Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis
Figures
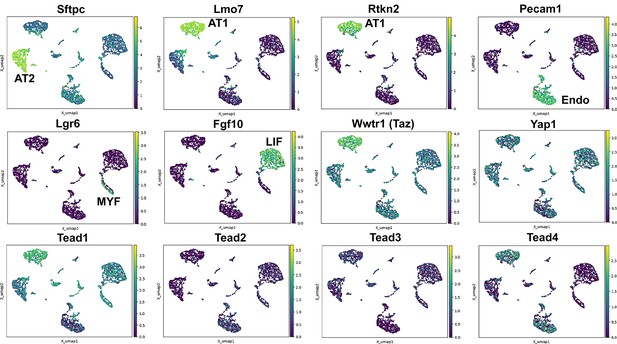
Hippo pathway expression in the lung.
Single nuclei RNA-seq was performed on E18.5 lungs. (Top and middle row) Cell types were identified based on gene expression: Sftpc (AT2); Lmo7, Rtkn2 (AT1); Pecam1 (endothelial); Lgr6 (myofibroblast/airway smooth muscle); Fgf10 (lipofibroblast). (MIddle and bottom row) Tead 1–4, Wwtr1 (Taz), and Yap1 mapped to these cell types.
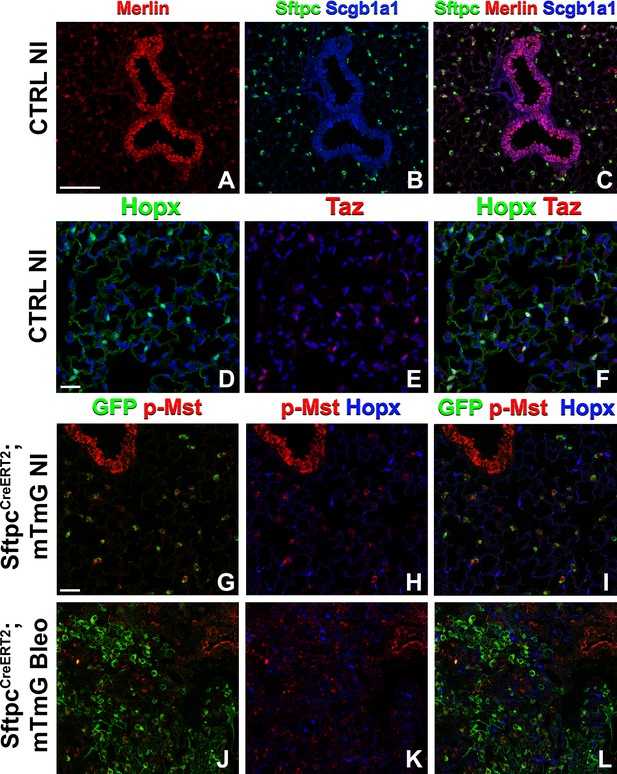
The Hippo pathway is active in alveolar type 2 (AT2) cells but not in AT1 cells.
Left lung lobes of 8-week-old mice were inflation fixed, embedded in paraffin, and sectioned. (A–C) Immunostaining for Sftpc (A–C), Merlin (A, C), and Scgb1a1 (B, C) and (D–F) immunostaining for Hopx (D, F) and Taz (E, F) on control non-injured (NI) lungs. (G–L) At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and left lung lobes were inflation fixed, embedded in paraffin, and sectioned 9 weeks after being placed on normal chow (at 20 weeks of age). Intratracheal bleomycin was administered 3 weeks after mice were removed from tamoxifen and placed on normal chow. Bleomycin-injured lungs were harvested at 6 weeks after injury (at 20 weeks of age). Immunostaining for GFP (G–L), phosphorylated-Mst (p-Mst; G, I, J, L), and Hopx (H, I, K, L) on non-injured (NI) SftpcCreERT2; mTmG (G–I) and on bleomycin injured SftpcCreERT2; mTmG (J–L) lungs. Representative images are presented. Scale bars, 100 µm (A–C), 50 µm (D–F), 50 µm (G–L).
