The large GTPase Sey1/atlastin mediates lipid droplet- and FadL-dependent intracellular fatty acid metabolism of Legionella pneumophila
Figures
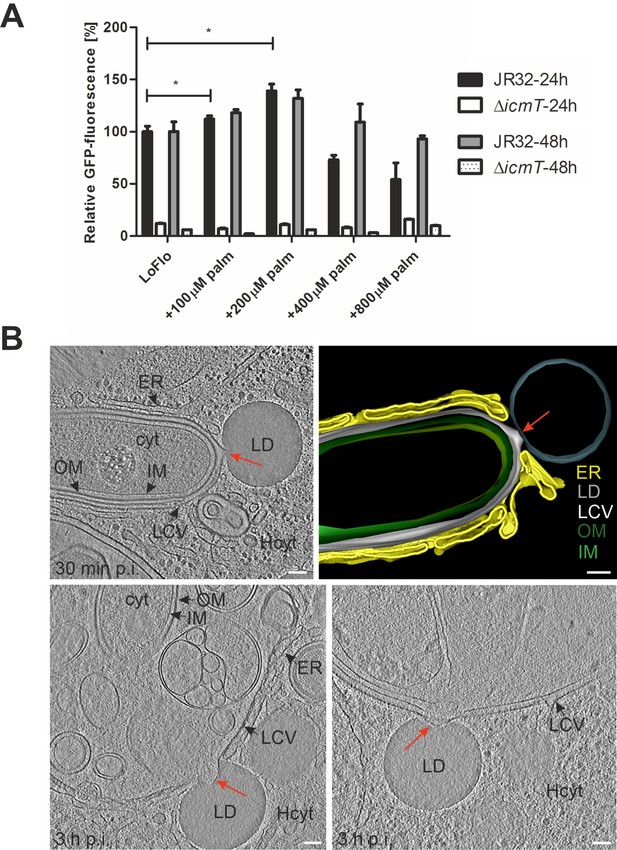
Palmitate-induced lipid droplets interact with LCVs in D. discoideum.
(A) D. discoideum Ax3, untreated (LoFlo medium) or treated with increasing concentrations of sodium palmitate (100–800 µM, 3 hr), were infected (MOI 10) with GFP-producing L. pneumophila wild-type JR32 or ΔicmT (pNT28). The GFP-fluorescence was measured with a microtiter plate reader at 1 hr, 24 hr, and 48 hr p.i. Data show the relative fluorescence increase between 1 hr and 24 hr or 48 hr p.i. (JR32: black/grey bar; ΔicmT: white/dotted bar). Data represent means ± SD of three independent experiments (*p<0.05). (B) Representative cryotomograms of D. discoideum Ax3, fed (3 hr) with 200 µM sodium palmitate and infected (MOI 100) with L. pneumophila JR32 for 30 min (top) or 3 hr (bottom). Intimate LCV-LD interactions are clearly visible (red arrows). Reconstruction of LCV-LD interaction observed at 30 min p.i. (top; right). OM, outer membrane; IM, inner membrane; LCV, Legionella-containing vacuole (limiting membrane); ER, endoplasmic reticulum; LD, lipid droplet; cyt, L. pneumophila cytoplasm; Hcyt, host cell cytoplasm. Scale bars: 100 nm.

Cytotoxicity of palmitate.
(A) D. discoideum Ax3 or Δsey1, untreated, treated with 70% ethanol (EtOH, 1 hr) or with increasing concentrations of sodium palmitate (100–800 µM, overnight), were stained with Zombie Aqua dye, fixed with PFA and analyzed by flow cytometry to assess cell viability (nevents = 10,000). Data represent means ± SD of three independent experiments (*p<0.05; ***p<0.001).
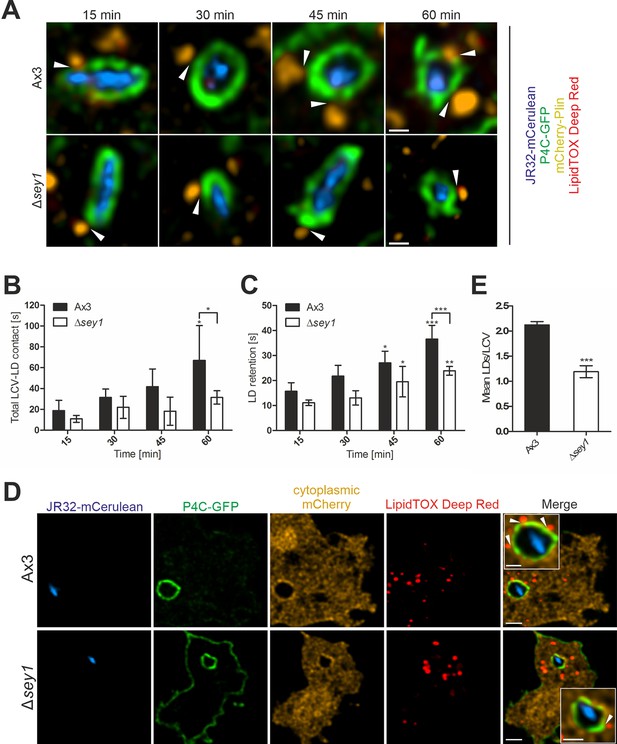
Sey1 promotes LDs recruitment to intact LCVs in D. discoideum.
