Scavenger receptor endocytosis controls apical membrane morphogenesis in the Drosophila airways
Figures
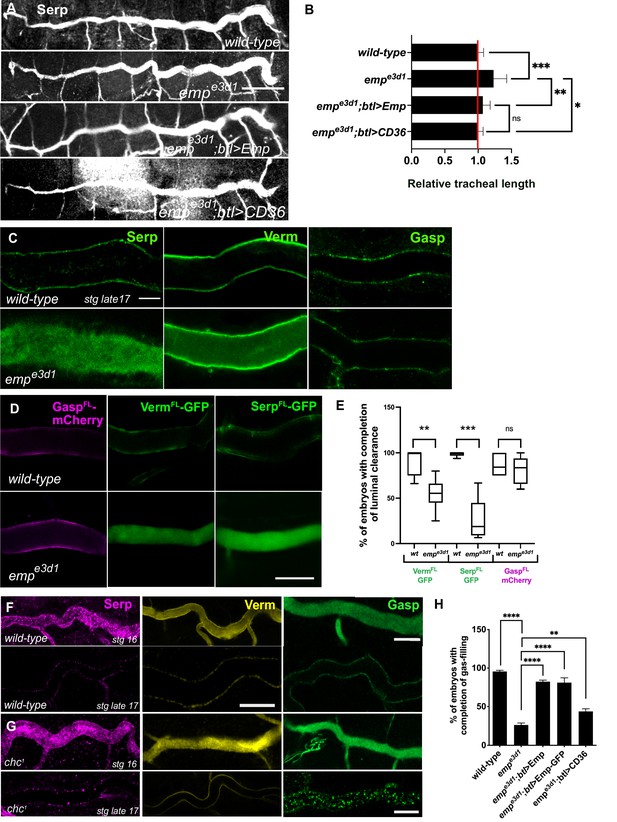
empe3d1 mutants show over-elongation of the tracheal tubes and severe luminal clearance defect of Serp.
(A) Images showing the dorsal trunk (DT) of wild-type, empe3d1, empe3d1;btl>Emp, empe3d1;btl>CD36 embryos stained for the luminal marker Serp. (B) Graph showing the tracheal DT length in wild-type embryos (n = 16), empe3d1 mutants (n = 17), empe3d1;btl>Emp (n = 19) and empe3d1;btl>CD36 (n = 15) embryos. (C) Confocal images showing the DT of wild-type and empe3d1 mutant at late stage 17 embryos, stained for the endogenous luminal proteins Serp, Verm, and Gasp. (D) Confocal images showing the DT of live btl>GaspFL-mCherry (magenta), btl>VermFL-GFP and btl>SerpFL-GFP (green) in wild-type, and empe3d1 mutant at 20.0 h AEL. (E) Plots showing the percentage of embryos with completion of luminal clearance in btl>VermFL-GFP (green, n = 59), empe3d1;btl>VermFL-GFP (green, n = 37), btl>SerpFL-GFP (green, n = 58), empe3d1;btl>SerpFL-GFP (green, n = 15), btl>GaspFL-mCherry (magenta, n = 28) and empe3d1;btl>GaspFL-mCherry (magenta, n = 18). Representative confocal images showing the tracheal DT of wild-type (F) and chc1 (G) mutant embryos, stained for the endogenous luminal markers Serp (magenta), Verm (yellow), and Gasp (green) before and after luminal clearance, stage 16 and late stage 17 (n ≥ 8, for each genotype were analyzed). (H) Bar graph shows the percentage of embryos that fill with gas in wild-type (n = 177), empe3d1 (n = 182), empe3d1; btl>Emp (n = 119), empe3d1;btl>Emp-GFP (n = 178), and empe3d1; btl>CD36 (n = 70) embryos. Error bars denote standard error of the mean (SEM), p > 0.05 not significant (ns), **p < 0.005, ***p < 0.0005, and ***p < 0.0001 (unpaired two-tailed t-tests). Scale bars, (A) 50 μm, (C, D) 10 μm, and (F) 5 μm.
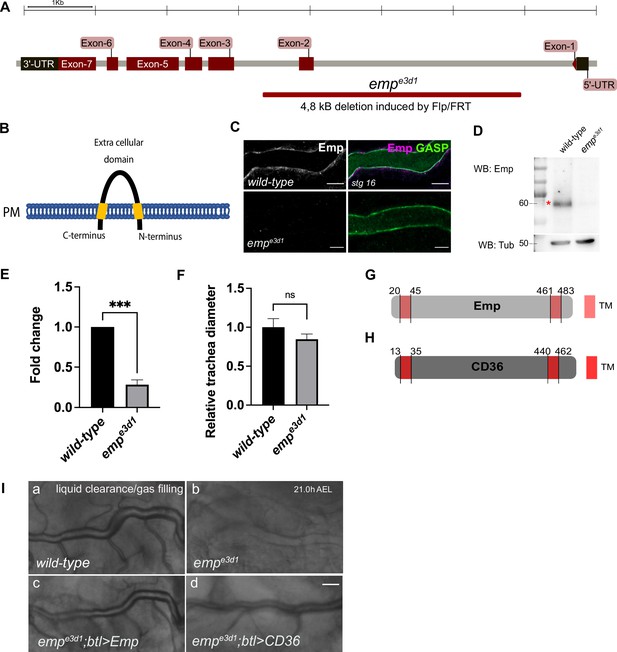
Conserved function of Emp and its human homolog CD36.
