Diet-induced loss of adipose hexokinase 2 correlates with hyperglycemia
Figures
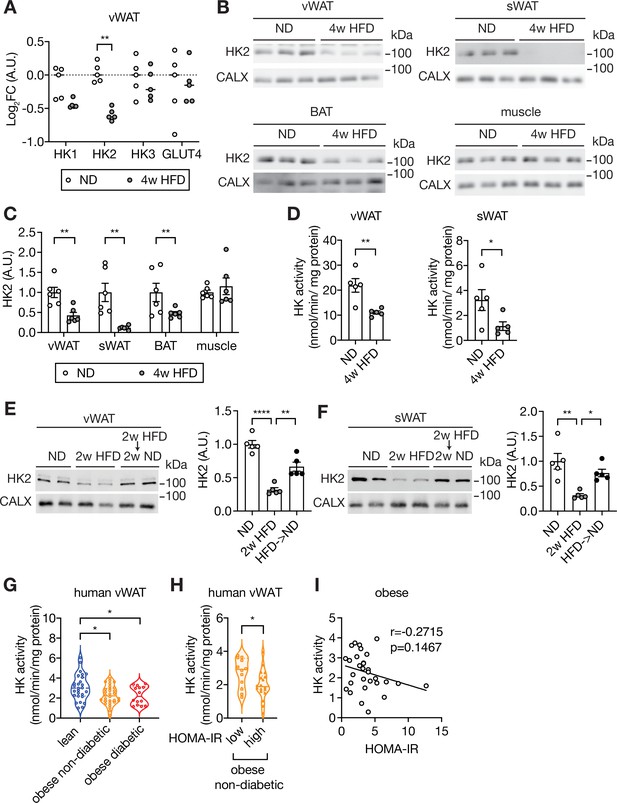
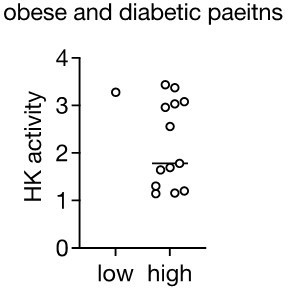
Loss of HK2 in obese mouse and obese human.
(A) The Log2 fold change (FC) of Hexokinase and GLUT4 protein expression in visceral white adipose tissue (vWAT) of normal diet (ND)- and 4 week high-fat diet (HFD)-fed wild-type C57BL/6JRj mice. Multiple t test, **q<0.0001. n=5 (ND) and 5 (HFD). (B) Immunoblot analyses of vWAT, subcutaneous WAT (sWAT), brown adipose tissue (BAT), and skeletal muscle from ND- and 4 week HFD-fed mice. CALX serves as a loading control. n=6 (ND) and 6 (HFD). (C) Quantification of panel B. Data is normalized to the loading control. Student’s t test. **p<0.01. (D) Hexokinase (HK) activity of vWAT and sWAT from ND- and 4 week HFD-fed mice. Student’s t test, *p<0.05, **p<0.01. n=5 (ND) and 5 (HFD). (E–F) Immunoblot analyses of vWAT (E) and sWAT (F) from ND-, 2 week HFD-, and 2 week HFD +2 week ND-fed mice. n=5 (ND), 5 (HFD), and 6 (HFD +ND). Data is normalized to the loading control. One-way ANOVA. *p<0.01, **p<0.01, ****p<0.0001. (G) Hexokinase (HK) activity of vWAT from lean, obese non-diabetic, and obese diabetic patients. Two-way ANOVA, *p<0.05. n=27 (lean), 30 (obese), and 14 (obese diabetic). (H) Comparison of vWAT HK activity from low or high HOMA-IR obese non-diabetic patients. Student’s t tests, *p<0.05. n=12 (low, HOMA-IR <2.9) and 18 (high, HOMA-IR >2.9). (I) Pearson’s correlation analyses of hexokinase activity and homeostatic assessment for insulin resistance (HOMA-IR) in obese patients. See Figure 1—source data 1.
-
Figure 1—source data 1
Uncropped blots and source data for graphs for Figure 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig1-data1-v1.zip
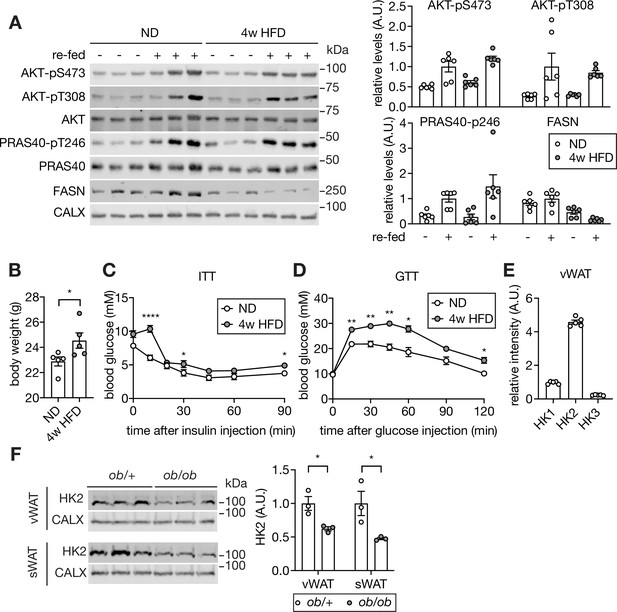
Supporting data 1 for Figure 1.