(A) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila JR32 (pNP99). Infected cells were recorded for 60 s each at the times indicated. Examples are shown for contact between LDs and the LCV membrane (white arrowheads). Scale bars: 0.5 µm. (B) Quantification of (A), total contact time of LDs with the LCV recorded for 60 s at the indicated time points p.i. (nLCV-LD contacts > 30). Data represent means ± SD of three independent experiments (*p<0.05). (C) Quantification of (A), retention time of single LDs with the LCV recorded for 60 s at the indicated time points p.i. (nLCV-LD contacts > 30). Data represent means ± SD of three independent experiments (*p<0.05, **p<0.01; ***p<0.001). (D) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and cytosolic mCherry (pDM1042), fed overnight with 200 µM sodium palmitate and infected (MOI 10, 1 hr) with mCerulean-producing L. pneumophila JR32 (pNP99), fixed with PFA and stained with LipidTOX Deep Red. Examples are shown for contact between LDs and the LCV membrane (white arrowheads). Scale bars: overview (2 µm), inset (1 µm). (E) Quantification of (D), mean number of LDs contacting a single LCV (nLCVs >102). Data represent means ± SD of three independent experiments (***p<0.001).
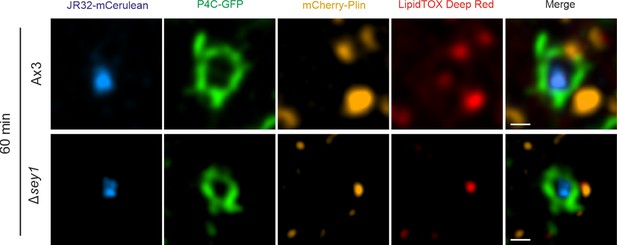
Sey1 promotes LD recruitment to LCVs in D. discoideum.
Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila JR32 (pNP99). Infected cells were recorded for 60 s each at the time indicated. Single fluorescence channels of the 60 min p.i. recordings (Figure 2A) are shown. Scale bars: 0.5 µm.
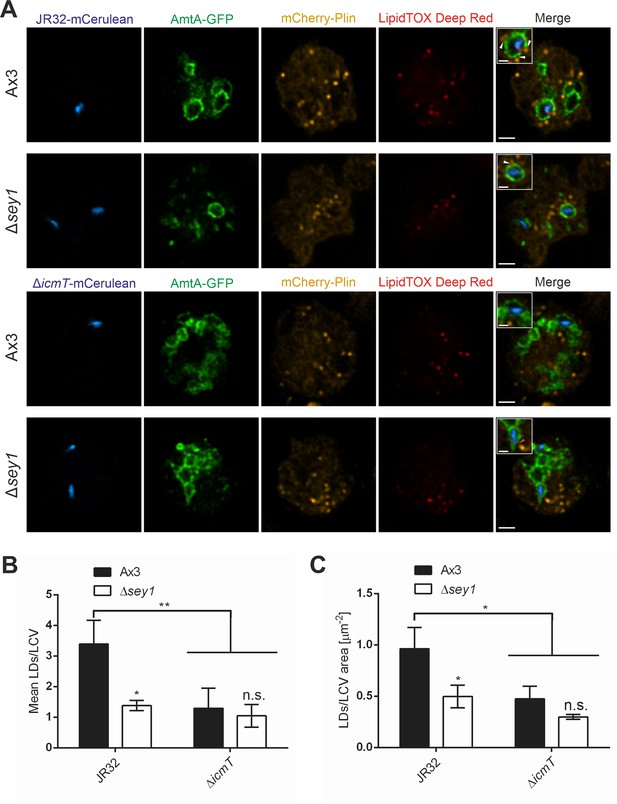
The L. pneumophila T4SS promotes Sey1-dependent LCV-LD interactions.
