Unconventional secretion of α-synuclein mediated by palmitoylated DNAJC5 oligomers
Figures
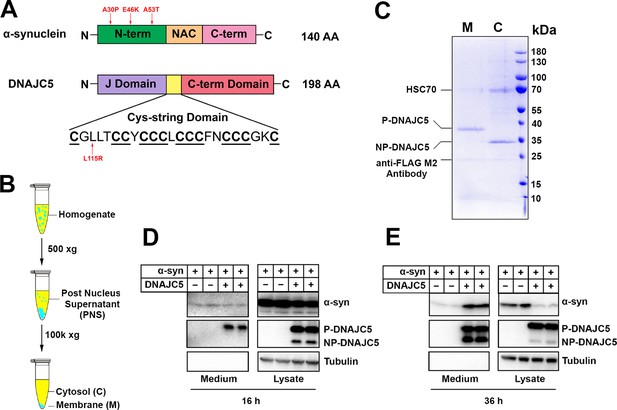
Reconstitution of α-syn secretion regulated by palmitoylated DNAJC5 in HEK293T cells.
(A) Schematic diagrams of α-syn and DNAJC5. Domains are highlighted in different colors. Red arrows indicate known disease-causing mutations on each protein. (B) Membrane and cytosol fractionation scheme. Briefly, homogenized HEK293T cells were centrifuged at low speed to prepare a post-nuclear supernatant (PNS). High-speed centrifugation was then performed to separate the sedimentable membrane (M) from cytosol (C). (C) Partition of palmitoylated DNAJC5 (P-DNAJC5) and non-palmitoylated DNAJC5 (NP-DNAJC5) between the membrane (M) and cytosol (C) fractions. DNAJC5 was immunoprecipitated from cytosol and membrane with anti-FLAG resin and evaluated by Coomassie-blue stained SDS-PAGE. (D) α-syn secretion 16 h after transfection. The secretion of P-DNAJC5 in the medium was detected. (E) α-syn secretion 36 h after transfection. NP-DNAJC5 was also secreted in the medium together with α-syn.
-
Figure 1—source data 1
Uncropped immunoblot and gel images corresponding to Figure 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig1-data1-v2.zip
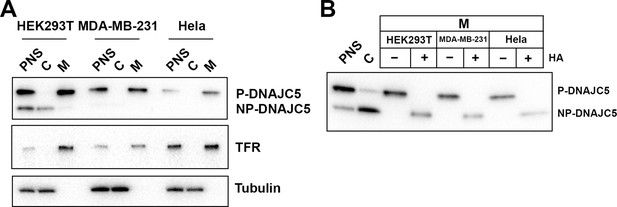
Validation of palmitoylation of DNAJC5 in various cell lines.
(A) Membrane and cytosol fractionation of various cell lines (HEK293T, MDA-MB-231, and Hela) transfected with DNAJC5. The fractionation was performed as depicted in Figure 1B. C, cytosol; M, membrane; PNS, post-nuclear supernatant. Transferrin receptor (TFR) was used as a membrane marker. Tubulin was used as a cytosol marker. (B) In vitro depalmitoylation assay. Sedimented membranes (M) from different DNAJC5-transfected cell lines were collected and resuspended in 0.25 M Tris pH 7.2 buffer or 0.25 M Hydroxylamine (HA) pH 7.2 buffer. After overnight incubation at room temperature, the samples were examined by SDS-PAGE followed by anti-DNAJC5 immunoblot. PNS and C were used to compare the mobility of P-DNAJC5 and NP-DNAJC5, respectively.
-
Figure 1—figure supplement 1—source data 1
Uncropped immunoblot images corresponding to Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig1-figsupp1-data1-v2.zip
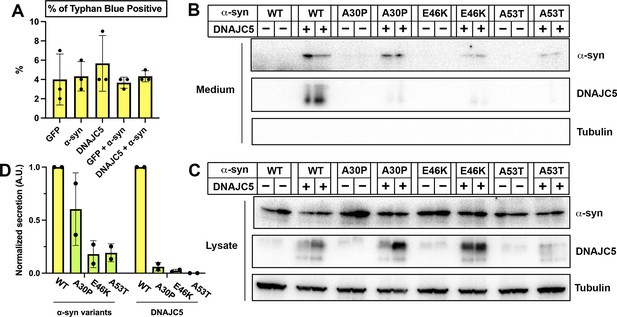
Secretion of α-syn variants.