(A) Insulin signaling in visceral white adipose tissue (vWAT) of wild-type mice fed normal diet (ND) or 4 week high-fat diet (HFD). Mice were fasted overnight or fasted overnight and re-fed for 3 hours. CALX serves as a loading control. n=6 (ND, fasted), 6 (ND, re-fed), 6 (HFD, fasted), and 6 (HFD, re-fed). (B) Body weight of ND- and 4 week HFD-fed wild-type C57BL/6JRj mice. Student’s t test, *p<0.05. n=5 (ND) and 5 (HFD). (C) Insulin tolerance test (ITT) on ND- or 4 week HFD-fed wild-type C57BL/6JRj mice. The mice were fasted for 6 hours and injected with insulin (0.5 U/kg body weight). Two-way ANOVA. *p<0.05, ****p<0.0001. n=5 (ND) and 5 (HFD). (D) Glucose tolerance test (GTT) on ND- or 4 week HFD-fed wild-type C57BL/6JRj mice. Mice were fasted for 6 hours and i.p. injected with glucose (2 g/kg body weight). Two-way ANOVA. *p<0.05, **p<0.01. n=5 (ND) and 8 (HFD). (E) HK2 is the most abundant hexokinase in vWAT. Protein expression of HK1, HK2, and HK3 in vWAT of C57BL/6 mice (Supplementary file 1) was normalized to HK1 expression. n=5. (F) Immunoblot analyses of HK2 in vWAT and sWAT from ob/+ and ob/ob mice. CALX serves as a loading control. n=3 (ob/+) and 3 (ob/ob). See Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Uncropped blots and source data for graphs for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig1-figsupp1-data1-v1.zip
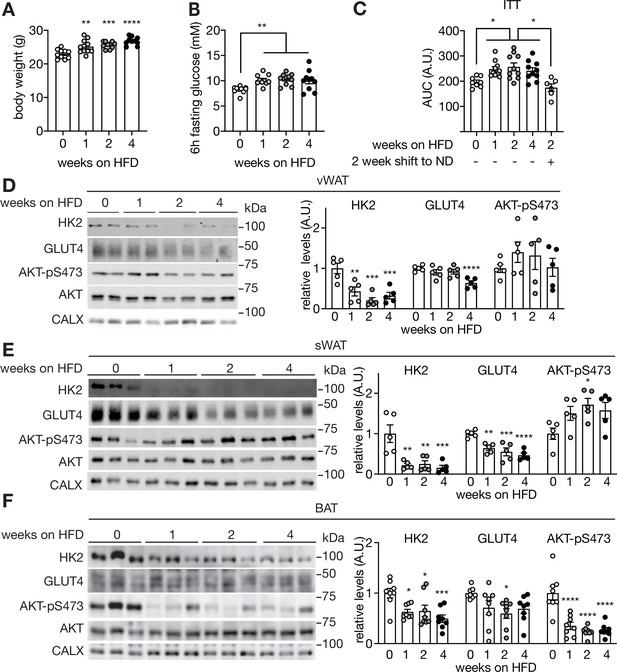
Supporting data 2 for Figure 1.
(A-B) Body weight (A) and fasting glucose levels (B) of mice fed a HFD for 0, 1, 2, or 4 weeks. One-way ANOVA compared to 0 week HFD-fed mice, **p<0.01, ***p<0.001, ****p<0.0001. n=10 (C) ITT on mice fed a HFD for 0, 1, 2, 4 weeks, and mice fed 2 week HFD and 2 week ND. The mice were fasted for 6 hours and injected with insulin (0.75 U/kg body weight). One-way ANOVA, *p<0.05. n=8 (0 week ND), 10 (1 week HFD), 10 (2 week HFD), 10 (2 week HFD), and 6 (2 week HFD +2 week ND). (D–F) Immunoblots of vWAT(D), sWAT(E), and BAT (F) from mice fed a HFD for 0, 1, 2, or 4 weeks. Mice were fasted for 6 hours and injected with insulin (0.75 U/kg body weight). n=5. See Figure 1—figure supplement 2—source data 1.
-
Figure 1—figure supplement 2—source data 1
Uncropped blots and source data for graphs for Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig1-figsupp2-data1-v1.zip
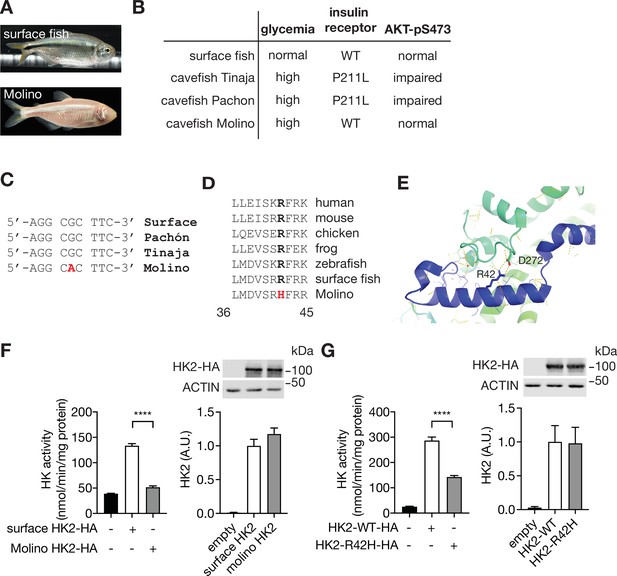
A loss-of-function HK2 variant in hyperglycemic Mexican cavefish.