(A) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing AmtA-GFP (pHK121) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate and infected (MOI 10, 1 hr) with mCerulean-producing L. pneumophila JR32 (top) or ΔicmT (bottom) (pNP99), fixed with PFA and stained with LipidTOX Deep Red. Examples are shown for contact between LDs and the LCV membrane (white arrowheads) or no contact (red arrowhead). Scale bars: overview (2 µm), inset (1 µm). (B) Quantification of (A), mean number of LDs contacting a single LCV (left) and ratio of LD number contacting one LCV divided by the LCV area (right) (nLCVs = 30). Data represent means ± SD of three independent experiments. (n.s., not significant; *p<0.05; **p<0.01).
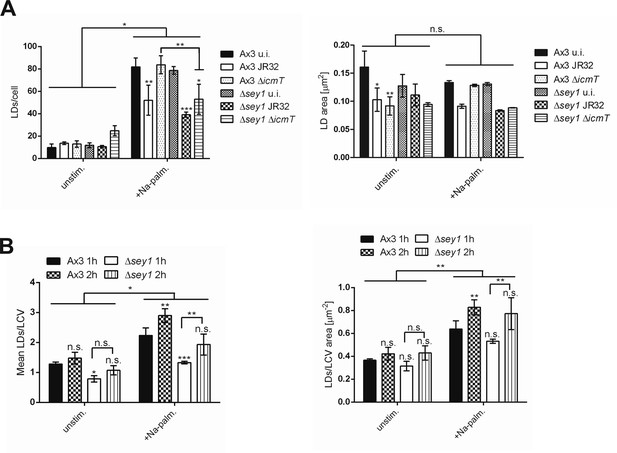
Palmitate promotes lipid droplet biogenesis.
(A) Quantification of LD number (left) (ncells >30) or LD area (right) (nLDs >250) in unstimulated or sodium palmitate-stimulated (200 µM, overnight) D. discoideum Ax3 or Δsey1 producing AmtA-GFP (pHK121) and mCherry-Plin (pHK102), uninfected or infected with mCerulean-producing L. pneumophila JR32 or ΔicmT (pNP99), fixed with PFA and stained with LipidTOX Deep Red. Data represent means ± SD of three independent experiments (n.s., not significant; *p<0.05; **p<0.01; ***p<0.001). (B) Quantification of mean number of LDs contacting a single LCV (left) (nLCVs >80) or ratio of LD number contacting one LCV divided by the LCV area (right) (nLCVs >80). Analysis was performed in unstimulated or sodium palmitate-stimulated (200 µM, overnight) D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), infected (MOI 10, 1 hr or 2 hr) with mCerulean-producing L. pneumophila JR32 (pNP99), fixed with PFA and stained with LipidTOX Deep Red. Data represent means ± SD of three independent experiments (n.s., not significant; *p<0.05; **p<0.01; ***p<0.001).