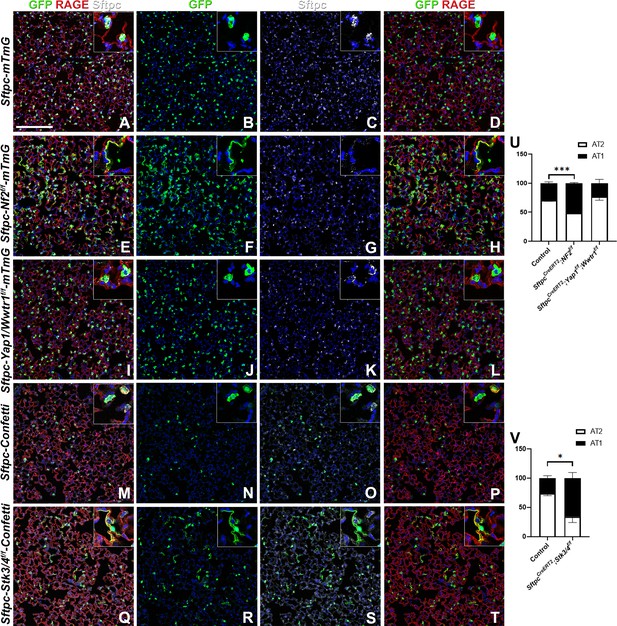
The Hippo pathway actively maintains alveolar type 2 (AT2) cells during homeostasis.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and left lung lobes were inflation fixed, embedded in paraffin, and sectioned 9 weeks after being placed on normal chow (at 20 weeks of age). (A–T) Immunostaining for GFP (B, F, J, N, R), Sftpc (C, G, K, O, S), and RAGE (D, H, L, P, T) on SftpcCreERT2; mTmG (A-D, n=4) SftpcCreERT2; Nf2f/f;mTmG (E-H, n=3), SftpcCreERT2; Yap1f/f; Wwtr1f/f; mTmG (I-L, n=4), SftpcCreERT2; Confetti (M-P, n=3), SftpcCreERT2; Stk3/4f/f;Confetti (Q-T, n=3). Representative images are presented. Scale bar, 200 µm. (U) Image analysis GFP+/Sftpc+ (AT2) and GFP+/RAGE+ (AT1) on staining in A-L and (V) M-T. Data are mean ± SEM. Student’s t-test was used to determine significance. *p<0.05, **p<0.01, ****p<0.0001.
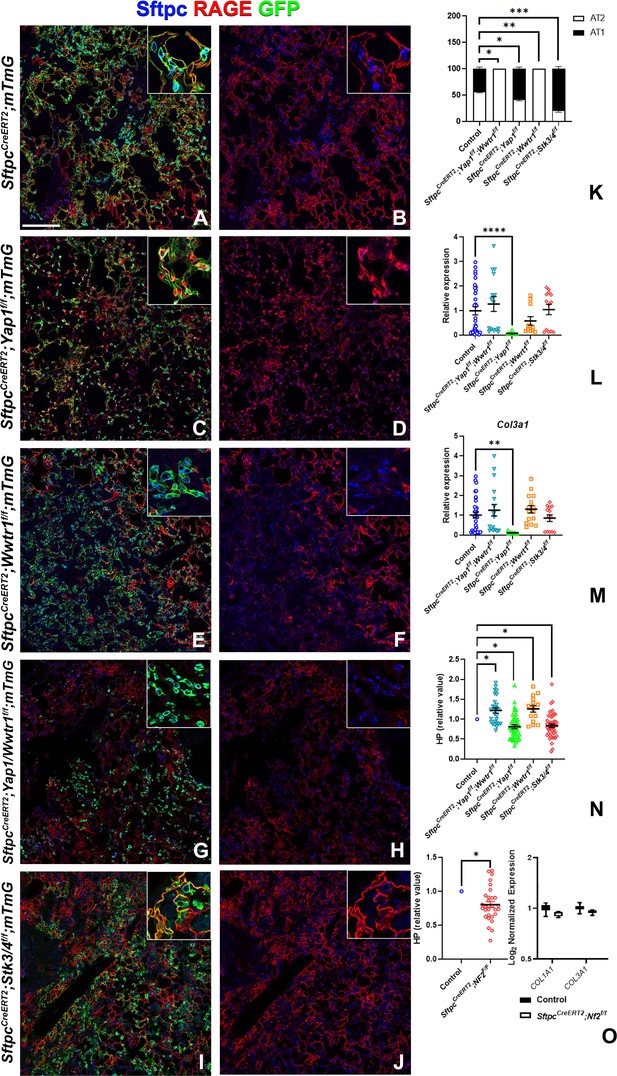
Taz is required for alveolar type 2 (AT2) into AT1 cell differentiation following bleomycin injury.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. (A–J) Immunostaining for GFP, Sftpc, and RAGE on SftpcCreERT2; mTmG (A, B, n=3), SftpcCreERT2; Yap1f/f;mTmG (C, D, n=4), SftpcCreERT2; Wwtr1f/f; mTmG (E, F, n=5), SftpcCreERT2;Yap1f/f; Wwtr1f/f; mTmG (G, H, n=4), SftpcCreERT2; Stk3/4f/f; mTmG (I, J, f/f=6). Representative images are presented. Scale bar, 200 µm. (K) Image analysis GFP+/Sftpc+ (AT2) and GFP+/RAGE+ (AT1) on staining in A-J. (L–M) qPCR analysis for Col1a1 (L) and Col3a1 (M) on control (n=33), SftpcCreERT2; Yap1f/f; Wwtr1f/f (n=15), SftpcCreERT2; Yap1f/f (n=9), SftpcCreERT2; Wwtr1f/f (n=11), SftpcCreERT2; Stk3/4 f/f (n=13). Values are represented as 2-ΔΔCt normalized to Control. (N) Hydroxyproline analysis for soluble collagen on control, SftpcCreERT2; Yap1f/f; Wwtr1f/f (n=27, control n=25), SftpcCreERT2; Yap1f/f (n=40, control n=37), SftpcCreERT2; Wwtr1f/f (n=15, control n=10), SftpcCreERT2; Stk3/4 f/f (n=46, control n=53). Values are normalized to each genotype’s Cre- control. (O) Hydroxyproline analysis for soluble collagen on control (n=15) and SftpcCreERT2; Nf2f/f (n=30) and Log2 normalized values for RNA expression for Col1a1 and Col3a1 from NanoString analysis (control n=21, SftpcCreERT2; Nf2f/f n=6). Values are normalized to control. (K-N) Data are mean ± SEM. (O) Data are mean ± SEM (left panel) or box and whisker plot (right panel). Student’s t-test was used to determine significance. *p<0.05, **p<0.01, ****p<0.0001.
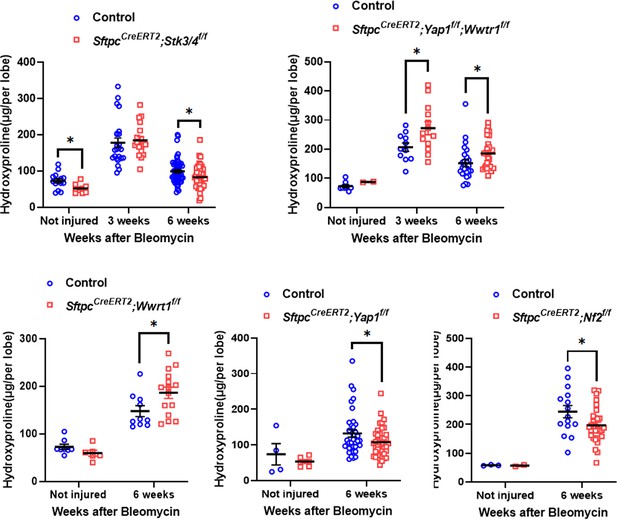
Inactivation of Stk3/4 promotes fibrosis resolution 6 weeks after bleomycin injury.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 3 or 6 weeks post-injury (at 17 or 20 weeks of age, respectively), mice were euthanized and lungs were flash frozen. Hydroxyproline analysis for soluble collagen on non-injured, 3 weeks post-injury, and 6 weeks post-injury control, SftpcCreERT2; Yap1f/f; Wwtr1f/f, SftpcCreERT2; Yap1f/f, SftpcCreERT2; Wwtr1f/f, SftpcCreERT2; Stk3/4 f/f, and SftpcCreERT2; Nf2f/f. Data are mean ± SEM. Student’s t-test was used to determine significance. *p<0.05.