(A) Surface fish and Mexican cavefish Molino. (B) Comparisons of phenotypes in surface fish, Pachón, Tinaja, and Molino. (C) DNA sequence of the Molino variant. (D) Amino acid sequence alignment of the HK2-R42H mutation within vertebrates. (E) Structural analyses revealed the presence of a salt bridge between Arginine 42 (R42) and Aspartic acid 272 (D272) in the human HK2 (PDB: 2MTZ). (F) HK activity and immunoblot analyses for lysates of HEK293T cells expressing surface or Molino HK2. Student’s t test, ****p<0.0001. N=4. (G) HK activity and immunoblots for lysates of HEK293T cells expressing control, HK2-WT, or HK2-R42H. Student’s t test, ****p<0.0001. N=4. See Figure 2—source data 1.
-
Figure 2—source data 1
Uncropped blots and source data for graphs for Figure 2.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig2-data1-v1.zip
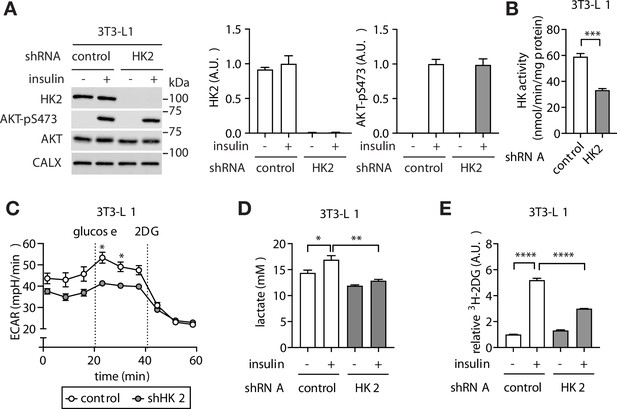
Loss of adipose HK2 causes reduced glucose disposal in adipocytes.
(A) Immunoblot analyses of control and HK2 knockdown 3T3-L1 adipocyte lysates. Cells were stimulated with 100 nM insulin for 25 min. N=4. (B) HK activity of control and HK2 knockdown 3T3-L1 adipocytes. Student’s t test, ***p<0.001. N=3. (C) Extracellular acidification rate of control and HK2 knockdown 3T3-L1 adipocytes in response to glucose (10 mM) and 2-deoxyglucose (2DG, 50 mM). Two-way ANOVA. *p<0.05. N=2. (D) Lactate secreted into media by control and HK2 knockdown 3T3-L1 adipocytes treated with or without 100 nM insulin. One-way ANOVA. *p<0.05, **p<0.01. N=3. (E) 2DG uptake in control and HK2 knockdown 3T3-L1 adipocytes treated with or without 100 nM insulin. One-way ANOVA. ****p<0.0001. N=3. See Figure 3—source data 1.
-
Figure 3—source data 1
Uncropped blots and source data for graphs for Figure 3.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig3-data1-v1.zip
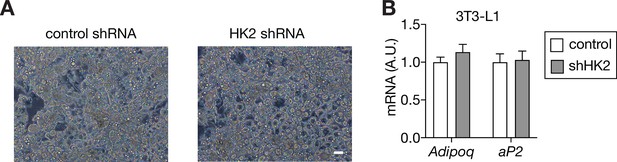
Supporting data for Figure 3.
(A) Bright filed images of control and HK2 knockdown 3T3-L1 adipocytes. (B) mRNA levels of mature adipocyte markers in control and HK2 knockdown 3T3-L1 adipocytes. N=3. See Figure 3—figure supplement 1—source data 1.
-
Figure 3—figure supplement 1—source data 1
Source images and source data for graphs for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig3-figsupp1-data1-v1.zip
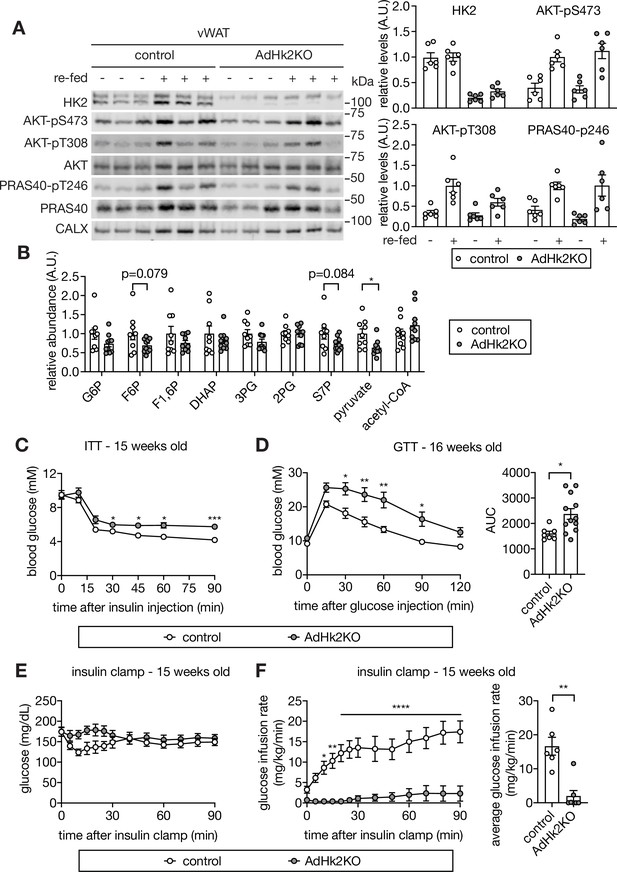
Loss of adipose HK2 causes hyperglycemia in mice.