(A) Trypan blue cell vital staining after transfection with various constructs used in Figure 1. Ratios of trypan blue positive cells indicate the toxicity caused by transfection. Error bars represent standard deviations of three samples. (B) Secretion of α-syn variants into conditioned medium. Medium was collected, concentrated, and evaluated by SDS-PAGE and immunoblot. (C) Expression of α-syn variants in HEK293T cells. HEK293T cells were co-transfected with Parkinson’s disease (PD)-causing α-syn mutant (A30P, E46K, and A53T) and DNAJC5. (D) Quantification of normalized secretion (amount in medium divided by amount in lysate) of various α-syn variants and DNAJC5. The quantification was based on immunoblot in (B) and (C).
-
Figure 1—figure supplement 2—source data 1
Uncropped immunoblot images corresponding to Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig1-figsupp2-data1-v2.zip
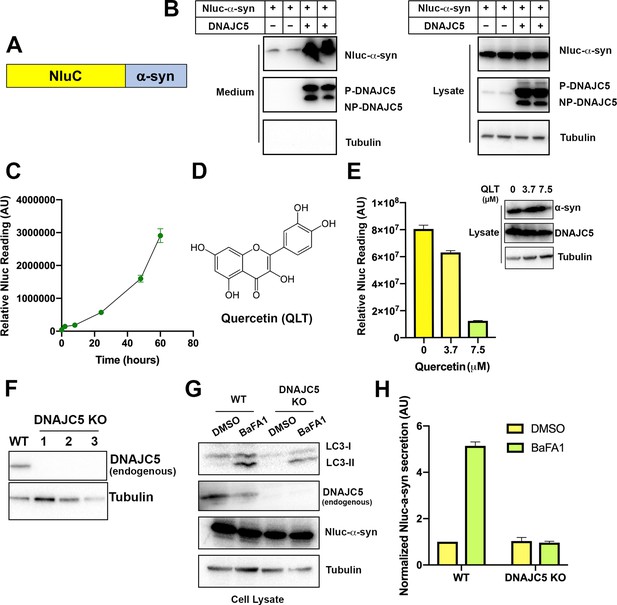
Secretion of α-syn is partially dependent on endogenous DNAJC5 in HEK293T cells.
(A) Schematic diagram of nanoluciferase (Nluc)-fused α-syn. (B) Nluc-α-syn secretion was stimulated by DNAJC5 expression. Plasmids encoding Nluc-α-syn and DNAJC5 were co-transfected into HEK293T cells. Expression and secretion of proteins were detected with immunoblot 36 hr after transfection. (C) Time-dependent of accumulation of Nluc-α-syn in the medium without DNAJC5 overexpression. After transfecting HEK293T cells with Nluc-α-syn alone, fractions of medium were collected at indicated time points. (D) Chemical structure of quercetin (QLT), a reported DNAJC5 inhibitor. (E) QLT inhibited endogenous Nluc-α-syn secretion in a concentration-dependent manner. AU, arbitrary unit. Secretion assay similar to (B) was performed with treatment of indicated concentration of QLT. Amounts of secreted proteins were quantified with nanoluciferase assay 36 hr after transfection. Immunoblot of α-syn and DNAJC5 in cell lysate after QLT treatment was shown on the right. Error bars represent standard deviations of three samples. (F) Validation of DNAJC5 knockout (KO) cell line generated by CRISPR. Wildtype (WT) HEK293T cell was used as a control. In all three single clones of DNAJC5 KO cell lines, DNAJC5 was not detectable by immunoblot. (G) Treatment of bafilomycin A1 (BaFA1) induced LC3 lipidation in cells. After 24 hr of 100 nM BaFA1 treatment, media were collected, and cells were lysed for evaluation with SDS-PAGE followed by immunoblot. The accumulation of the lipidated form of LC3 (LC3-II) was used to indicate the inhibition of autophagy and lysosomal degradation in cells. (H) Quantification of α-syn secretion from WT and DNAJC5 KO HEK293T cells after 24 hr treatment of BaFA1. The normalized secretion was calculated as nanoluciferase reading from media divided by the reading from cell lysate. Error bars represent standard deviations of three samples.