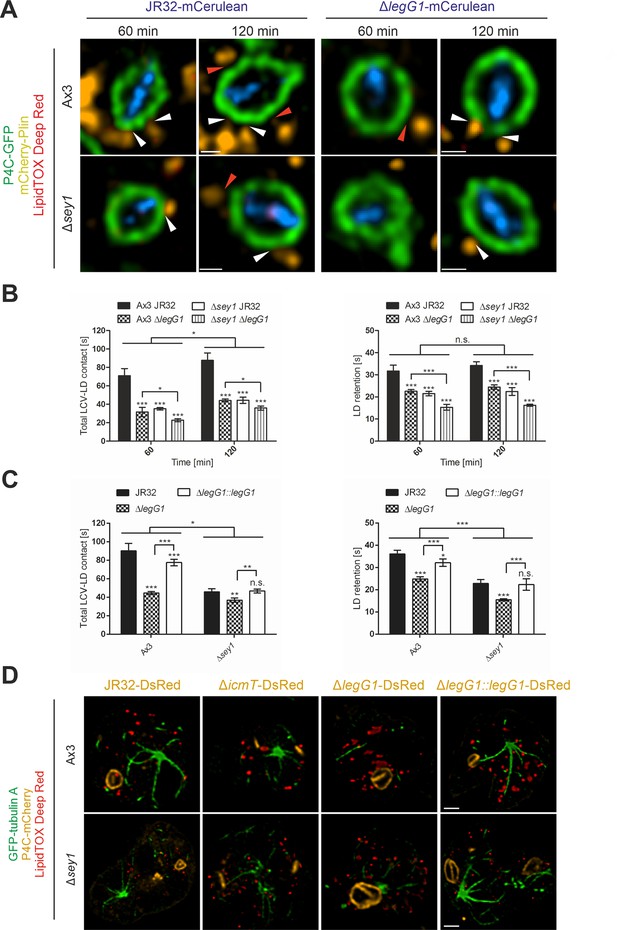
L. pneumophila LegG1 promotes Sey1-dependent LCV-LD interactions.
(A) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with L. pneumophila JR32 or ΔlegG1 producing mCerulean (pNP99). Infected cells were recorded for 60 s each at the times indicated. Examples are shown for contact between LDs and the LCV membrane (white arrowheads) or no contact (red arrowheads). Scale bars: 0.5 µm. (B) Quantification of (A), total contact time of LDs with the LCV (left) or retention time of single LDs with the LCV (right) recorded for 60 s at the indicated time points p.i. (nLCV-LD contacts > 30). Data represent means ± SD of three independent experiments (n.s., not significant; *p<0.05; ***p<0.001). (C) Quantification of total contact time of LDs with the LCV (left) or retention time of single LDs with the LCV (right) recorded for 60 s at 120 min p.i. (nLCV-LD contacts > 30). D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with L. pneumophila JR32 or ΔlegG1 producing DsRed (pSW001), or ΔlegG1 producing DsRed and M45-LegG1 (pER005; ΔlegG1::legG1) were analysed. Data represent means ± SD of three independent experiments (n.s., not significant; *p<0.05; **p<0.01; ***p<0.001). (D) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing GFP-tubulin A (pLS110) and P4C-mCherry (pWS032), fed overnight with 200 µM sodium palmitate and infected (MOI 10, 1 hr) with L. pneumophila JR32, ΔicmT or ΔlegG1 producing DsRed (pSW001), or ΔlegG1 producing DsRed and M45-LegG1 (pER005; ΔlegG1::legG1), fixed with PFA and stained with LipidTOX Deep Red. Scale bars: 2 µm.
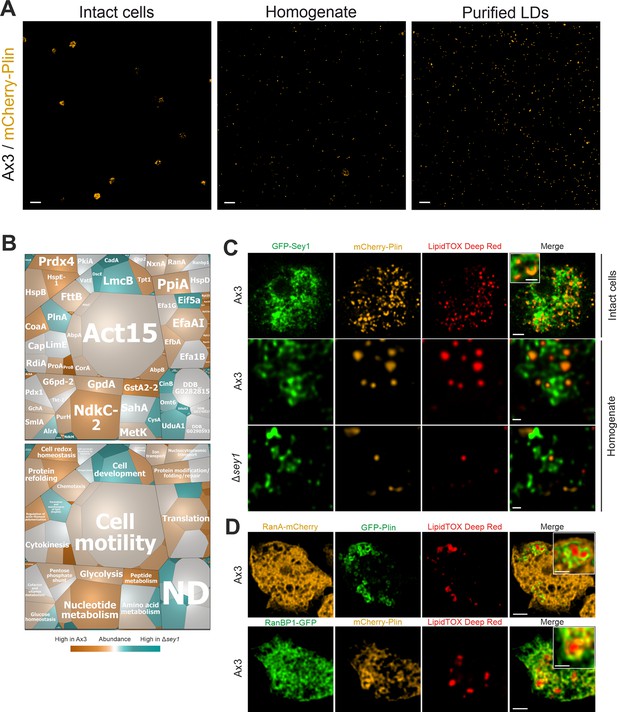
Purification of LDs, proteomics and localization of Sey1, RanA, and RanBP1.
(A) Qualitative analysis of LDs purification. Representative fluorescence micrographs of intact D. discoideum Ax3 producing mCherry-Plin (pHK102), cell homogenate or purified LDs. Scale bars: 10 µm. (B) Voronoi treemaps displaying abundance of proteins on isolated LDs from D. discoideum Ax3 and Δsey1. The treemaps were generated by taking into account proteins exclusively identified on LDs from Ax3 or Δsey1, or proteins showing significant difference in abundance between the two samples. Additionally, the 50 most abundant proteins in each sample were also included. The mosaic tiles are represented as (top) single proteins or (bottom) grouped in functional domains, which in turn are assembled according to general function (black frames). Individual iBAQ values of proteins on D. discoideum Ax3 LDs are represented by the area of the mosaic tiles (large tiles: more abundant, small tiles: less abundant). Comparison of abundances of proteins on Ax3 LDs with Δsey1 LDs is represented by the color gradient of the mosaic tiles (orange: more abundant on Ax3 LDs, grey: equally abundant on Ax3 and Δsey1 LDs, blue: more abundant on Δsey1 LDs). ND, not defined. Representative data for three independent experiments. (C) Representative fluorescence micrographs of intact or homogenized D. discoideum Ax3 or Δsey1 producing GFP-Sey1 (pBS001) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate and stained with LipidTOX Deep Red. Scale bars: intact cells (2 µm, inset: 1 µm), homogenate (0.5 µm). (D) Representative fluorescence micrographs of D. discoideum Ax3 producing RanA-mCherry (pLS221) and GFP-Plin (pHK101) (top) or RanBP1-GFP (pLS222) and mCherry-Plin (pHK102) (bottom), fed overnight with 200 µM sodium palmitate and stained with LipidTOX Deep Red. Scale bars: overview (2 µm), inset (1 µm).
