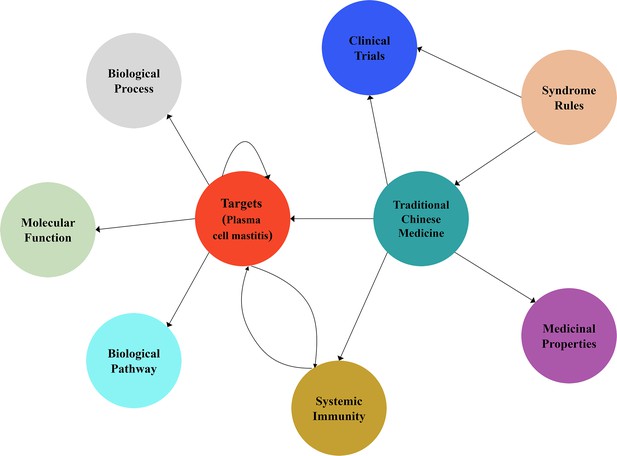
Schematic diagram of the knowledge graph for the drug discovery of plasma cell mastitis in the present work.
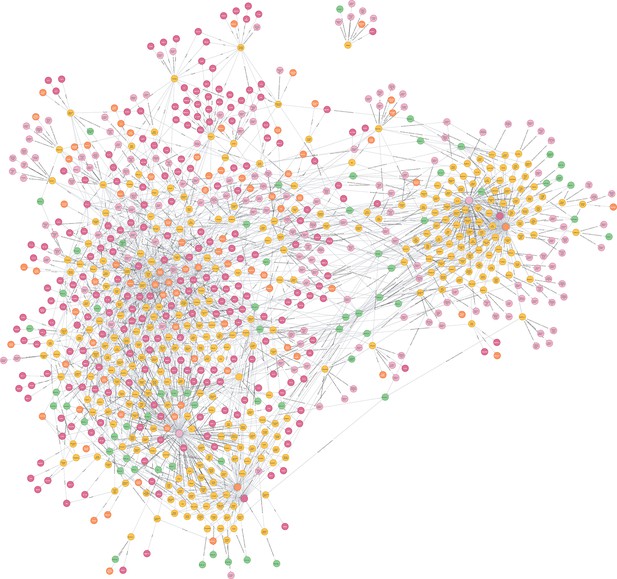
Snapshot of the medical knowledge graph toward immunotherapy.
The knowledge graph was constructed using Neo4j and Python tools (http://www.ikgg.org/kghti). The yellow color represents the herbal drug entity, the pink color represents the cellular targets, and the green color represents the attributes of herb drugs.
-
Figure 1—figure supplement 1—source data 1
Full resolution file for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig1-figsupp1-data1-v3.zip
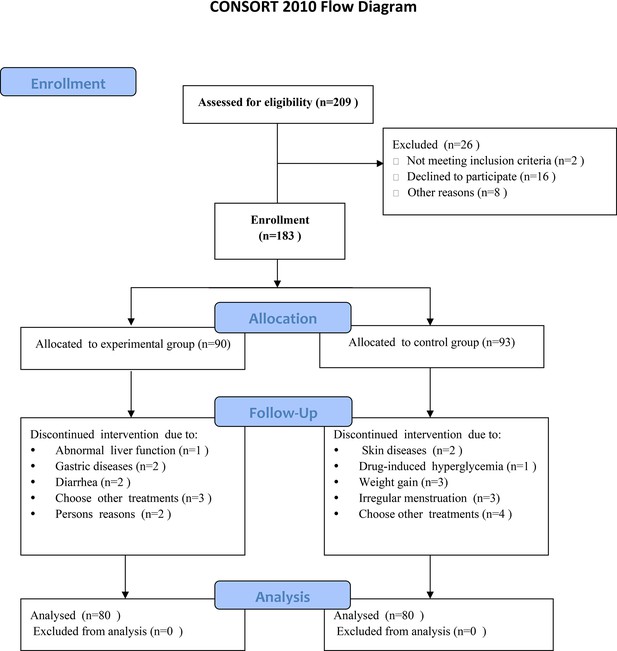
CONSORT flowchart diagram of the clinical trial for the herbal drug combination.