-
Figure 1—figure supplement 3—source data 1
Uncropped immunoblot images corresponding to Figure 1—figure supplement 3.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig1-figsupp3-data1-v2.zip
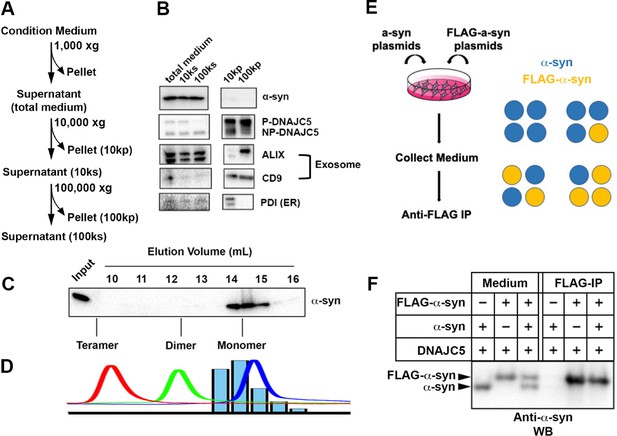
Characterization of secreted α-syn.
(A) Medium fractionation scheme. (B) Secreted α-syn was soluble. Differential centrifugation was performed with conditioned medium from HEK293T cells transfected with DNAJC5 and α-syn. Alix and CD9, exosome markers. PDI, an endoplasmic reticulum (ER) marker, was used as exosome-negative control. (C) Gel filtration fractionation of medium. Conditioned medium was concentrated and subjected to gel filtration fractionation. Fractions were evaluated by anti-α-syn immunoblot. (D) Chromatograms of tandem α-syn monomer (blue curve), dimer (green curve), and tetramer (red curve) were overlaid. In comparison, the relative intensity of secreted α-syn in each fraction was plotted as blue bars. (E) Schematic diagram of co-immunoprecipitation (co-IP) of secreted α-syn and FLAG-α-syn. Shown here is possible interaction between α-syn (blue circle) and FLAG-α-syn (yellow circle) in a representative tetrameric conformation. (F) Anti-FLAG immunoprecipitation (FLAG-IP) of media from cells transfected with indicated plasmids. Both the medium input and FLAG-IP samples were evaluated with anti-α-syn immunoblot (anti-α-syn WB).
-
Figure 2—source data 1
Uncropped immunoblot corresponding to Figure 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig2-data1-v2.zip
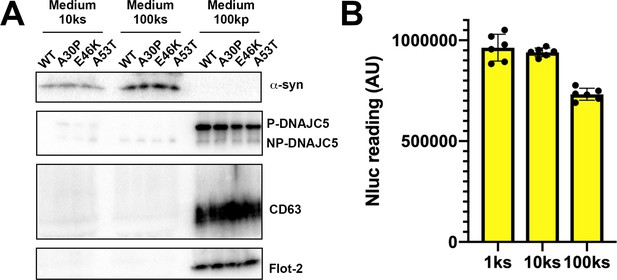
Solubility of secreted α-syn variants.
(A) Medium fractionation of secreted α-syn PD mutants (A30P, E46K, and A53T). After differential centrifugation of medium, supernatant (10ks and 100ks) and pellet fractions (100kp) were evaluated by immunoblot. (B) Medium fractionation of basal secreted Nluc-α-syn without DNAJC5 overexpression. Similar fractionation assay was performed in (A) with medium from HEK293T cells transfected with Nluc-α-syn alone. Secreted Nluc-α-syn was quantified with a nanoluciferase assay. AU, arbitrary unit.