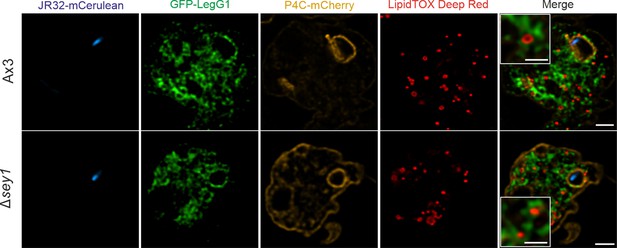
Cellular localization of GFP-LegG1.
Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing GFP-LegG1 (pLS117) and P4C-mCherry (pWS032), fed overnight with 200 µM sodium palmitate and infected (MOI 5, 2 hr) with mCerulean-producing L. pneumophila JR32 (pNP99), fixed with PFA and stained with LipidTOX Deep Red. Scale bars: overview (2 µm), inset (1 µm).
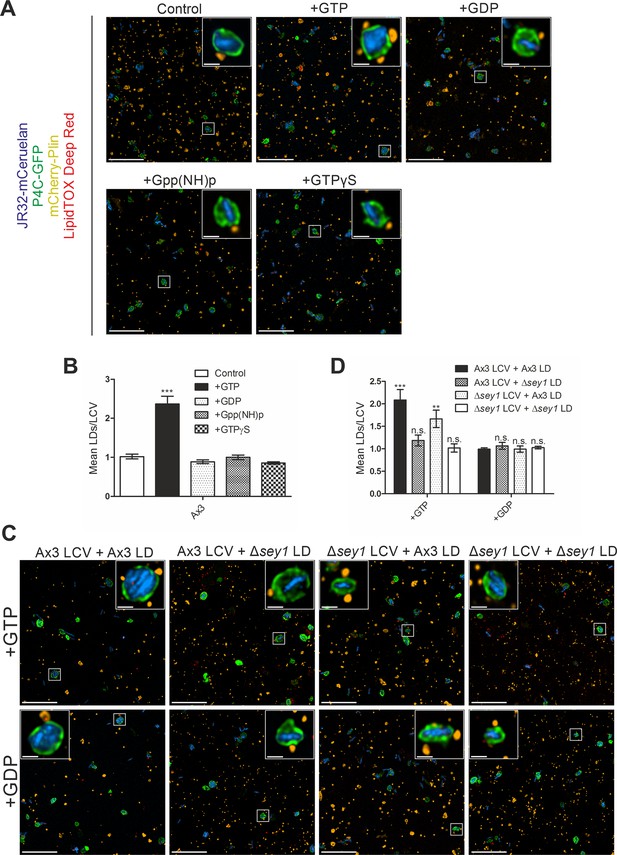
Sey1 and GTP promote LCV-LD interactions in vitro.
(A) Representative fluorescence micrographs of LCVs isolated from D. discoideum Ax3 producing P4C-GFP (pWS034), infected (MOI 50, 1 hr) with mCerulean-producing L. pneumophila JR32 (pNP99) and mixed with LDs from D. discoideum Ax3 producing mCherry-Plin (pHK102) fed overnight with 200 µM sodium palmitate. LCVs and LDs were co-incubated (1 hr, 30 °C) in presence of 5 mM MgCl2 and 5 mM GTP, GDP, Gpp(NH)p or GTPγS and fixed with PFA prior to imaging. Scale bars: overview (10 µm), inset (1 µm). (B) Quantification of (A), mean number of LDs contacting a single LCV in vitro (nLCVs = 150). Data represent means ± SD of three independent experiments (***p<0.001). (C) Representative fluorescence micrographs of LCVs isolated from D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034), infected (MOI 50, 1 hr) with mCerulean-producing L. pneumophila JR32 (pNP99) and mixed with LDs from D. discoideum Ax3 or Δsey1 producing mCherry-Plin (pHK102) fed overnight with 200 µM sodium palmitate. LCVs and LDs were co-incubated (1 hr, 30 °C) in presence of 5 mM MgCl2 and 5 mM GTP (top) or GDP (bottom) and fixed with PFA prior to imaging. Scale bars: overview (10 µm), inset (1 µm). (D) Quantification of (C), mean number of LDs contacting a single LCV in vitro (nLCVs = 150). Data represent means ± SD of three independent experiments (n.s., not significant; **p<0.01; ***p<0.001).