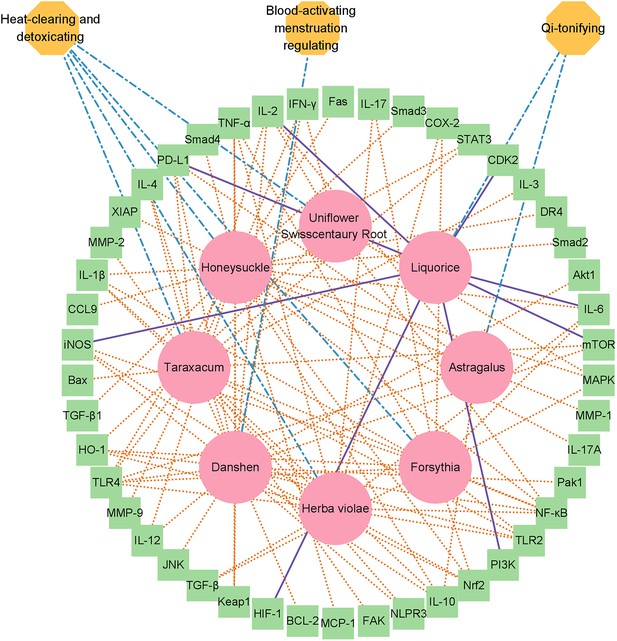
Network diagram of the herbal drug combination consisting of eight entities, including ‘Honeysuckle,’ ‘Taraxacum,’ ‘Astragalus,’ ‘Danshen,’ ‘Forsythia,’ ‘Herba violae,’ ‘Liquorice,’ and ‘Uniflower swisscentaury root,’ displayed in red circles.
In total, 46 cellular targets related to systemic immunity were hit by the drug combination such as NLPR3, IL-17, TLR4, STAT3, IL-6, iNOS, and TLR2, which are displayed in the green box model. Three major medicinal attributes (properties) were identified for these herbal drug entities, including ‘heat-clearing and detoxicating,’ ‘Qi-tonifying,’ and ‘blood-activating menstruation regulation’.
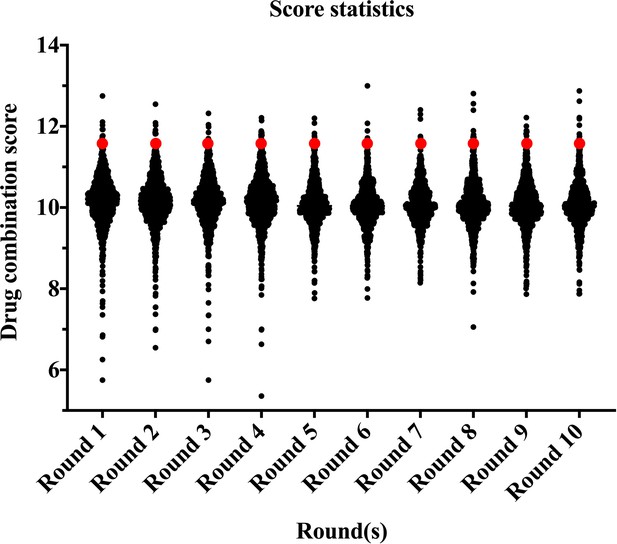
The score statistics of 10 rounds of random herbal drug combinations (1000 random combination from each round) for the treatment of plasma cell mastitis.
It is noteworthy that the scoring results of the 10 rounds are presented as normal distributions. The herbal drug combination identified for the clinical trial was among the top 20 choices in all the 10 rounds and is marked with red dots.