(A) Schematic view of the emp (CG2727) locus. The Flp/FRT-induced 4.8 kb deletion in empe3d1. (B) The extracellular domain of the Emp protein selected to generate the anti-Emp antibody. (C) Confocal images of dorsal trunk (DT), showing the Emp antibody specificity by immunofluorescence staining. (D) Western blot from wild-type and empe3d1 lysates embryos, probed for Emp and α-Tubulin. The red star (*) indicates the Emp corresponing band. (E) The expression levels of emp mRNA were calculated by qRT-PCR from wild-type or empe3d1 embryos at stage 16. (F) Bar plots showing the relative tracheal diameter of wild-type (n = 10) and empe3d1 (n = 8) embryos for Tr7-8. *** denotes p < 0,0001 (E) and ns (not significant) p > 0.05, (F) (unpaired two-tailed t-tests). Graphical illustration of the Emp (G) and human CD36 (H) protein domains. (I) Widefield images of DT at late stage 17 of living wild-type, empe3d1, empe3d1; btl>Emp and empe3d1; btl>CD36 embryos showing gas-filing phenotype.
-
Figure 1—figure supplement 1—source data 1
This zip archive contains the raw unedited western blot shown in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/84974/elife-84974-fig1-figsupp1-data1-v2.zip
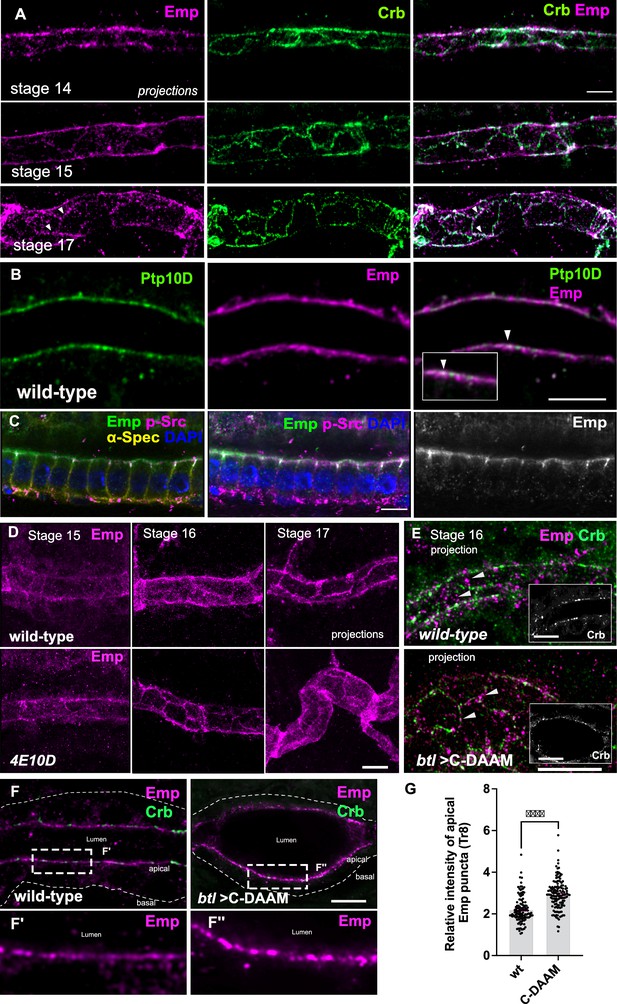
Dynamic apical distribution of Emp during tube maturation.
(A) Confocal images showing dorsal trunk (DT) projections, from stage 14, 15, and 17 embryos, stained for Emp and Crb (Crumbs). (B) Confocal images of the DT in wild-type embryos stained for Emp and Ptp10D. Inset denotes a region of the apical membrane (arrowheads) with Emp and Ptp10D co-localization. (C) Confocal images of embryonic gut cells stained for Emp, p-Src, α-Spectrin (α-Spec), and 4′,6-diamidino-2-phenylindole ( DAPI) showing the subcellular localization of Emp in stage 17. (D) DT projections, from stage 15, 16, and 17 of wild-type and Ptp4E10D (4E10D) stained for Emp. (E) Confocal image-projections of the DT cortex (depth: 5.2 μm) in wild-type and btl>C-DAAM/+ (stage early-16) embryos stained for Emp and Crb. Emp is prematurely enriched at the AJs in btl>C-DAAM/+, but not in wild-type embryos (arrowheads). Images in insets are single longitudinal sections depicting the luminal borders of the tubes. Note: btl>C-DAAM/+, displays irregular tubes with ellipsoid dilations. (F) Confocal images showing the tracheal DT of wild-type and btl>C-DAAM/+ embryos stained for Emp and Crb. Insets show magnified regions of (F), indicated by the white rectangles. The apical or basal sides of the tracheal cells are indicated. (G) Bar plot showing the relative intensity of apical Emp puncta at DT (Tr8) in wild-type (n = 168 puncta, 5 embryos) and btl>C-DAAM (n = 237 puncta, 6 embryos). Statistically significant shown in p-values, ***p < 0.0005 (unpaired two-tailed t-tests). Scale bars, 5 μm (A, E, F) and 10 μm (B, C, D).
Emp localization.