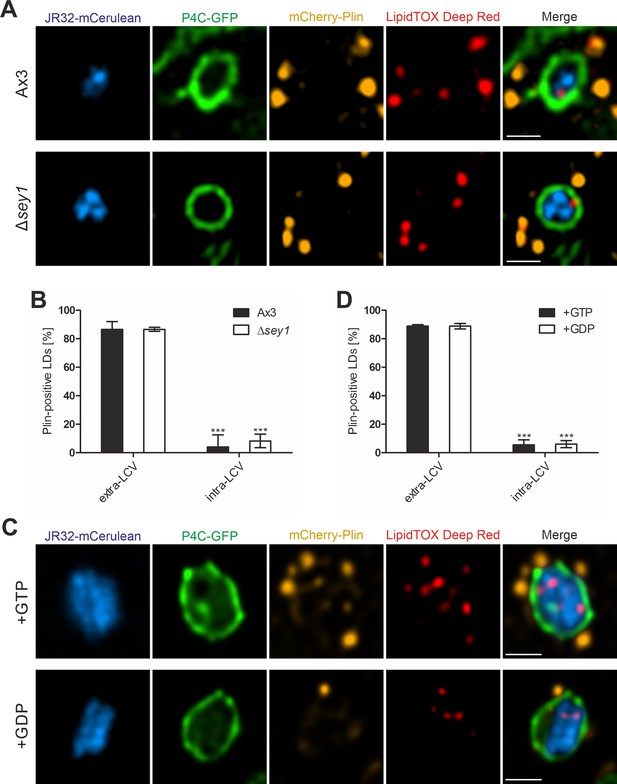
LDs shed perilipin upon LCV membrane crossing independently of Sey1 and GTP.
(A) Representative fluorescence micrographs of D. discoideum Ax3 or Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5, 1 hr) with mCerulean-producing L. pneumophila JR32 (pNP99). Scale bars: 1 µm. (B) Quantification of (A), percentage of extravacuolar and intravacuolar LDs staining positive for mCherry-Plin (nLDs >24). Data represent means ± SD of three independent experiments (***p<0.001). (C) Representative fluorescence micrographs of LCVs isolated from D. discoideum Ax3 producing P4C-GFP (pWS034), infected (MOI 50, 1 hr) with mCerulean-producing L. pneumophila JR32 (pNP99) and mixed with LDs from D. discoideum Ax3 producing mCherry-Plin (pHK102) fed overnight with 200 µM sodium palmitate. LCVs and LDs were co-incubated (1 hr, 30 °C) in presence of 5 mM MgCl2 and 5 mM GTP or GDP and fixed with PFA prior to imaging. Scale bars: 1 µm. (D) Quantification of (C) percentage of extravacuolar and intravacuolar LDs staining positive for mCherry-Plin (nLDs = 180). Data represent means ± SD of three independent experiments (***p<0.001).

Sey1 and L. pneumophila FadL promote intracellular replication and 13C16-palmitate catabolism.
(A) D. discoideum Ax3 amoeba were infected (MOI 1) with L. pneumophila JR32, ΔicmT, ΔfadL or ΔfadL::fadL (chromosomal integration of fadL in ΔfadL), and intracellular bacterial replication was assessed by colony forming units (CFU) for 6 days. Data represent means ± SD of three independent experiments in technical triplicates (***p<0.001). (B) D. discoideum Ax3 or ∆sey1 were left untreated (empty symbols) or fed overnight with 200 µM sodium palmitate (filled symbols) and infected (MOI 1) with GFP-producing L. pneumophila JR32 or ΔfadL (pNT28) for 6 days. Intracellular bacterial replication was assessed by quantification of relative fluorescence units (RFU). Data represent means ± SD of three independent experiments in technical hextuplicates (**p<0.01; ***p<0.001). (C) D. discoideum Ax3 or Δsey1 producing calnexin-GFP (CnxA-GFP, pAW016) were infected (MOI 50, 1 hr) with mCerulean-producing L. pneumophila JR32 or ΔfadL (pNP99) and washed to remove extracellular bacteria. At 5 hr p.i., 200 µM [U-13C16]palmitate was added to the infected amoeba for 10 hr. The infected cells were lysed and centrifuged to separate bacteria from cell debris. 13C-excess (mol%) in key metabolites of the L. pneumophila fraction (‘fraction 2’) was analyzed by GC/tandem MS. Data show means ± SD of technical triplicates (*p<0.05; ***p<0.001) and are representative for two independent experiments.