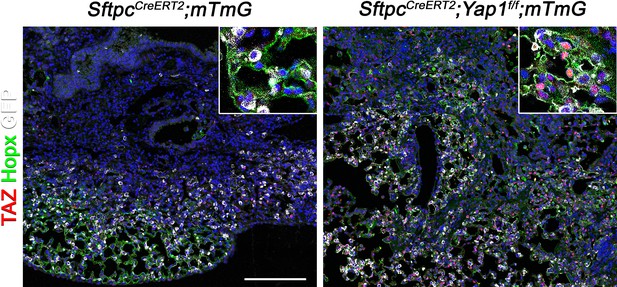
Inactivation of Yap1 in alveolar type 2 (AT2) induces Taz upregulation.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Immunostaining for GFP, Taz, and Hopx on control SftpcCreERT2; mTmG and SftpcCreERT2; Yap1f/f; mTmG lungs. Scale bar, 200 µm.
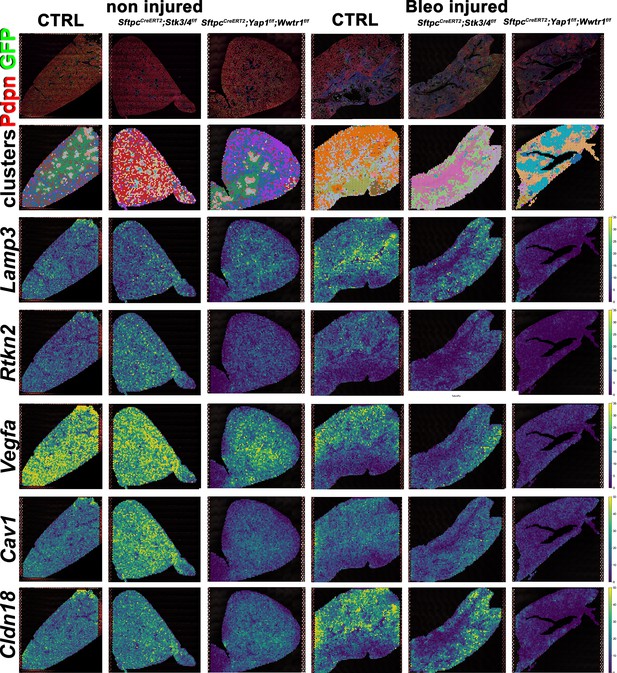
Decreased alveolar epithelial regeneration following bleomycin injury upon inactivation of Yap/Taz in alveolar type 2 (AT2) cells.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Spatial transcriptomics was performed on injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f;Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/4f/f; mTmG and non-injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/f/ff/f; mTmG lungs. Immunofluorescence colocalization of Pdpn (red) and GFP (green) on non-injured lungs and lungs 6 weeks after bleomycin injury. Projection of spot clusters onto immunofluorescence image of the tissue sample. Spatial gene expression transcripts of cell type-specific markers were mapped onto spot coordinates. Lamp3 (AT2) and Rtkn2, Vegfa, Cav1, Cldn18 (AT1) markers. Color scale reflects the abundance of indicated transcripts.
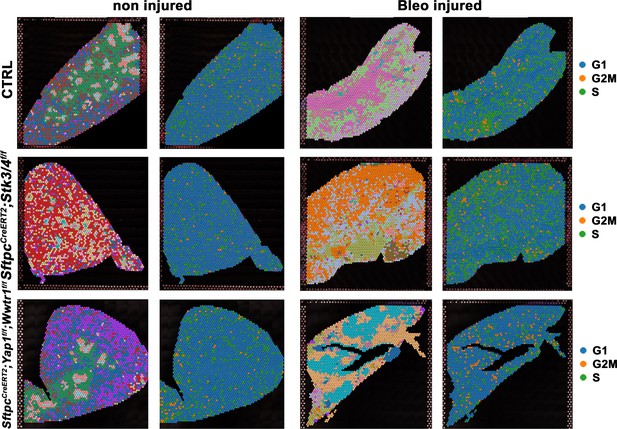
Alveolar type 2 (AT2) inactivation of Yap1/Wwtr1 reduces the S phase after bleomycin injury.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Projection of spot clusters onto tissue samples of injured (6 weeks after bleomycin injury) control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/4f/f; mTmG and non-injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/ f/ff/f; mTmG lungs. Cell replication phase within spatial RNAseq data with G1 in blue, G2M in orange, and S phase in green.