(A) Immunoblot analyses of HK2 expression and insulin signaling in vWAT of control and adipose-specific Hk2 knockout (AdHk2KO) mice. Mice were fasted overnight or fasted overnight and re-fed for 3 hours. Quantification data is normalized to a loading control. n=6. (B) Fold change (AdHk2KO/control) of metabolites in glycolysis and the pentose phosphate pathway in vWAT of control and AdHk2KO mice. Student’s t test. *p<0.05. n=9 (control) and 10 (AdHk2KO). (C) Insulin tolerance test (ITT) on control and AdHk2KO mice. The mice were fasted for 6 hours and injected with insulin (0.5 U/kg body weight). Two-way ANOVA. *p<0.05, ***p<0.001. n=6 (control) and 10 (AdHk2KO). (D) Glucose tolerance test (GTT) on control and AdHk2KO mice. Mice were fasted for 6 hours and injected with glucose (2 g/kg body weight). Two-way ANOVA for glucose curves and Student’s t test for AUC. *p<0.05, **p<0.01. n=7 (control) and 12 (AdHk2KO). (E–F) Hyperinsulinemic-euglycemic clamp studies on control and AdHk2KO mice. Mice were fasted for 6 hours. Under insulin clamp, euglycemia was maintained (E) by manipulating glucose infusion rate (F). Bar graph shows average glucose infusion rate under euglycemia. Two-way ANOVA for glucose infusion rate curve and Student’s t test for average glucose infusion rate, **p<0.01, ****p<0.0001. n=6 (control) and 7 (AdHk2KO). See Figure 4—source data 1.
-
Figure 4—source data 1
Uncropped blots and source data for graphs for Figure 4.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig4-data1-v1.zip
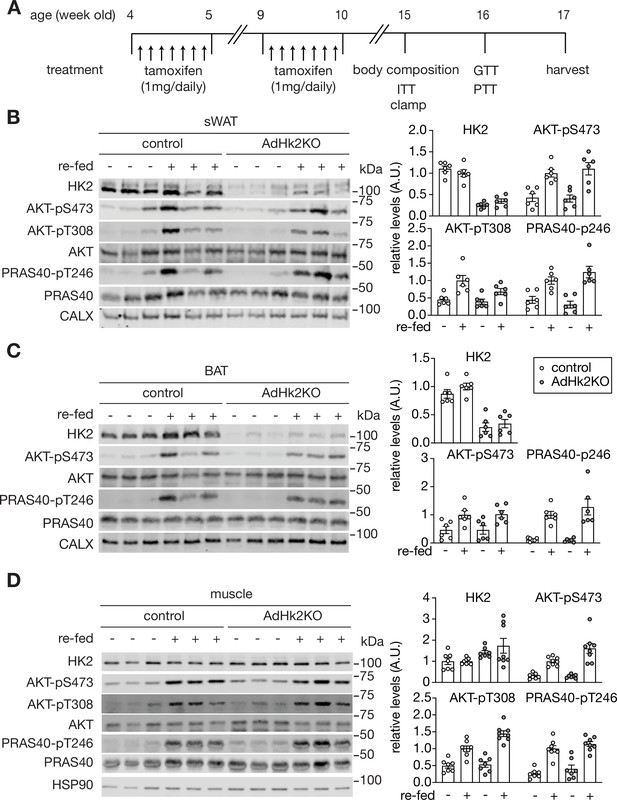
Supporting data 1 for Figure 4.
(A) Experimental design of tamoxifen treatment, body composition measurement, ITT, GTT, PTT, and clamp studies in control and AdHk2KO mice. Both control and AdHk2KO mice were treated with tamoxifen. (B) Immunoblot analyses of HK2 expression and insulin signaling in sWAT of control and adipose-specific Hk2 knockout (AdHk2KO) mice. Mice were fasted overnight or fasted overnight and re-fed for 3 hours. n=6. Source data Figure S3C. Quantification data is normalized to a loading control. (C) Immunoblot analyses of HK2 expression and insulin signaling in BAT of control and adipose-specific AdHk2KO mice. Mice were fasted overnight or fasted overnight and re-fed for 3 hours. n=6. Quantification data is normalized to a loading control. (D) Immunoblot analyses of HK2 expression and insulin signaling in skeletal muscle. of control and AdHk2KO mice. Mice were fasted overnight or fasted overnight and re-fed for 3 hours. n=7 (fasted control), 8 (refed control), 7 (fasted AdHk2KO), 8 (refed AdHk2KO). Quantification data is normalized to a loading control. See Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
Uncropped blots and source data for graphs for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig4-figsupp1-data1-v1.zip
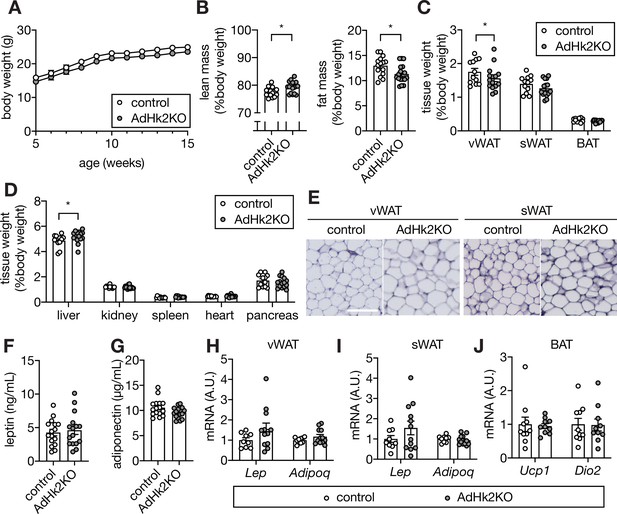
Supporting data 2 for Figure 4.