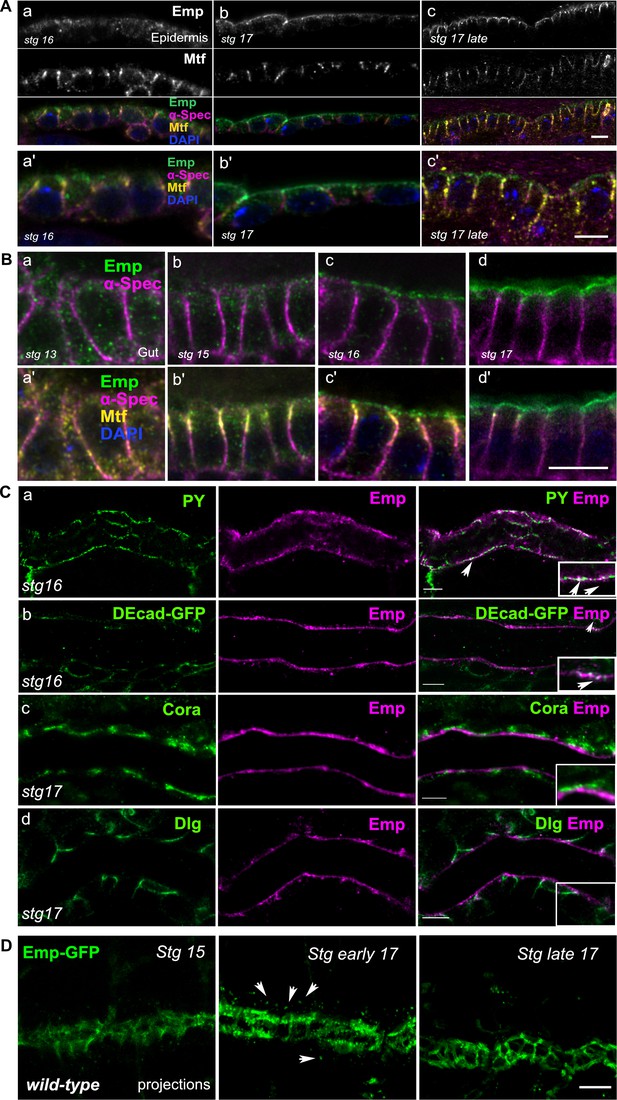
(A) Confocal images of embryonic epidermis stained for Emp, melanotransferrin (Mtf) and α-spectrin (α-Spec) showing the subcellular localization of Emp in stage 16 (a), stage 17 (b), and stage 17 late (c). (a’–c’) represent zoomed images from a–c. (B) Confocal images of embryonic gut stained for Emp, Mtf, and α-Spec in stage 13 (a–a’), stage 15 (b–b’), stage 16 (c–c’), and stage 17 (d–d’). (C) Confocal images of the tracheal dorsal trunk (DT), showing the relative localization of Emp and various cellular markers including PY (a), DE-cadherin (b), during stage 16, Cora (Coracle) (c), and Dlg (Disc Large) (d) during stage 17. Insets show zoomed cross section views of the DT of Emp co-localization with PY and DE-cadherin, as indicated by arrowheads. (D) Confocal frames of the DT of living wild-type embryos expressing Emp-GFP (green). The protein gradually accumulates at apical junctions through intracellular trafficking. Arrowheads indicates the intercellular Emp. Scale bars, 5 μm.
The apical localization of Emp in dorsal trunk (DT) and terminal branches.
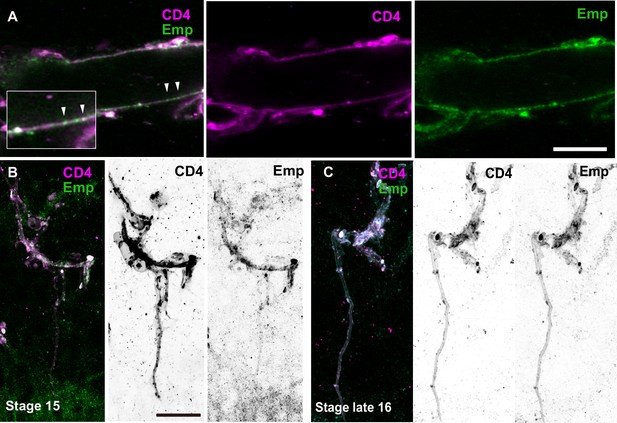
(A) Confocal images showing the DT of btl>CD4-tomato embryos stained for Emp (green) and RFP (magenta). (B) Projections of confocal images showing the terminal branches of btl>CD4-Tomato embryos stained for Emp (green) and RFP (magenta). Embryo developmental stage is indicated. Scale bars, 10 μm.
Low apical co-localization of Emp with the cortical actin bundles.
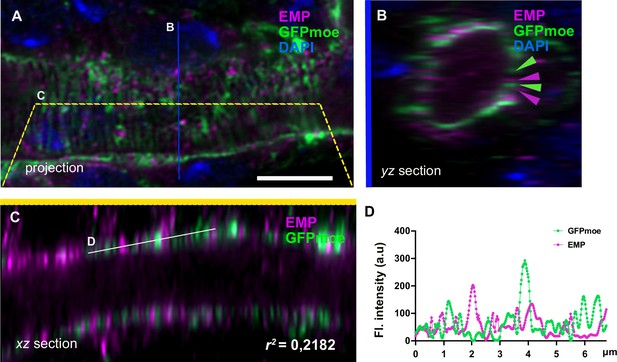
(A) Confocal image projection (A) of the tracheal dorsal trunk (DT) of btl>GFPmoe embryos stained for GFP and Emp, during stage 17.
(B–C) yz and zx sections of the projection (A) indicated by blue and yellow lines, respectively. The average Pearson’s correlation coefficient (r2) between GFP-moe (green) and Emp (magenta) of xz sections is indicated (r2 = 2182 ± 0.57, n = 5 embryos). (D) Intensity profile of a random region (5 pixels line) across the yz section as indicated by dash line. The apical intensity signals of EMP and GFP-moe are largely complementary. Scale bars, 10 μm.