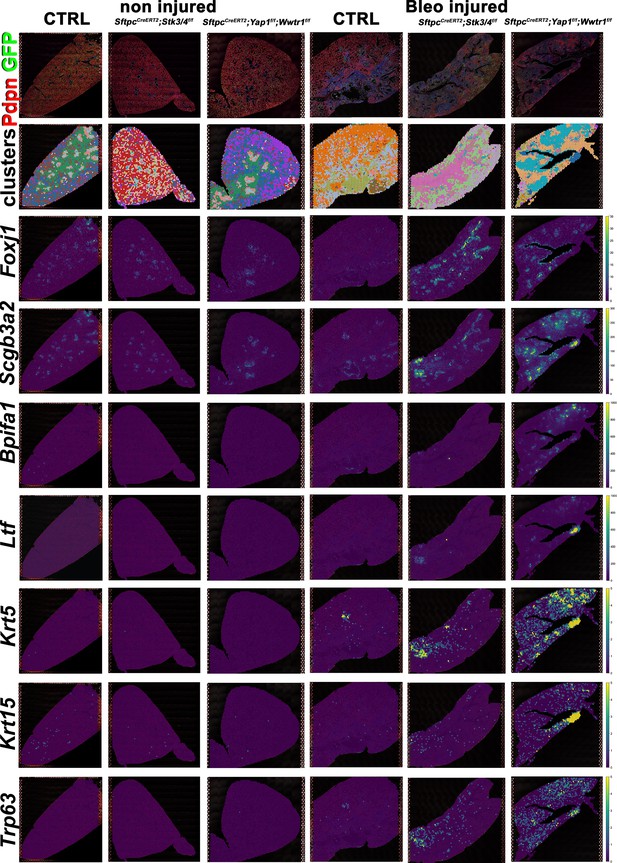
Increased bronchiolization upon Yap/Taz inactivation in alveolar type 2 (AT2) cells.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Spatial transcriptomics was performed on injured (6 weeks after bleomycin injury) control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f;Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/4f/f; mTmG and non-injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/ f/ff/f; mTmG lungs. Immunofluorescence colocalization of Pdpn (red) and GFP (green) on non-injured lungs and lungs 6 weeks after bleomycin injury. Projection of spot clusters onto immunofluorescence image of the tissue sample. Spatial gene expression transcripts of cell type-specific markers were mapped onto spot coordinates. Foxj1 (ciliated), Scgb3a2 (serous and club), Bpifa1, Ltf (serous) and Krt5, Krt15, Trp63 (basal) markers. Color scale reflects the abundance of indicated transcripts.
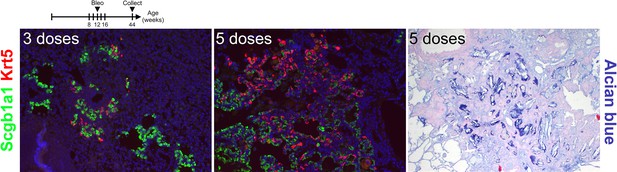
Repetitive bleomycin doses induce bronchiolization.
Mice were administered bleomycin intratracheally beginning at 8 weeks of age. Every 2 weeks mice received additional doses of bleomycin for either 3 or 5 doses. Left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Immunostaining for Scgb1a1 and Krt5 or Alcian blue on injured lungs.