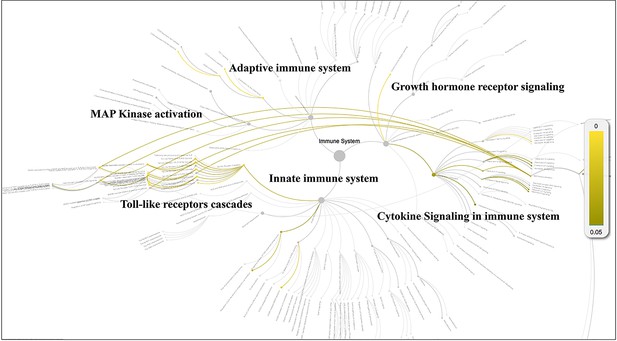
Pathway analysis for the potential cellular targets of the herbal drug combination via Reactome Knowledgebase.
The statistically significant pathways are highlighted and displayed in yellow color (p<0.05).
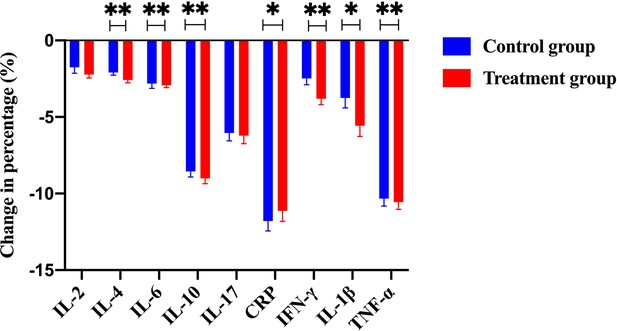
The change of percentage for numerous immunological cytokines, including IL-2, IL-4, IL-6, IL-10, IL-17, CRP, IFN-γ, IL-1β, and TNF-α, from the control group and the treatment group (with Traditional Chinese Medicine [TCM] treatment).
Notably, a few key cytokines such as IL-2, IL-4, IFN-γ, IL-1β, and TNF-α were significantly downregulated in the treatment group compared to the control group.
-
Figure 4—source data 1
Patients’ raw data of serum cytokines from the control group (without Traditional Chinese Medicine [TCM] treatment) and the treatment group (with TCM treatment) in the clinical trial (ClinicalTrials.gov: NCT05530226).
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig4-data1-v3.zip
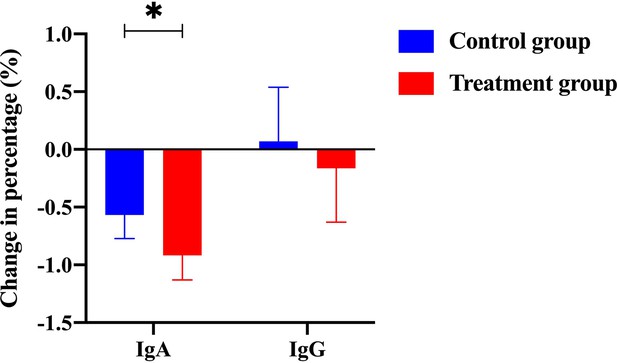
The change of percentage for IgA level and IgG level from the control group and the treatment group (with Traditional Chinese Medicine [TCM] treatment).
-
Figure 5—source data 1
Patients’ raw data of serum IgA and IgG level from the control group (without Traditional Chinese Medicine [TCM] treatment) and the treatment group (with TCM treatment) in the clinical trial (ClinicalTrials.gov: NCT05530226).
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig5-data1-v3.zip
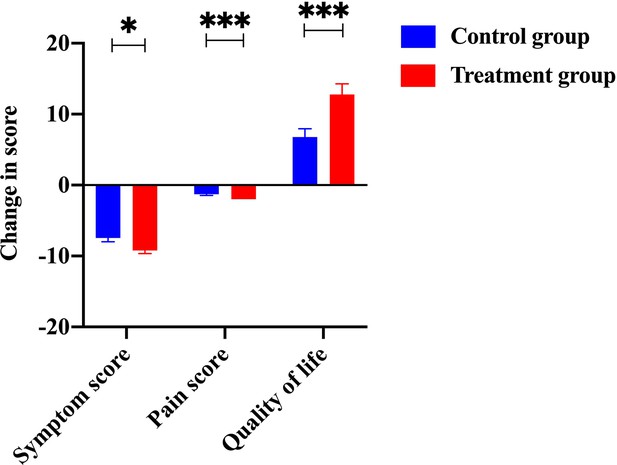
The change of scores from items, including symptom, pain, and living quality.