Alignment of FadL homologues.
FadL homologues of E. coli (E. co.), L. pneumophila (L. pn.), V. cholerae (V. ch.), and P. aeruginosa (P. ae.) were aligned using the ClustalW algorithm available in the Clustal Omega web browser. The symbols underneath the alignment indicate the degree of conservation: identical residues (*), highly similar residues (:), similar residues (.). The red box denotes the strictly conserved NPA motif in the “hatch” domain (amino acids 73–75 in L. pneumophila FadL, Lpg1810).
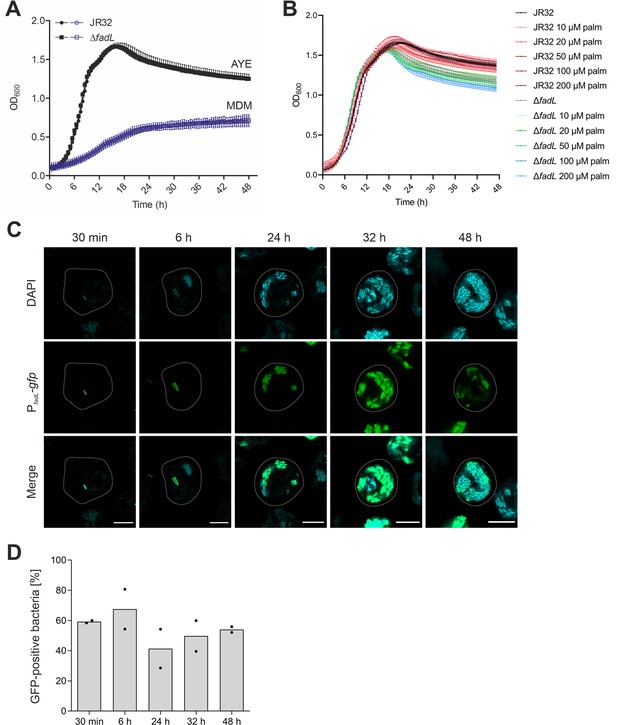
Growth of L. pneumophila ΔfadL in medium and expression of fadL in D. discoideum.
Growth of L. pneumophila JR32 or ΔfadL at 37 °C (A) in AYE medium (black) or MDM (violet), or (B) in AYE medium supplemented with the palmitate concentrations indicated. The OD600 was monitored for 48 hr in time intervals of 30 min using a microplate reader. Data represent means ± SD of three independent experiments. (C) Confocal microscopy of D. discoideum infected (MOI 5) for the time indicated with L. pneumophila JR32 harboring PfadL-gfp (pPS003). The cells were fixed in 4% PFA, permeabilized with ice-cold methanol, and stained with 1 µg/ml DAPI. Images shown are representative of two independent experiments. Scale bars: 5 μm. (D) Quantification of GFP-positive L. pneumophila JR32 (pPS003) growing in D. discoideum. At the time p.i. indicated, host cells were lysed with 0.1% Triton X-100, fixed in 4% PFA, stained with 1 µg/ml DAPI and analyzed by flow cytometry and the software FlowJo. Plotted are means and individual data points of two independent experiments.
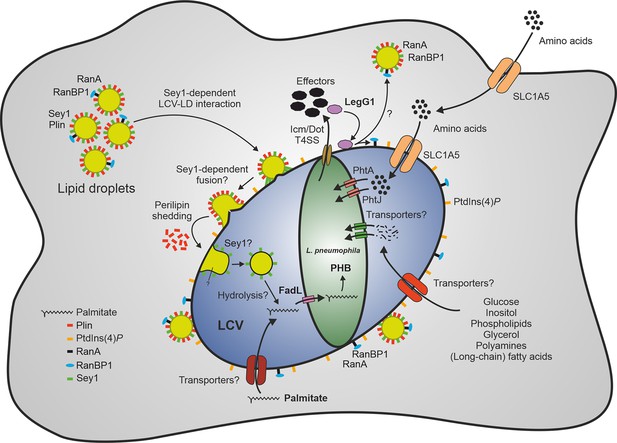
Sey1-, LegG1- and FadL-dependent catabolism of palmitate by L. pneumophila through lipid droplets.