(A-B) Body weight curve (A), lean and fat mass (B) of control and AdHk2KO mice. Student’s t test, *p<0.05. n=15 (control) and 17 (AdHk2KO). (C–D) Organ weight for fat tissues (C) and non-fat tissues (D) of control and AdHk2KO mice. Student’s t test, *p<0.05. n=12 (control) and 15 (AdHk2KO). (E) Hematoxylin and eosin staining of vWAT (left) or sWAT (right) of control and AdHk2KO mice. n=4 (control) and 5 (AdHk2KO). Bar = 100 µm. (F–G) Plasma leptin (F) and adiponectin (G) levels of control and AdHk2KO mice. n=16. (H–I) mRNA levels of Lep and Adipoq in vWAT (H) or sWAT (I) from control and AdHk2KO mice. n=9 (control) and 12 (AdHk2KO). (J) mRNA levels of Ucp1 and Dio2 in BAT from control and AdHk2KO mice. n=10 (control) and 10 (AdHk2KO). See Figure 4—figure supplement 2—source data 1.
-
Figure 4—figure supplement 2—source data 1
Source images and source data for graphs for Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig4-figsupp2-data1-v1.zip
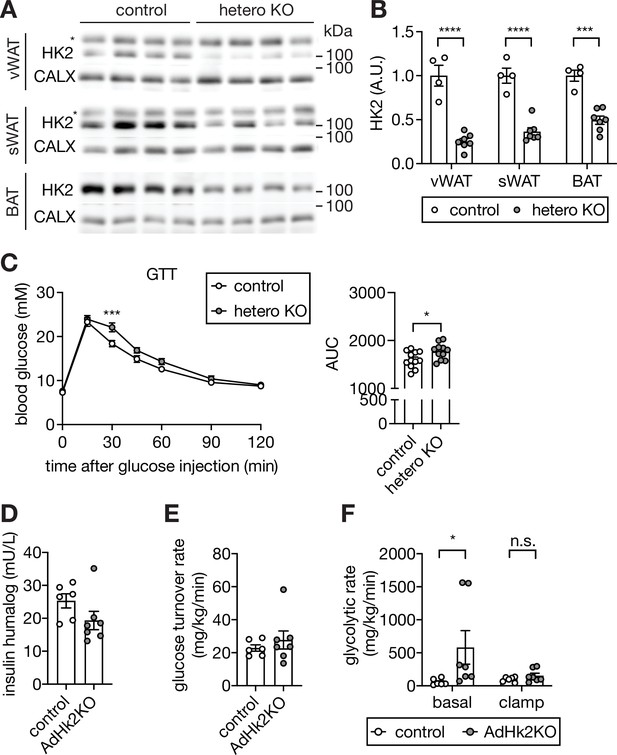
Supporting data 3 for figure 4.
(A-B) Immunoblot analyses of HK2 expression in vWAT, sWAT, and BAT of control and adipose-specific heterozygous Hk2 knockout (hetero KO) mice. n=4 (control) and 8 (hetero KO). Quantification of panel A (B). Data is normalized to a loading control. (C) Glucose tolerance test (GTT) on control and hetero KO mice. Mice were fasted for 6 hours and injected with glucose (2 g/kg body weight). Two-way ANOVA for glucose curves and Student’s t test for AUC. ***p<0.01. n=12 (control) and 11 (hetero KO). (D) The level of insulin humalog at the end of the clamp in control and AdHk2KO mice. (E) Glucose turnover rate during hyperinsulinemic-euglycemic clamp in control and. AdHk2KO mice. n=6 (control) and 7 (AdHk2KO). (F) Whole-body glycolytic rate at basal and during hyperinsulinemic-euglycemic clamp in control and AdHk2KO mice. One-way ANOVA, *p<0.05. n=6 (control) and 7 (AdHk2KO). See Figure 4—figure supplement 3—source data 1.
-
Figure 4—figure supplement 3—source data 1
Uncropped blots and source data for graphs for Figure 4—figure supplement 3.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig4-figsupp3-data1-v1.zip
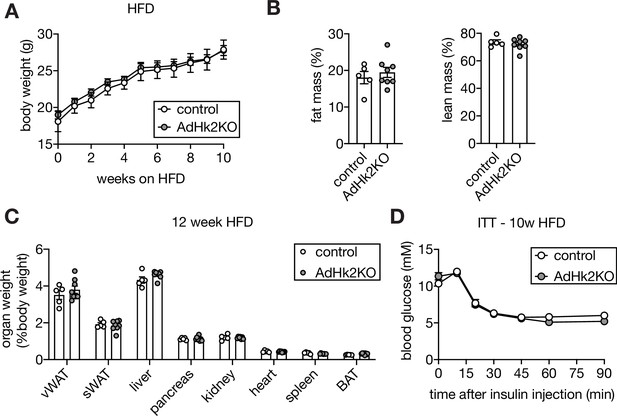
Supporting data 4 for figure 4.