The scores of symptoms, pain, and living quality from the treatment group were significantly improved compared to the control group.
-
Figure 6—source data 1
Patients’ raw data of symptom scores from the control group (without Traditional Chinese Medicine [TCM] treatment) and the treatment group (with TCM treatment) in the clinical trial (ClinicalTrials.gov: NCT05530226).
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig6-data1-v3.zip
-
Figure 6—source data 2
Patients’ raw data of pain scores from the control group (without Traditional Chinese Medicine [TCM] treatment) and the treatment group (with TCM treatment) in the clinical trial (ClinicalTrials.gov: NCT05530226).
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig6-data2-v3.zip
-
Figure 6—source data 3
Patients’ raw data of quality of life from the control group (without Traditional Chinese Medicine [TCM] treatment) and the treatment group (with TCM treatment) in the clinical trial (ClinicalTrials.gov: NCT05530226).
- https://cdn.elifesciences.org/articles/84414/elife-84414-fig6-data3-v3.zip
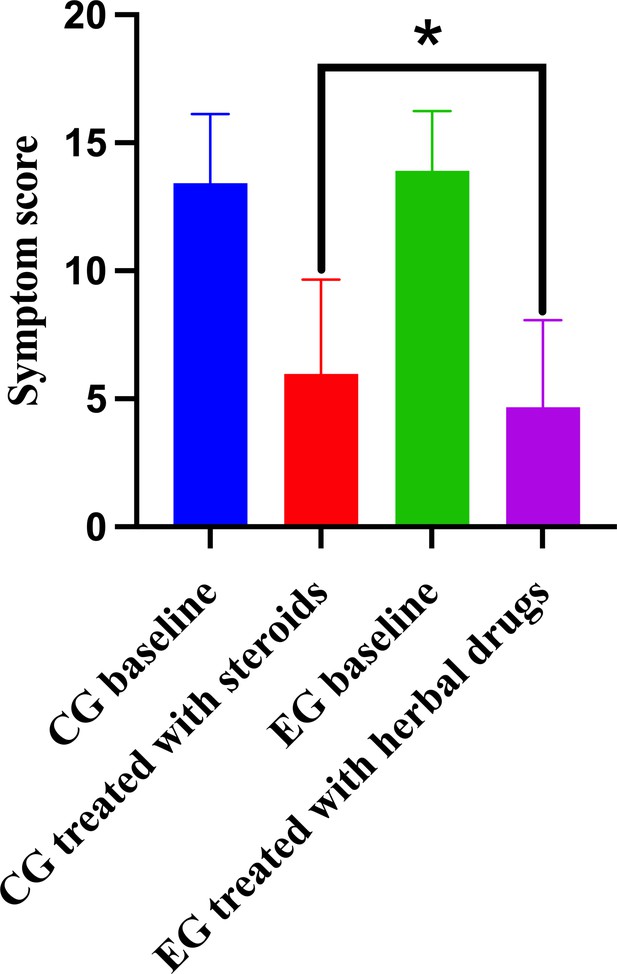
The comparison of symptom score between different groups.
Control group (CG) baseline, experimental group (EG) baseline, CG treated with methylprednisolone, and EG treated with herbal drug combinations.