The endosomal localization of Serp is strongly reduced in empe3d1 mutants.
(A) Confocal images of tracheal dorsal trunk (DT) of wild-type embryos expressing endogenous tagged YFP-Rab (knock-in) proteins, YRab5, YRab7, and YRab11 stained with Emp, Serp, and GFP. Insets show zoomed cross section views of the DT (y–z plane) of Emp co-localization with YRab5, YRab7, and YRab11, as indicated with red arrowheads. (B) Confocal images showing the tracheal DT, stained for Serp and endogenous YRab5, YRab7, and YRab11 in empe3d1 mutant. (C) Scatter plots representing the co-localization between the YRabs and Serp in wild-type and in emp3ed1 mutants calculated by Pearson correlation coefficient (r2). (D) Schematic representation of Serp and Gasp domain organization. The following abbreviations are used: SP, signal peptide (blue); LDLr, low-density lipoprotein receptor (black); ChtB, chitin-binding domain (black); GFP (green); Cht BD2, chitin-binding domain (black); and mCherry (magenta). btl>SerpFL-GFP represents the full length of Serp, btl>SerpLDLr-GFP represents the LDLr-domain of Serp, btl>SerpCBD-GFP expresses ChtB domain of Serp, btl>GaspFL-mCherry represents the full-length Gasp protein and btl>GaspLDLr-mCherry represents the full-length Gasp protein with addition of the LDLr-domain. (E) Confocal images showing the DT of live btl>SerpFL-GFP, btl>SerpLDL-GFP, btl>SerpCBD-GFP (green) embryos before (18.0 h AEL) and after (20.0 h AEL) luminal protein clearance. btl>SerpCBD-GFP embryos show incomplete luminal GFP clearance compared to btl>SerpFL-GFP or btl>SerpLDLr-GFP. (F) Plots showing the percentage of embryos with completion of luminal clearance in btl>SerpFL-GFP (green, n = 58); empe3d1;btl>SerpFL-GFP (green, n = 15); btl>SerpLDLr-GFP (green, n = 3 2); empe3d1;btl>SerpLDLr-GFP (green, n = 26); btl>SerpCBD-GFP (green, n = 45); empe3d1; btl>SerpCBD-GFP (green, n = 28); btl>GaspFL-LDLr-mCherry (magenta, n = 57); and empe3d1;btl>GaspFL-LDLr-mCherry (magenta, n = 56), from at least five independent experiments. The median (horizontal line) is shown in the plots with the data range from 25th to 75th percentile. Error bars denote standard error of the mean (SEM), *p < 0.05, ** p < 0.01, and ***p < 0.0005 (unpaired two-tailed t-tests). Scale bars, 5 μm (A, B) and 10 μm (E).
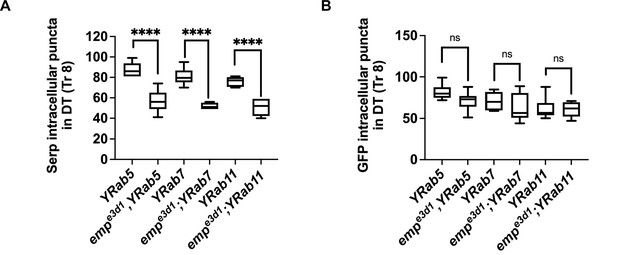
Quantification of Serp internalization.
Boxplots of Serp (A) and GFP (B) intracellular puncta of tracheal cells in YRab5, empe3d1;YRab5, YRab7, empe3d1;YRab7, YRab11, and empe3d1;YRab11 embryos at late stage 16. The boxplot (A, B) shows the median (horizontal line) and the data range from 25th to 75th percentile p > 0.05 not significant (ns) and ****p < 0.0001 (unpaired two-tailed t-tests).
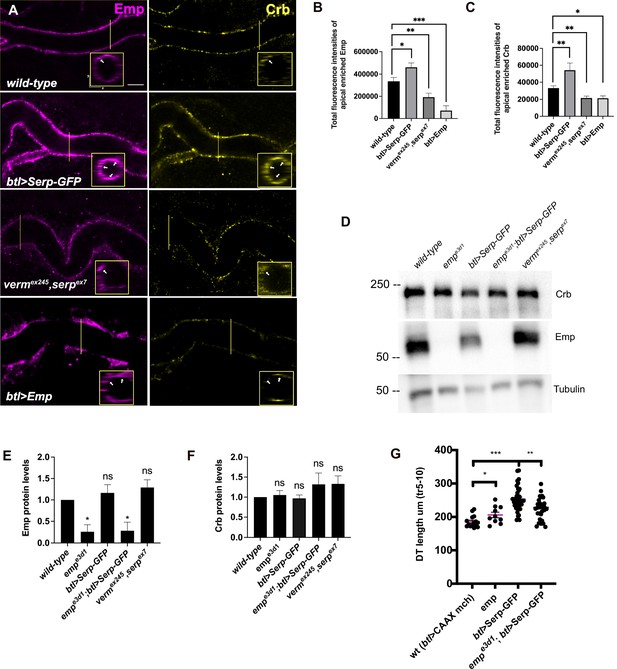
Serp-GFP overexpression induces apical Emp accumulations and tracheal over-elongation.