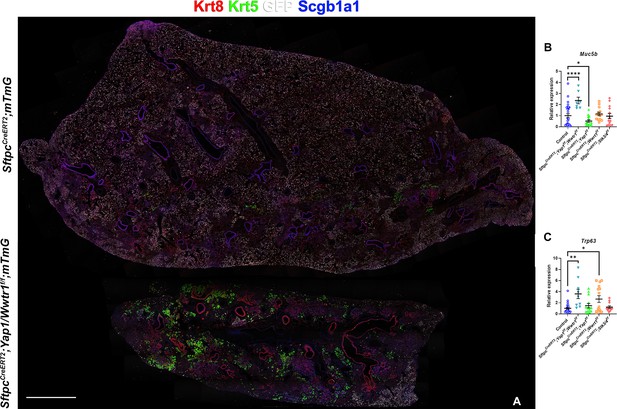
Inactivation of Yap and Taz in alveolar type 2 (AT2) cells enhances bronchiolization following bleomycin injury.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. (A) Immunostaining for GFP, Krt8, Krt5, and Scgb1a1 on control SftpcCreERT2; mTmG and SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG. Scale bar, 666 µm. (B–C) qPCR analysis for Muc5b (B) and Trp63 (I), on control (n=29), SftpcCreERT2; Yap1f/f; Wwtr1f/f (n=7), SftpcCreERT2; Yap1f/f (n=14), SftpcCreERT2; Wwtr1f/f (n=14), SftpcCreERT2; Stk3/4 f/f (n=14) lungs. Data are mean ± SEM. Student’s t-test was used to determine significance. *p<0.05, **p<0.01, ****p<0.0001.
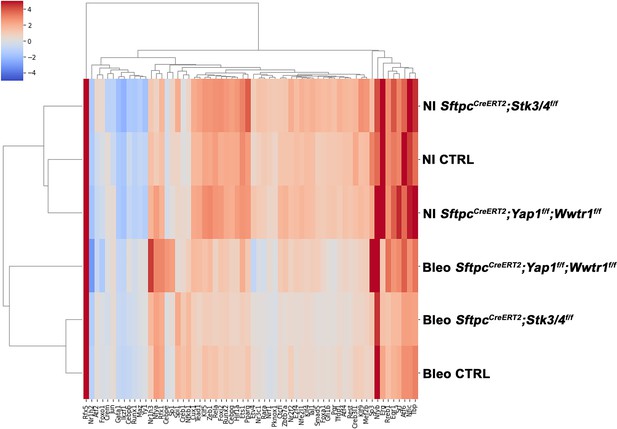
Predicted transcription factor expression.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Heatmap showing unique transcription factor activity in injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/4f/f; mTmG and non-injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yapf/f; Tazf/f, mTmG, SftpcCreERT2; Stk3/ f/ff/f; mTmG lungs. Color scale at the left reflects the intensity of the predicted transcription factor score.
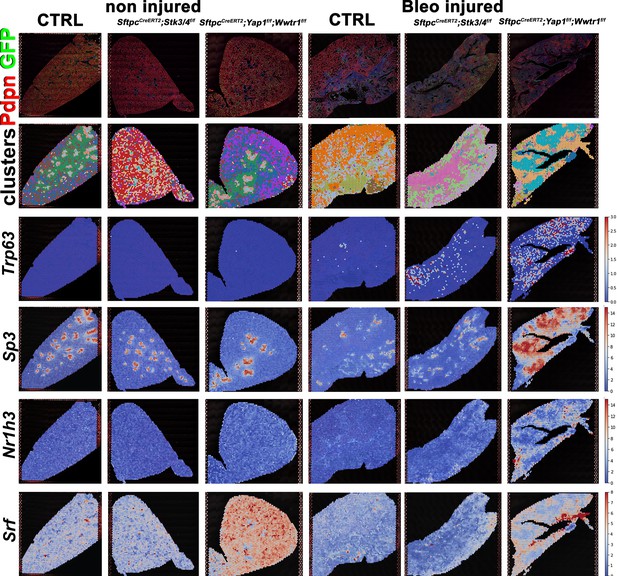
Predicted transcription factor expression is altered based on genotype and injury status.
At 8 weeks of age, mice were placed on tamoxifen chow for 3 weeks, and following a 3-week washout period, mice were non-injured or injured with intratracheal administration of bleomycin. At 6 weeks post-injury (at 20 weeks of age), left lung lobes were inflation fixed, embedded in paraffin, and sectioned. Projection of spot clusters onto tissue samples of injured (6 weeks after bleomycin injury) control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/4f/f;mTmG and non-injured control SftpcCreERT2; mTmG, SftpcCreERT2; Yap1f/f; Wwtr1f/f, mTmG, SftpcCreERT2; Stk3/ f/ff/f; mTmG lungs. Spatial transcription factor activity prediction scores within spatial RNAseq data, red indicates increased intensity of prediction factor activity scores, where blue is decreased intensity.