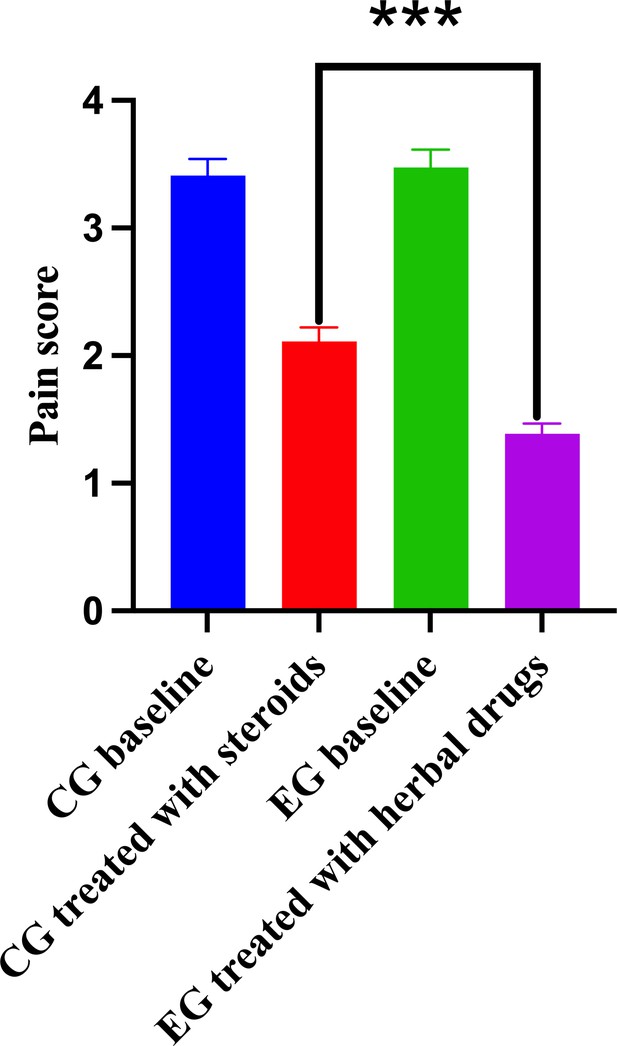
The comparison of pain score between different groups.
Control group (CG) baseline, experimental group (EG) baseline, CG treated with methylprednisolone, and EG treated with herbal drug combinations.
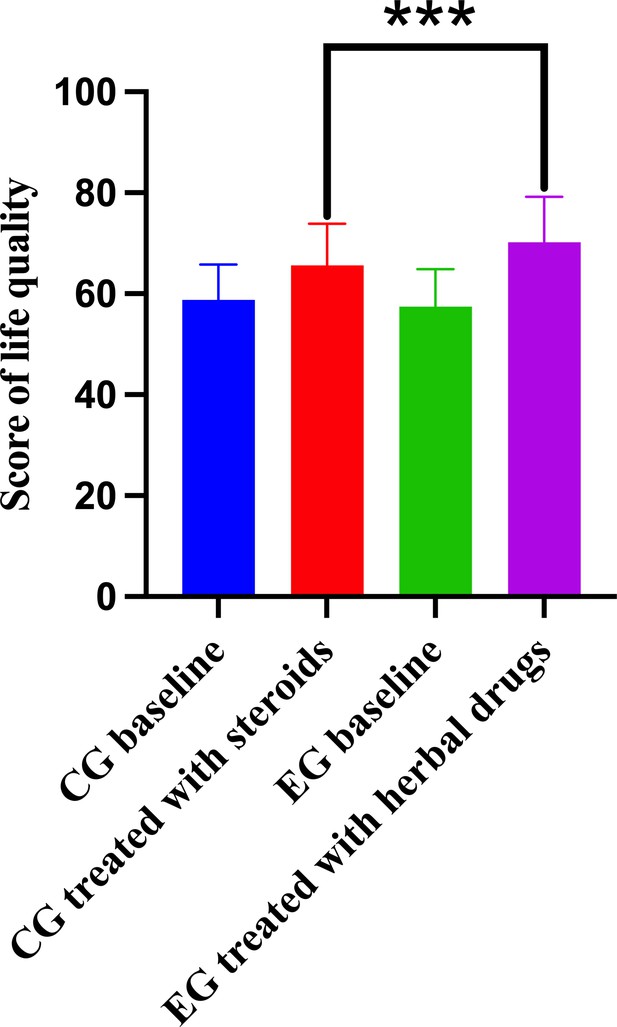
The comparison of score of life quality between different groups.