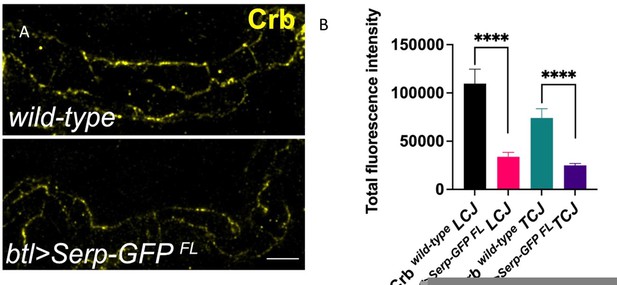
(A) Confocal images stained for Emp and Crb, in wild-type, btl>Serp-GFP, vermex245,serpex7 mutant, and btl>Emp embryos. Inset shows zoomed view of Emp and Crb signals. The arrows indicate the accumulation of Emp and Crb in yz plane. Bar plots showing total fluorescence intensities of apical enriched Emp (B) or Crb (C) in wild-type (n = 15), btl>Serp-GFP (n = 12), vermex245,serpex7 (n = 10) mutant, and btl>Emp (n = 7) embryos. (D) Representative western blot from protein lysates of wild-type, empe3d1, btl>Serp-GFP, empe3d1;btl>Serp-GFP and vermex245,serpex7 mutants, blotted for Emp and α-Tubulin. (E) and (F) show the quantification of protein levels of Emp and Crb, respectively, based on four independent western blot experiments (n = 4). (G) Plots representing the length of the tracheal dorsal trunk (DT) in µm (tr 5–10) from btl>CAAX-mcherry (control, n = 15), emp e3d1 (n = 10), btl>Serp-GFP (n = 37) and emp e3d1;btl>Serp-GFP (n = 29) embryos. Statistical significance shown in p-values; ****p < 0.0001, ***p < 0.0005, **p < 0.01, *p < 0.05, and p > 0.05 not significant (ns) (unpaired two-tailed t-tests). Scale bars, 5 μm.
-
Figure 4—source data 1
This zip archive contains the raw unedited western blot shown in Figure 4D.
- https://cdn.elifesciences.org/articles/84974/elife-84974-fig4-data1-v2.zip
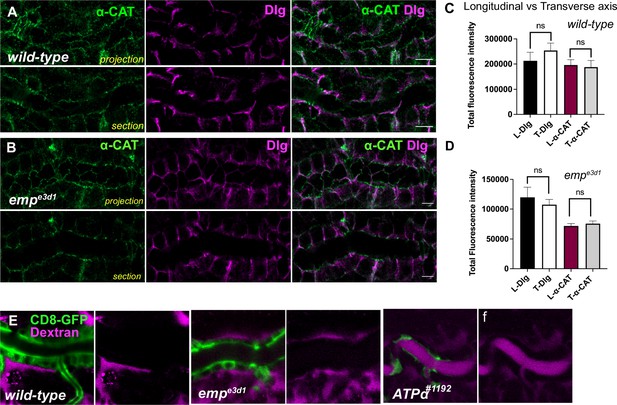
Epithelial cell integrity is not affected in empe3d1 mutants.
(A) and (B) are confocal images of tracheal dorsal trunk (DT) stained for α-CAT (green) and Dlg (magenta), respectively. Projections and sections of the tracheal tube in wild-type and empe3d1 embryos is shown. (C) and (D) plots showing the total fluorescence intensity of α-CAT and Dlg stainings in the Longitudinal (L-Dlg and L-α-CAT) and Transverse (T-Dlg and T-α-CAT) axis in wild-type and empe3d1 embryos, respectively. (E) Selected confocal time-lapse images showing the tracheal clearance of Dextran-TR in living wild-type, empe3d1 and ATPα mutant embryos expressing the membrane marker btl>CD8-GFP during stage 16. Statistical significance shown in p-values p > 0.05 not significant (ns) (Mann–Whitney test). Scale bars, 5 μm (A, C, D) and 10 μm (G).
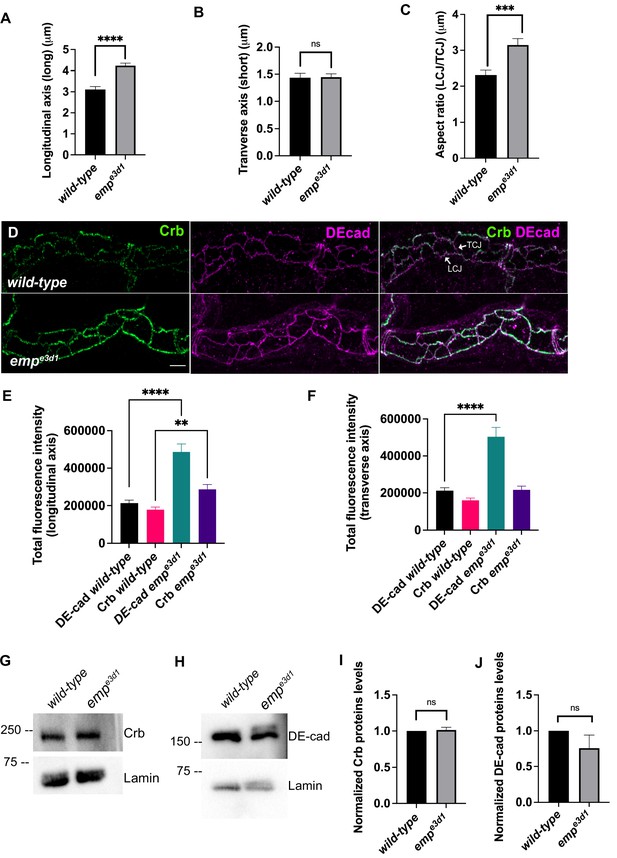
Emp modulates the Crb and DE-cad levels to control tracheal tube elongation.