(A-B) Body weight curve (A), lean and fat mass (B) of HFD-fed control and AdHk2KO mice. n=5 (control) and 8 (AdHk2KO). (C) Organ weights of HFD-fed control and AdHk2KO mice. n=5 (control) and 8 (AdHk2KO). (D) Insulin tolerance test (ITT) on HFD-fed control and AdHk2KO mice. The mice were fasted for 6 hours and injected with insulin (0.5 U/kg body weight). n=5 (control) and 8 (AdHk2KO). See Figure 4—figure supplement 4—source data 1.
-
Figure 4—figure supplement 4—source data 1
Source data for graphs for Figure 4—figure supplement 4.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig4-figsupp4-data1-v1.zip
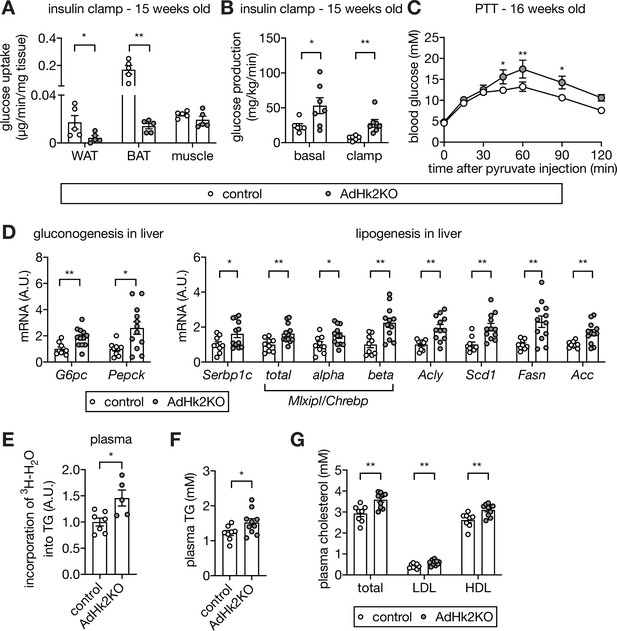
Loss of adipose HK2 causes reduced glucose disposal in adipose tissue and de-represses glucose production in liver.
(A) Glucose uptake measured at the end of hyperinsulinemic-euglycemic clamp. 2DG was injected 30 min prior to organ collections. The 2DG values were normalized by tissue mass used for the assay. Mann-Whitney test, *p<0.05, **p<0.01. n=5 (control) and 5 (AdHk2KO) mice. (B) Endogenous glucose production under basal and hyperinsulinemic-euglycemic clamp conditions. Mann-Whitney test, *p<0.05, **p<0.01. n=6 (control) and 7 (AdHk2KO) mice. (C) Pyruvate tolerance test (PTT) on control and AdHk2KO mice. Mice were fasted for 15 hours and injected with pyruvate (2 g/kg body weight). Two-way ANOVA, *p<0.05, **p<0.01. n=5 (control) and 6 (AdHk2KO). (D) mRNA levels of gluconeogenic genes (left) and lipogenic genes (rignt) in liver of control and AdHk2KO mice. Multiple t test, *p<0.05, **p<0.01. n=9 (control) and 12 (AdHk2KO). (E) De novo-synthesized plasma TG. Mice were treated with 3H-H2O and incorporation of 3H in plasma TG was measured. Student’s t test, *p<0.05. n=7 (control) and 5 (AdHk2KO). (F) Plasma triglyceride (TG) levels in control and AdHk2KO mice. Student’s t test, *p<0.05. n=8 (control) and 10 (AdHk2KO). (G) Plasma cholesterol, low density lipoprotein (LDL), or high density lipoprotein (HDL) levels in control and AdHk2KO mice. Multiple t test, **p<0.01. n=8 (control) and 10 (AdHk2KO). See Figure 5—source data 1.
-
Figure 5—source data 1
Source data for graphs for Figure 5.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig5-data1-v1.zip
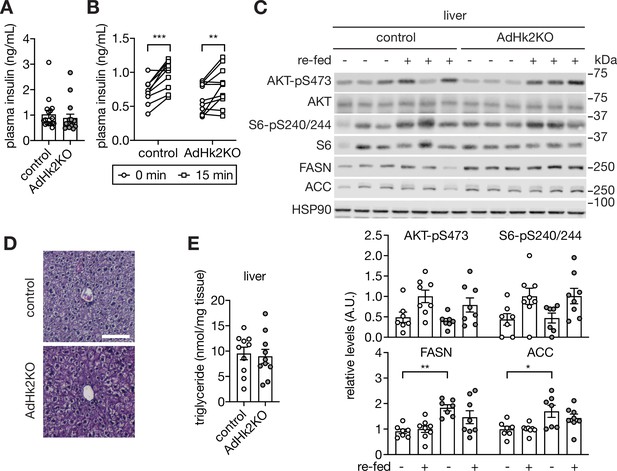
Supporting data 1 for figure 5.