Control group (CG) baseline, experimental group (EG) baseline, CG treated with methylprednisolone, and EG treated with herbal drug combinations.
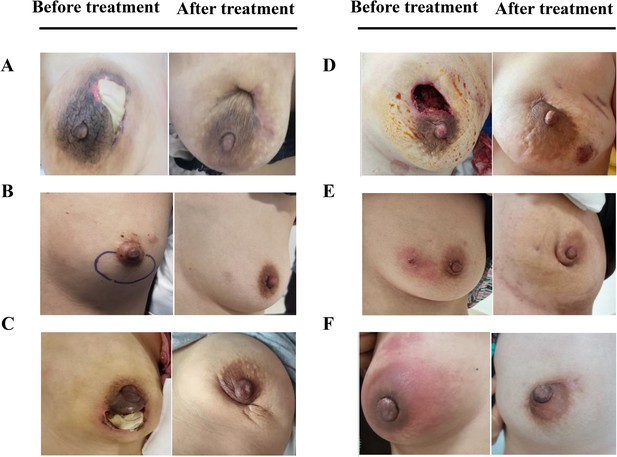
The comparison of whole breast for six representative plasma cell mastitis (PCM) patients in the experimental group (EG) before treatment and after treatment.
(A-F), Six selected PCM patients in the clinical trial to reveal the effects of herbal drug combination. Written informed consents have been provided by all the patients (including written informed consent for the publication of the images in this figure), and detailed patient information is provided in Supplementary file 3.
Tables
Comparison of operation rate, recurrence rate, and incidence of adverse reactions between the two groups (EG: experimental group; CG: control group).
| Groups | N (patients) | Operation | Recurrence | Incidence of adverse events |
|---|---|---|---|---|
| EG | 80 | 25 (31.25%) | 3 (3.75%) | 5 (6.25%) |
| CG | 80 | 47 (58.75%) | 10 (12.5%) | 9 (11.25%) |
| X2 | 12.22 | 4.103 | 1.252 | |
| p | <0.001 | 0.043 | 0.263 |
Clinical symptom scores between the two groups in the clinical trial (EG: experimental group; CG: control group; The clinical symptom rating scale for plasma cell mastitis (PCM) is displayed in Supplementary file 1B).
| Groups | N(patients) | Baseline | Treated | t | p |
|---|---|---|---|---|---|
| EG | 80 | 13.90 ± 2.37 | 4.68 ± 3.40 | 22.19 | <0.001 |
| CG | 80 | 13.423 ± 2.70 | 5.98 ± 3.68 | 14.282 | <0.001 |
| t | 1.189 | 2.323 | |||
| p | 0.236 | 0.021 |
Additional files
-
Supplementary file 1
Baseline charateristics and rating scale for the clinical trial.
(A) Baseline characteristics of patients in the clinical trial. (B) Clinical symptom rating scale for plasma cell mastitis (PCM).
- https://cdn.elifesciences.org/articles/84414/elife-84414-supp1-v3.docx
-
Supplementary file 2
Clinical protocol for the clinical trial study.
- https://cdn.elifesciences.org/articles/84414/elife-84414-supp2-v3.docx
-
Supplementary file 3
Some detailed information for the six patients displayed in Figure 7.
- https://cdn.elifesciences.org/articles/84414/elife-84414-supp3-v3.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/84414/elife-84414-mdarchecklist1-v3.docx
-
Source code 1
Python source code for the scoring system of knowledge graph to assess and select appropriate drug combinations.
- https://cdn.elifesciences.org/articles/84414/elife-84414-code1-v3.zip