(A) and (B) bar plots showing the length (in μm) of longitudinal and transverse cell axis, respectively, in wild-type and empe3d1 embryos. (C) Bar plots showing the aspect ratio, between longitudinal over transverse axis (LCJ/TCJ) in wild-type (n = 30), and empe3d1 (n = 30), mutant embryos. ****p < 0.0001, ***p < 0.0005, and p > 0.05 not significant (ns) (unpaired two-tailed t-tests). (D) Projection images of wild-type and empe3d1 mutant embryos, from stage 16, stained for Crb and DE-cadherin. (E) and (F) show quantifications of the fluorescence intensities of Crb and DE-Cadherin along longitudinal (E) and transverse (F) axis in wild-type (n = 78), and empe3d1 (n = 66), mutants. ****p < 0.0001 and **p < 0.01 (Mann–Whitney test). Representative western blot from protein lysates of wild-type and empe3d1 mutants, blotted with anti-Crb (G) or anti-DE-cad (H) and anti-Lamin (control). (I) and (J) are quantifications of Crb and DE-cad protein levels, respectively, based on three independent western blot experiments (n = 3). Statistical significance shown in p-values; p > 0.05 not significant (ns) (unpaired two-tailed t-tests). Scale bars, 5 μm.
-
Figure 5—source data 1
This zip archive contains the raw unedited western blot shown in Figure 5G, H.
- https://cdn.elifesciences.org/articles/84974/elife-84974-fig5-data1-v2.zip
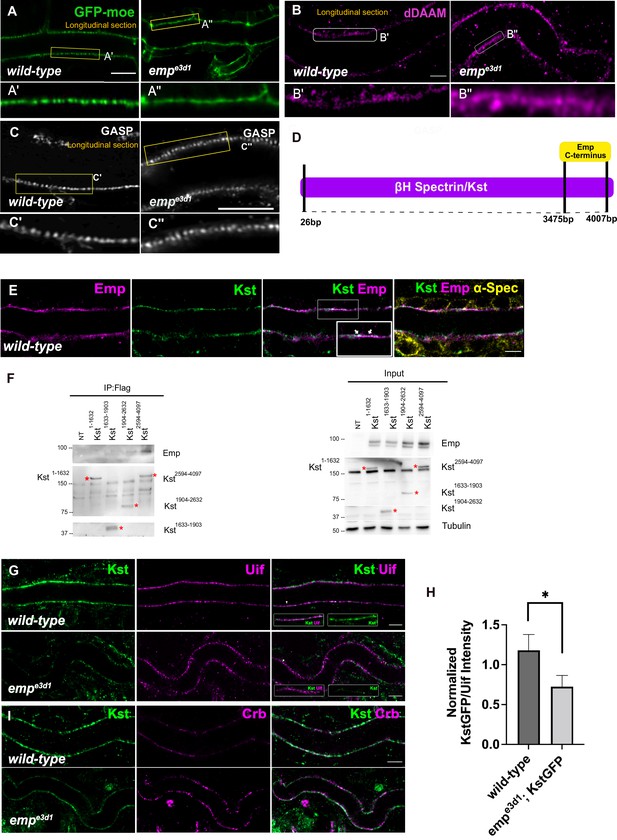
Loss of Emp affects apical F-actin organization.
(A) Confocal images showing live dorsal trunk (DT) of wild-type and empe3d1 mutant embryos from 17.5 h AEL expressing the actin reporter btl>moe-GFP. (A’, A’’) zoomed views of areas indicated by the rectangular frames of (A) panel. (B) Confocal images of dDAAM stainings in wild-type and empe3d1 mutant embryos. (B’, B’’) shows magnified regions of (B), indicated with white rectangle. (C) Confocal images of GASP stainings showing the DT in wild-type and empe3d1 mutant embryos at stage 17. (C', C'') are magnified regions of the apical extracellular matrix (ECM) as indicated by the rectangular frames in (C) panel. (D) Schematic view of the interaction domain of Emp C-terminus in 3475–4007 bp region of βH-Spectrin/Kst, as obtained by the Y2H screen. The tested Y2H prey-clones were covered the 26–4007 bps of the gene. (E) Confocal images showing the DT of Kst-Venus expressing embryos (wild-type) stained for Emp, GFP (kst), and α-Spec (α-Spectrin). (F) Co-immunoprecipitation of Flag-tagged Kst constructs from transfected S2 cells lysates, blotted with anti-Emp and anti-Flag. Input 2% is indicated. Red stars (*) denote the coresponding Kst band. st band. (G) Confocal images showing endogenous Kst-Venus in the DT of wild-type and empe3d1 mutants, stained for GFP (kst) and Uif (Uninflatable). (H) Bar plot showing Kst-GFP levels in wild-type (n = 5), and empe3d1 (n = 5), mutant embryos in relation to Uif. (I) Confocal images showing wild-type and empe3d1 mutant embryos stained for GFP (kst) and Crb. Statistical significance shown in p-value, * p < 0.05 (unpaired two-tailed t-tests) (H). Scale bars, 5 μm (B, D, F, H) and 10 μm (A).
-
Figure 6—source data 1
This zip archive contains the raw unedited western blots shown in Figure 6E.