-
Figure 2—figure supplement 1—source data 1
Uncropped immunoblot images corresponding to Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig2-figsupp1-data1-v2.zip
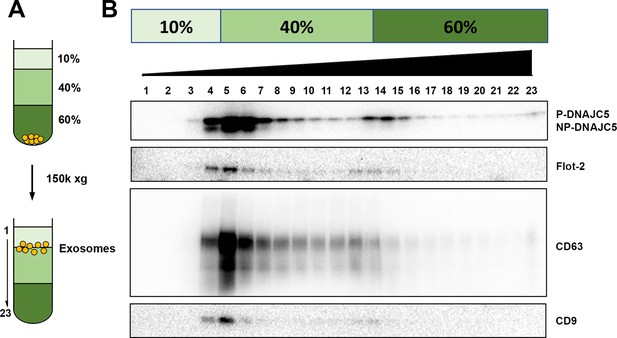
DNAJC5 enriched in buoyant EV fraction.
(A) Schematic diagram of EV flotation protocol. Briefly, high-speed pellet fractions of growth medium were resuspended in 60% sucrose buffer and overlaid sequentially with 40% and 10% sucrose buffer. The tubes were centrifuged at 150,000 (150k)×g at 4°C for overnight. Buoyant EVs floated at the 10%/40% sucrose interface, separated from other insoluble materials. (B) Immunoblots across the sucrose step gradient. DNAJC5 as well as other classical exosome markers (Flot-2, CD63, and CD9) were highly enriched in fractions 4–6 at the 10%/40% interface.
-
Figure 2—figure supplement 2—source data 1
Uncropped immunoblot images corresponding to Figure 2—figure supplement 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig2-figsupp2-data1-v2.zip
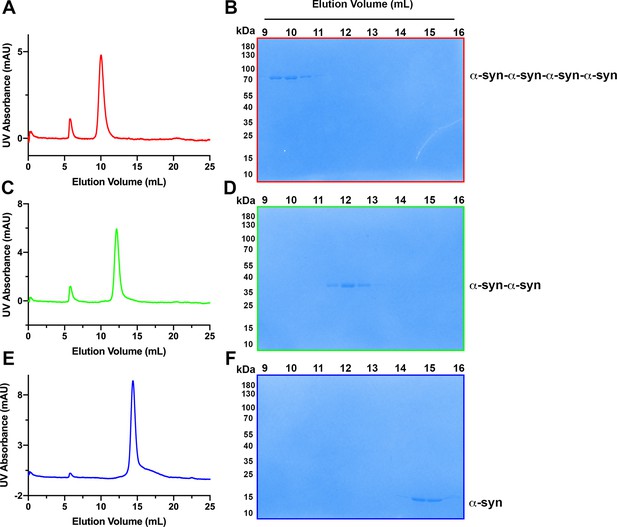
Assessment of tandem α-syn oligomers by gel filtration chromatography.
(A) Chromatogram of purified tetrameric α-syn tandem oligomer (α-syn-α-syn-α-syn-α-syn). (B) Coomassie-blue stained SDS-PAGE of fractions from gel filtration of tetramericα-syn tandem oligomer (α-syn-α-syn-α-syn-α-syn). (C) Chromatogram of purified dimeric α-syn tandem oligomer (α-syn-α-syn). (D) Coomassie-blue stained SDS-PAGE of fractions from gel filtration of dimericα-syn tandem oligomer (α-syn-α-syn). (E) Chromatogram of purified WT α-syn (α-syn). (F) Coomassie-blue stained SDS-PAGE of fractions from gel filtration of WTα-syn (α-syn).
-
Figure 2—figure supplement 3—source data 1
Uncropped gel images corresponding to Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig2-figsupp3-data1-v2.zip
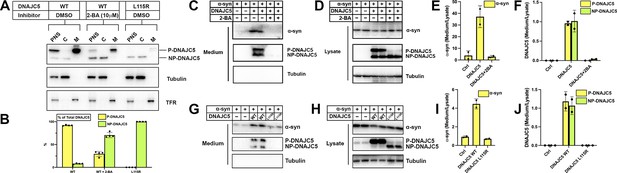
Disruption of palmitoylation of DNAJC5 inhibited α-syn secretion.