(A) Plasma insulin levels of ad libitum fed control and AdHk2KO mice. n=16 (control) and 16 (AdHk2KO). (B) Plasma insulin levels of before (0 min) or after (15 min) glucose (2 g/kg body weight) injection in control and AdHk2KO mice fasted for 6 hours. Two-way ANOVA, **p<0.01, ***p<0.001. n=10 (cotnrol) and 11 (AdHk2KO). (C) Immunoblot analyses of insulin signaling and lipogenic enzymes in liver of control and AdHk2KO mice. Mice were fasted overnight or fasted overnight and refed for 3 hours. Quantification of panel A. n=7 (fasted control), 8 (refed control), 7 (fasted AdHk2KO), 8(refed AdHk2KO). (D) Hematoxylin and eosin staining of liver of control and AdHk2KO mice. n=4 (control) and 5 (AdHk2KO). Bar = 100 µm. (E) Hepatic triglyceride levels of control and AdHk2KO mice. n=10 (control) and 10 (AdHk2KO). See Figure 5—source data 1.
-
Figure 5—figure supplement 1—source data 1
Uncropped immunoblots, source images and source data for graphs for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig5-figsupp1-data1-v1.zip
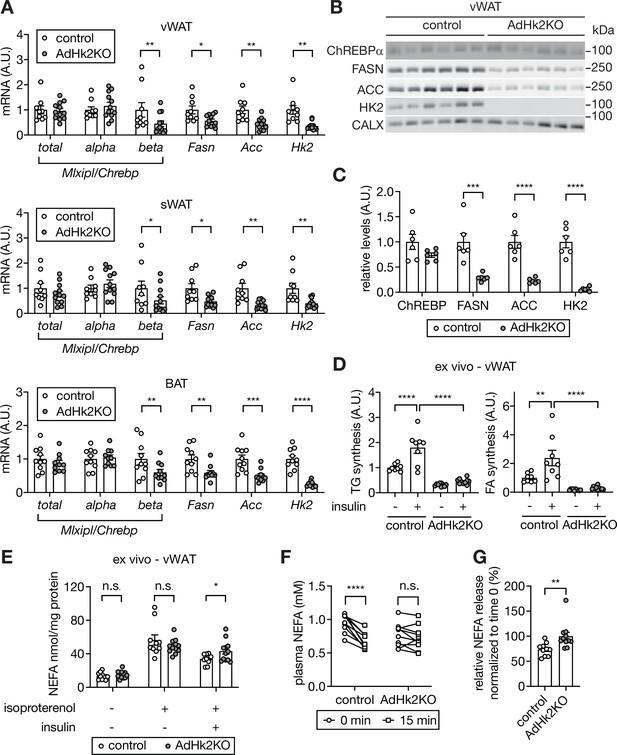
Decreased lipogenesis and enhanced fatty acid release in adipose tissue in AdHk2KO mice.
(A) mRNA levels of fatty acid synthesis genes in vWAT (top), sWAT(middle), and BAT (bottom) from control and AdHk2KO mice. Multiple t test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n=9 (control) and 12 (AdHk2KO) for vWAT and sWAT. n=10 (control) and 10 (AdHk2KO) for BAT. (B) Immunoblot analyses of fatty acid synthesis enzymes in vWAT of control and Adk2KO mice. n=6. (C) Quantification of panel B. Data is normalized to loading controls. Student’s t test. **p<0.01. ****p<0.0001. (D) De novo lipogenesis of vWAT explants of control and AdHk2KO mice. vWAT explants were treated with 3H-H2O in the absence or presence of 100 nM insulin for 1 hour. Two-way ANOVA, **p<0.01, ****p<0.0001. n=8 (control) and 10 (AdHk2KO). (E) Non-esterified fatty acid (NEFA) release of vWAT explants of control and AdHk2KO mice. vWAT explants were treated with or without 10 µM isoproterenol in the absence or presence of 100 nM insulin. Multiple t test, *p<0.05. n.s., not significant. n=10 (control) and 12 (AdHk2KO). (F) Plasma NEFA levels in control and AdHk2KO mice before (0 min) and after (15 min) glucose injection. Mice were fasted for 6 hours and injected with glucose (2 g/kg body weight). Two-way ANOVA, **p<0.001. n.s., not significant. n=10 (control) and 11 (AdHk2KO). (G) Plasma NEFA levels in control and AdHk2KO mice after (15 min) glucose injection was normalized by plasma NEFA levels at 0 min (before glucose injection) in E. Student’s t test, **p<0.01. n=10 (control) and 11 (AdHk2KO). See Figure 6—source data 1.
-
Figure 6—source data 1
Uncropped blots and source data for Figure 6.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig6-data1-v1.zip
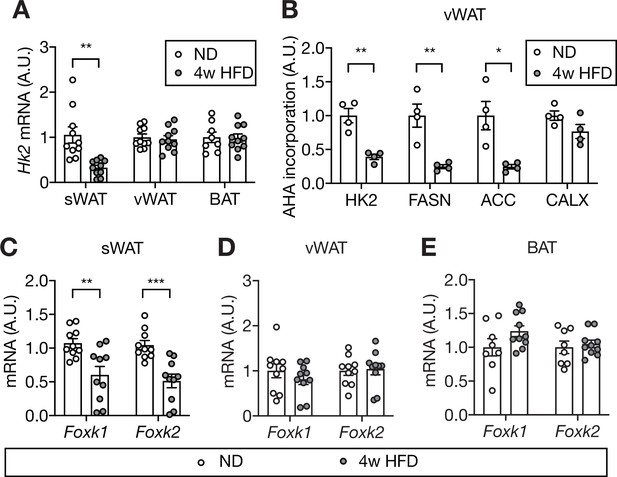
Mechanism of HFD-induced HK2 down-regulation in adipose tissue.