- https://cdn.elifesciences.org/articles/84974/elife-84974-fig6-data1-v2.zip
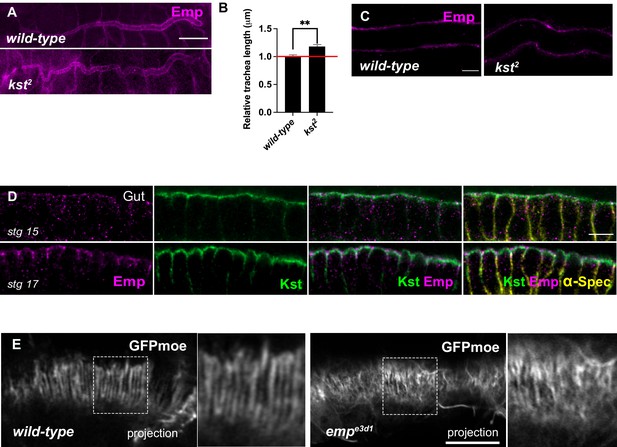
Tube elongation is defected in kst2 mutants.
(A) Confocal images showing the dorsal trunk (DT) of wild-type and kst2 embryos stained for Emp. (B) Bar plots are quantifications of the tracheal tube length in wild-type (n = 6) and kst2 (n = 10) mutant embryos. Error bars denote standard error of the mean (SEM), **p < 0.005 (unpaired two-tailed t-tests). (C) Confocal images showing the tracheal DT of wild-type and kst2 embryos stained for Emp. (D) Confocal images showing the localization of Kst in the gut during stg 15 and stg 17, stained for Emp, GFP (Kst), and α-Spec. (E) Airy-scan confocal images of the DT in a wild-type and emp mutant embryo expressing the actin reporter btl>moe-GFP (gray). Images were acquired from living embryos during luminal endocytosis, 18 h AEL. Side-panels depict zoomed view of areas indicated by rectangular frames. Scale bars, 50 μm (A), 10 µm (E), and 5 µm (C, D).
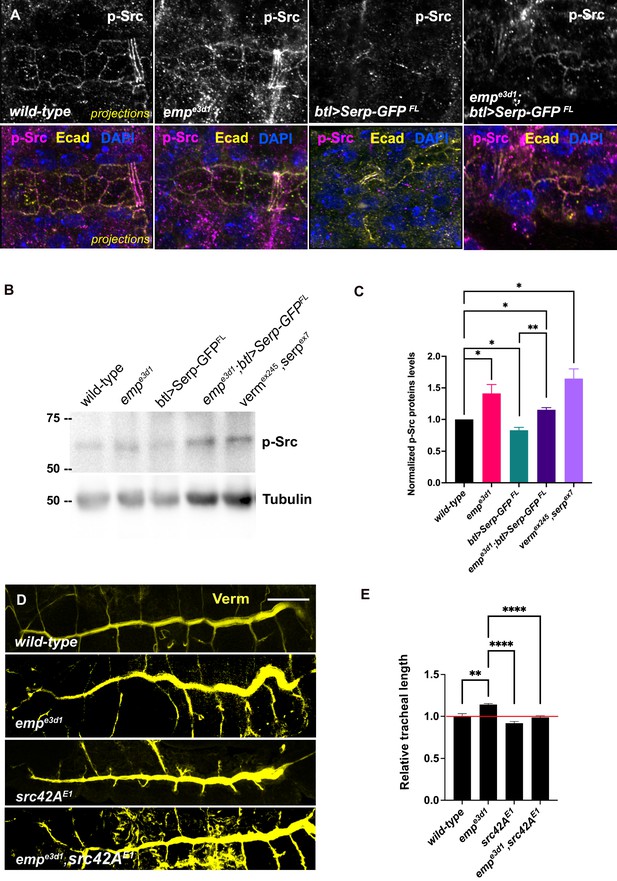
Elevated p-Src levels in empe3d1 mutants.
(A) Confocal images showing projection of the tracheal dorsal trunk (DT) of wild-type, empe3d1 mutants, btl>Serp-GFP and empe3d1;btl>Serp-GFP embryos at late stage 16 to early stage 17, stained for endogenous p-Src. (B) Representative western blot from protein lysates of wild-type, empe3d1 mutants, btl>Serp-GFP, empe3d1;btl>Serp-GFP and verm,serp double mutant embryos, blotted with anti-p-Src and anti-α-Tubulin. (C) Quantifications of p-Src protein levels based on three independent western blot experiments (n = 3). *p < 0.05 and **p < 0.01 (unpaired two-tailed t-tests). (D) Representative images of tracheal length size (DT), in wild-type, empe3d1, src42AE1, and empe3d1,src42AE1 embryos stained for the luminal marker Verm. (E) Plots show the quantification of tracheal DT length of wild-type (n = 6), empe3d1 (n = 7), src42AE1 (n = 10), and empe3d1;src42AE1 (n = 7) embryos. Error bars denote standard error of the mean (SEM) wild-type, *p < 0.05, **p < 0.005, and ****p < 0.0001 (unpaired two-tailed t-tests). Scale bars, 10 and 50 μm for images (A) and (D), respectively.
-
Figure 7—source data 1
This zip archive contains the raw unprocessed western blots shown in Figure 7B.