(A) Inhibition of DNAJC5 palmitoylation by 2-bromopalmitic acid (2-BA) or introduced mutation L115R. Cellular fractionation was performed with HEK293T cells transfected with WT DNAJC5 and treated with 10 μm 2-BA, or transfected with DNAJC5 L115R mutant. C, cytosol; M, membrane; PNS, post-nuclear supernatant; TFR, transferrin receptor. (B) Quantification of the percentage of P-DNAJC5 and NP-DNAJC5 in different conditions as shown in (A). Error bars represent standard deviations of three experiments. (C) α-syn secretion was blocked with 2-BA treatment. HEK293T cells transfected with indicated plasmids were treated with DMSO or 10 μm 2-BA. Media fractions were collected and secretion was evaluated by SDS-PAGE and immunoblot. (D) Palmitoylation of DNAJC5 was blocked in HEK293T cells treated with 2-BA. (E) Quantification of normalized α-syn secretion in HEK293T cells after 2-BA treatment. The quantification was based on immunoblot in (C) and (D). The α-syn secretion was calculated as the amount of α-syn in media divided by the amount in lysate. (F) Quantification of normalized DNAJC5 secretion in HEK293T cells after 2-BA treatment. The quantification was based on immunoblot in (C) and (D). The DNAJC5 secretion was calculated as the amount of DNAJC5 in media divided by the amount in lysate. (G) DNAJC5 L115R mutant reduced α-syn secretion compared with WT DNAJC5. Secretion assay with HEK293T cells transfected with indicated plasmids encoding DNAJC5 variant was performed similar to (C). (H) DNAJC5 L115R was non-palmitoylated in HEK293T cells. (I) Quantification of normalized α-syn secretion in HEK293T cells transfected with DNAJC5 L115R mutant. The quantification was based on immunoblot in (G) and (H). (J) Quantification of normalized DNAJC5 secretion in HEK293T cells transfected with DNAJC5 L115R mutant. The quantification was based on immunoblot in (G) and (H).
-
Figure 3—source data 1
Uncropped immunoblot corresponding to Figure 3.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig3-data1-v2.zip
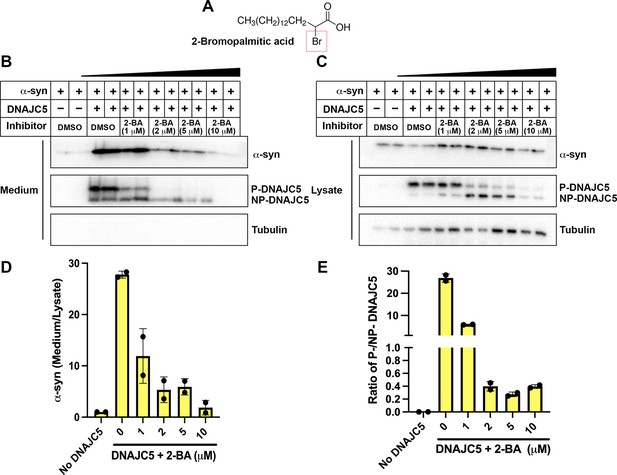
Dose-dependent inhibition of α-syn secretion by 2-bromopalmitic acid (2-BA).
(A) Chemical structure of palmitoylation inhibitor 2-bromopalmitic acid (2-BA). The single bromo substituent at position 2 is highlighted by a red dashed square. (B) α-syn secretion in the medium was inhibited by 2-BA in a dose-dependent manner. About 10 μm 2-BA was serially diluted by DMSO into 5 μm, 2 μm, and 1 μm solution. HEK293T cells were first transfected with DNAJC5 and α-syn. After medium replacement, 2-BA of indicated concentration was added to cell culture. Media were collected after 36 hr and followed by sample preparation, SDS-PAGE and immunoblot. (C) With increasing concentration of 2-BA, P-DNAJC5 decreased and NP-DNAJC5 increased in HEK293T cells. (D) Quantification of normalized α-syn secretion upon increasing concentration of 2-BA. Quantification was based on immunoblot in (B) and (C). The α-syn secretion was calculated as the amount of α-syn in media divided by the amount in lysate. (E) Quantification of ratio of P-DNAJC5/NP-DNAJC5. The quantification was based on immunoblot in (C).