The large fusion GTPase Sey1 and GTP as well as the L. pneumophila RCC1 repeat effector LegG1 promote the recruitment of host LDs to LCVs. LegG1 activates the small GTPase RanA, leading to accumulation of RanBP1 and microtubule stabilization. Upon intimate contact of LDs with LCVs, perilipin is shed, and LDs cross the membrane to reach the LCV lumen, where LD constituents (e.g. triacylglycerols) are likely hydrolyzed, and free fatty acids are taken up by L. pneumophila. Palmitate is transported by FadL inside the bacteria and metabolized to acetyl-CoA, which is further aerobically catabolized in the tricarboxylic acid cycle to CO2 and/or anabolized to the storage compound polyhydroxybutyrate (PHB). Amino acids are transported by the host transporter SLC1A5 and the L. pneumophila transporters PhtA/PhtJ. Additional trans-membrane transporters for lipids (long-chain fatty acids, phospholipids), sugars (glucose, inositol), alcohols (glycerol), polyamines, and amino acids are likely present in the plasma membrane, LCV membrane and L. pneumophila membrane. LD, lipid droplet; LCV, Legionella-containing vacuole; PHB, polyhydroxybutyrate; Plin, perilipin; RanBP1, Ran-binding protein 1.
Videos
D. discoideum Ax3 infected with L. pneumophila JR32.
Representative movie of D. discoideum Ax3 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila JR32 (pNP99). Infected cells were recorded for 60 s each at the times indicated.
D. discoideum Ax3 infected with L. pneumophila ΔlegG1.
Representative movie of D. discoideum Ax3 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila ΔlegG1 (pNP99). Infected cells were recorded for 60 s each at the times indicated.
D. discoideum Δsey1 infected with L. pneumophila JR32.
Representative movie of D. discoideum Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila JR32 (pNP99). Infected cells were recorded for 60 s each at the times indicated.
D. discoideum Δsey1 infected with L. pneumophila ΔlegG1.
Representative movie of D. discoideum Δsey1 producing P4C-GFP (pWS034) and mCherry-Plin (pHK102), fed overnight with 200 µM sodium palmitate, stained with LipidTOX Deep Red and infected (MOI 5) with mCerulean-producing L. pneumophila ΔlegG1 (pNP99). Infected cells were recorded for 60 s each at the times indicated.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Legionella pneumophila Philadelphia-1) | lpg1810/fadL | GenBank | AE017354.1 | |
| Strain, strain background (Legionella pneumophila) | ER01 (ΔlegG1) | Rothmeier et al., 2013 | JR32 legG1::KanR | |
| Strain, strain background (Legionella pneumophila) | GS3011 (ΔicmT) | Segal and Shuman, 1998 | JR32 icmT3011::KanR | |
| Strain, strain background (Legionella pneumophila) | JR32 | Sadosky et al., 1993 | Derivative of wild-type Legionella pneumophila strain Philadelphia-1 | |
| Strain, strain background (Legionella pneumophila) | PS01 (ΔfadL) | This study | JR32 fadL::KanR | |
| Strain, strain background (Escherichia coli) | TOP10 | Invitrogen, Thermo Fisher Scientific | ||
| Strain, strain background (Dictyostelium discoideum) | Ax3 | Loovers et al., 2007 | Parental strain | |
| Strain, strain background (Dictyostelium discoideum) | Δsey1 | Hüsler et al., 2021 | Ax3, insertion in gene DDB_G0279823, BlsR | |
| Antibody | anti-SidC (rabbit polyclonal) | Weber et al., 2006 | 1:3000 | |
| Antibody | anti-rabbit IgG MACS micro-beads (goat polyclonal) | Miltenyi Biotec | Cat# 130-048-602 | 20 µl magnetic bead slurry per 0.5 ml concentrated cell homogenate |
| Chemical compound, drug | LipidTOX Deep Red | Invitrogen, Thermo Fisher Scientific | Cat# H34477 | 1:200 – 1:1000 |
Additional files
-
Supplementary file 1
Comparative proteomics analysis of LDs from D. discoideum Ax3 or Δsey1 mutant amoeba.
Dark blue: exclusively present on Δsey1 LDs, light blue: more abundant on Δsey1 LDs, grey: equally abundant on Ax3 and Δsey1 LDs, light orange: more abundant on Ax3 LDs, dark orange: exclusively present on Ax3 LDs.
- https://cdn.elifesciences.org/articles/85142/elife-85142-supp1-v2.xlsx
-
Supplementary file 2
Cells, bacterial strains, and plasmids used in this study.
- https://cdn.elifesciences.org/articles/85142/elife-85142-supp2-v2.docx
-
Supplementary file 3
Oligonucleotides used in this study.
- https://cdn.elifesciences.org/articles/85142/elife-85142-supp3-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85142/elife-85142-mdarchecklist1-v2.docx