- https://cdn.elifesciences.org/articles/84974/elife-84974-fig7-data1-v2.zip
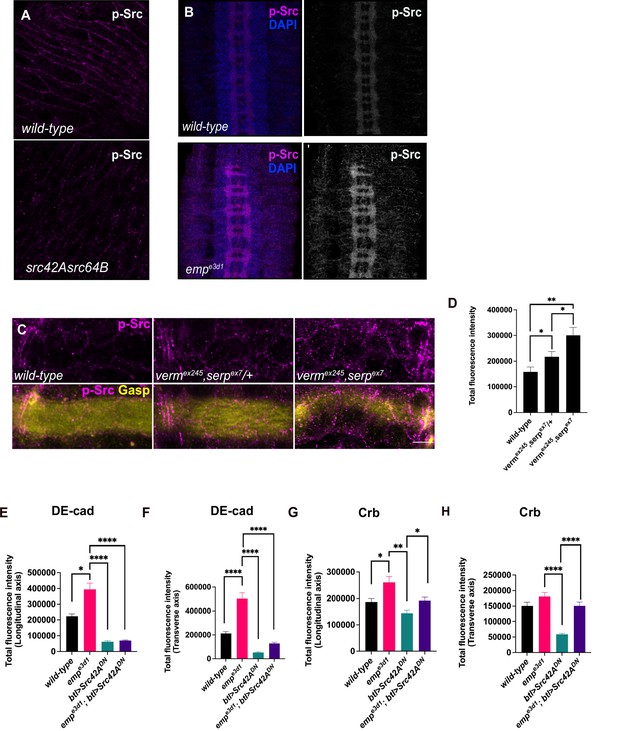
p-Src levels are increased in CNS of empe3d1 mutants.
(A) Confocal images of embryonic epidermis stained for p-Src in wild-type (a) and src42AE1;src64B (b) embryos, showing the specificity of p-Src antibody. (B) Images of the ventral nerve cord in wild-type (a, a’) and empe3d1 embryos (b, b’) stained for p-Src and DAPI. (C) Confocal images of the tracheal dorsal trunk (DT) stained for p-Src (magenta) and Gasp (yellow) in wild-type, vermex245,serpex7/+, and vermex245,serpex7 embryos. (D) Bar plots showing the total fluorescence intensity of p-Src displayed in (C). (E) and (F) bar plots showing the total fluorescence intensity of DE-cad from DT in the longitudinal and transverse axis, respectively, in wild-type, empe3d1, btl>src42ADN and empe3d1;btl>src42ADN embryos. (G) and (H) bar plots representing the total fluorescence intensity of Crb in the longitudinal and transverse axis, respectively, of the DT in embryos described in (C) and (D). Error bars denote standard error of the mean (SEM) wild-type, *p < 0.05, **p < 0.005, and ****p < 0.0001 (Mann–Whitney tests).

The proposed model of Emp regulation.
(A) 3D drawing shows the multicellular tube (dorsal trunk) of Drosophila airways. The annular ridges of the apical extracellular matrix (ECM) are indicated in relation to the longitudinal elongation. LJ: Longitudinal junctions; TJ: transverse junctions. (B) Schematic zoom of the apical ECM region (A) showing the formation of two apical domains in the membrane–cytoskeleton interface of tracheal cells at 12–16 h AEL. Transverse F-actin bundles (white color zone) restrict apical Emp endocytosis along the transverse tube axis. In F-actin bundle-free membrane region (green color zone), Emp is associated with βH-Spectrin and Serp promotes Emp clustering leading to endocytosis and recycling of Serp and others ‘passenger’ transmembrane proteins (i.e. Crb) along the longitudinal tube axis. (C) Drawing of the apical, SAR and AJ regions during luminal protein clearance (18–19 h). Emp clears luminal Serp by endocytosis and trans-locates to the SAR/AJ to restrict p-Src42 activity and to control DE-Cad, Crb levels at the SAR/AJ.
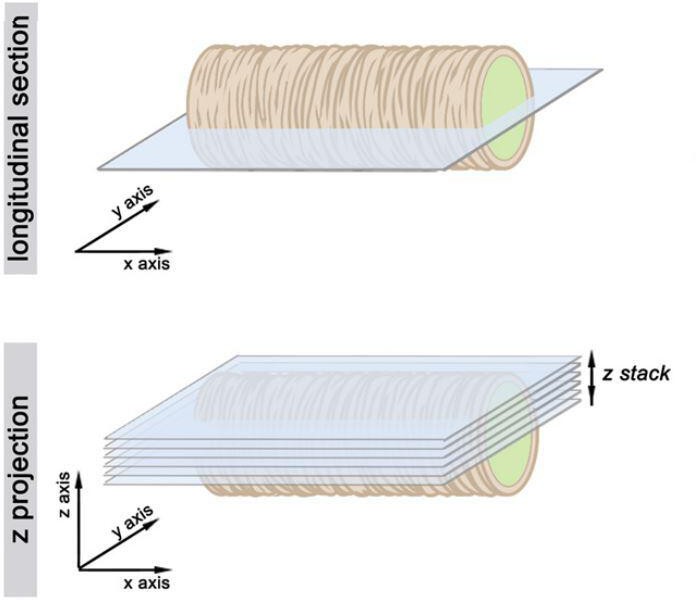
Schematic overview of the imaging in the apical surface of the tubes.
Single sections (upper row) and Z-stack maximum projections (lower row).
Additional files
-
Supplementary file 1
The fly strains used in this study.
- https://cdn.elifesciences.org/articles/84974/elife-84974-supp1-v2.docx
-
Supplementary file 2
The sequences of primers used for qPCR analysis.
- https://cdn.elifesciences.org/articles/84974/elife-84974-supp2-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/84974/elife-84974-mdarchecklist1-v2.pdf