-
Figure 3—figure supplement 1—source data 1
Uncropped immunoblot corresponding to Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig3-figsupp1-data1-v2.zip
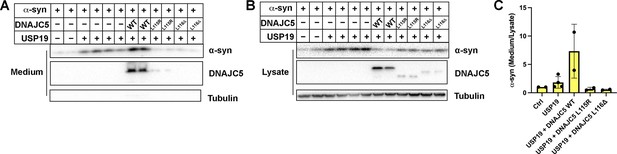
Blockage of USP19-induced α-syn secretion by DNAJC5 L115R or L116Δ mutant.
(A) USP19 induced α-syn secretion in the medium, which was further enhanced by WT DNAJC5 and blocked by two DNAJC5 palmitoylation-deficient mutants (L115R and L116Δ). (B) Both mutations, L115R and L115Δ, inhibited DNAJC5 palmitoylation in HEK293T cells. (C) Quantification of normalized α-syn secretion in HEK293T cell transfected with indicated constructs. The quantification was based on immunoblot in (A) and (B). α-Syn secretion was calculated as the amount of α-syn in media divided by the amount in lysate.
-
Figure 3—figure supplement 2—source data 1
Uncropped immunoblot corresponding to Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig3-figsupp2-data1-v2.zip
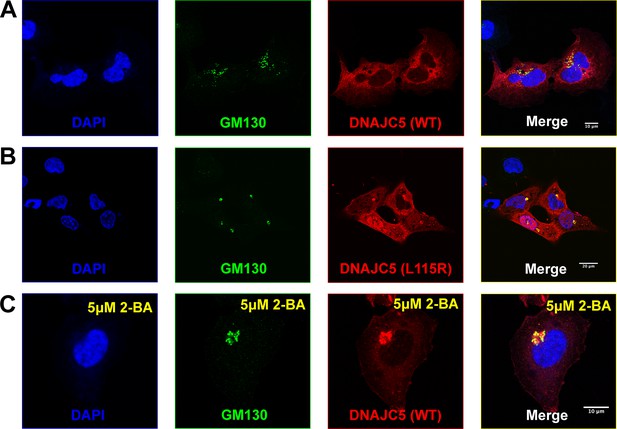
Golgi retention of DNAJC5 in secretion-deficient conditions.
(A) Immunofluorescence (IF) images of U2OS cells transfected with wildtype (WT) DNAJC5-HaloTag. Before fixation, HaloTag TMR ligands were added to the cell culture to label DNAJC5. Golgi apparatus was visualized by IF using anti-GM130 antibody. Nuclei were stained with DAPI. Scale bar: 10 μm. (B) IF images of U2OS cells transfected with DNAJC5 (L115R)-HaloTag. Same staining procedure was performed as in (A). Scale bar: 20 μm. (C) IF images of U2OS cells transfected with WT DNAJC5-HaloTag and treated with 5 μM 2-BA. After transfection with WT DNAJC5-HaloTag, cells were incubated with 5 μM 2-BA for 1 day to block palmitoylation of DNAJC5. Same staining procedure was performed as in (A). Scale bar: 10 μm.