(A) Hk2 mRNA levels in sWAT, vWAT, and BAT from ND- and 4 week HFD-fed mice. Student’s t test, **p<0.01. n=10 (sWAT from ND and HFD), 10 (vWAT from ND and HFD), 8 (BAT from ND), 10 (BAT from HFD). (B) Nascent polypeptides. vWATs isolated from ND- or 4 week HFD-fed mice were labled with L-azidohomoalanine (AHA), and AHA-incorporated polypeptides were measured by mass spectrometer. FASN and ACC serve as positive controls and CALX serves as a negative control. Multiple t test, *p<0.05, **p<0.01. n=4 (ND) and 4 (HFD). (C) Foxk1 and Foxk2 mRNA levels in sWAT from ND- or 4 week HFD-fed mice. Multiple t test, **p<0.01, ***p<0.001. n=10 (ND) and 10 (HFD). (D) Foxk1 and Foxk2 mRNA levels in vWAT of ND- or 4 week HFD-fed wild-type C57BL6JRj mice. No significant difference in multiple t test. n=10 (ND) and 10 (HFD). (E) Foxk1 and Foxk2 mRNA levels in BAT of ND- or 4 week HFD-fed wild-type C57BL6JRj mice. No significant difference in multiple t test. n=8 (ND) and 10 (HFD). See Figure 7—source data 1.
-
Figure 7—source data 1
Source data for graphs for Figure 7.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig7-data1-v1.zip
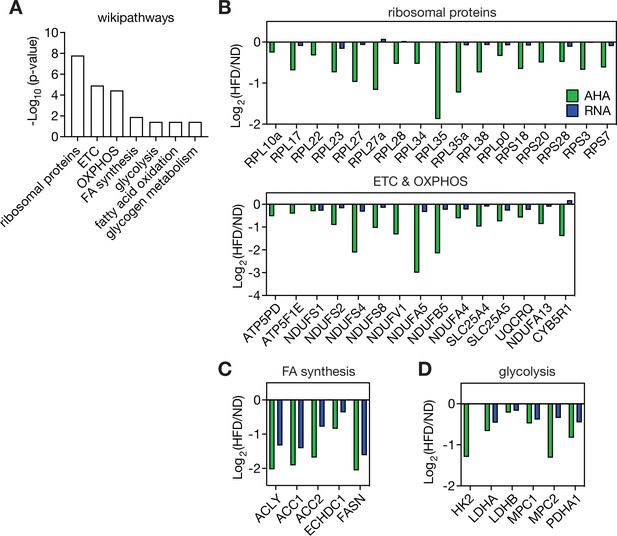
Transcriptionally and post-transcriptionally de-regulated proteins in vWAT of HFD-fed mice.
(A) Wikipathway analyses for proteins whose L-Azidohomoalanine (AHA) incorporation is positively correlated with that of HK2 (r>0.8). n=4. (B) Post-transcriptionally de-regulated proteins in vWAT of HFD-fed mice. ETC, electron transport chain. OXPHOS, oxidative phosphorylation. (C) Transcriptionally regulated proteins in vWAT of HFD-fed mice. FA, fatty acid. (D) AHA-incorporation of glycolytic proteins and their mRNA levels in vWAT of HFD-fed mice. See Figure 7—figure supplement 1—source data 1.
-
Figure 7—figure supplement 1—source data 1
Source data for graphs for Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/85103/elife-85103-fig7-figsupp1-data1-v1.zip
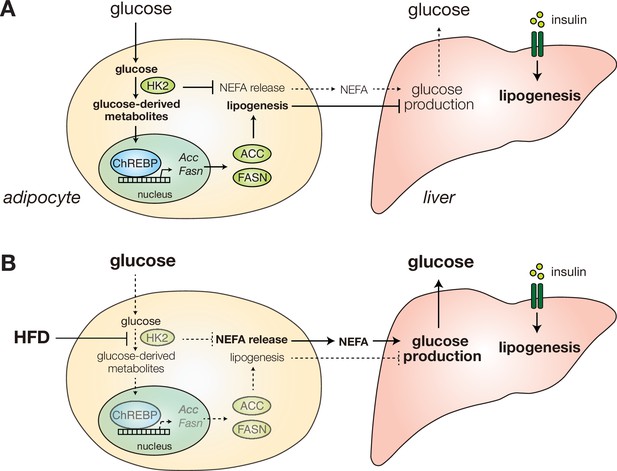
Diet-induced HK2 in adipose tissue promotes glucose intolerance.
(A) HK2 promotes lipogenesis and suppresses NEFA release in adipose tissue (left), suppressing hepatic glucose production (right) and thus maintaining glucose homeostasis. (B) Diet-induced loss of adipose HK2 triggers glucose intolerance via reduced glucose disposal in adipocytes (left) and de-repressed hepatic gluconeogenesis despite maintained lipogenesis (right).
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85103/elife-85103-mdarchecklist1-v1.pdf
-
Supplementary file 1
vWAT proteomics data from 4w HFD-fed mice, compared to ND-fed mice.
- https://cdn.elifesciences.org/articles/85103/elife-85103-supp1-v1.xlsx
-
Supplementary file 2
Patients' data.
- https://cdn.elifesciences.org/articles/85103/elife-85103-supp2-v1.xlsx
-
Supplementary file 3
AHA data and RNAseq data in vWAT from 4w HFD-fed mice, compared to ND-fed mice.
- https://cdn.elifesciences.org/articles/85103/elife-85103-supp3-v1.xlsx