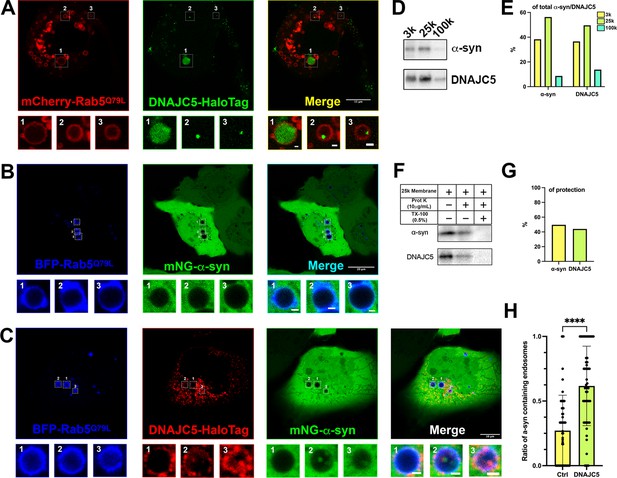
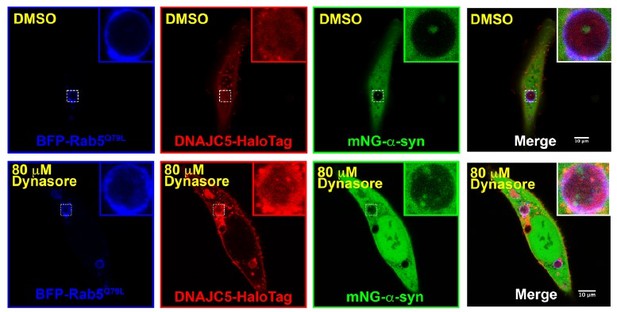
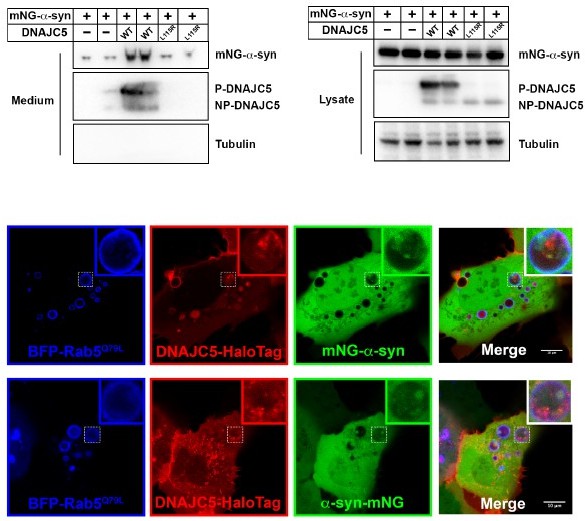
Topological localization of α-syn and DNAJC5 in enlarged endosomes.
(A) DNAJC5 was internalized inside enlarged endosomes. Live U2OS cells expressing mCherry-Rab5Q79L (red) showed circular enlarged endosomes labeled by Rab5 mutant. DNAJC5-HaloTag (green) was visualized by addition of HaloTag Oregon Green Ligand. Representative enlarged endosomes show diffuse (1) or punctate (2 and 3) internalized DNAJC5. Scale bar: 15 μm in overviews and 1 μm in magnified insets. (B) α-syn was excluded from enlarged endosomes. In live U2OS cells, expression of BFP-Rab5Q79L (blue) produced enlarged endosomes of similar morphology compared with mCherry-Rab5Q79L. mNeonGreen-α-syn (mNG-α-syn, green) was expressed both in the nucleus and cytosol. No mNG-α-syn was found inside enlarged endosomes (1–3). Scale bar: 20 μm in overviews and 1 μm in magnified insets. (C) α-syn enters into enlarged endosomes in the presence of DNAJC5. DNAJC5-HaloTag (red) and mNG-α-syn (green) were coexpressed in U2OS cells carrying BFP-Rab5Q79L (blue) mutant and imaged. No mNG-α-syn was internalized in endosome without DNAJC5-HaloTag inside (1). In contrast, mNG-α-syn was found inside endosomes with DNAJC5-HaloTag inside (2 and 3). Scale bar: 10 μm in overviews and 1 μm in magnified insets. (D) α-syn and DNAJC5 co-sedimented in membrane fractionation. HEK293T cell homogenate was sequentially centrifuged at increasing velocity from 3000×g (3k), 25,000×g (25k), and 100,000×g (100k). The 25k membrane fraction had the highest amount of both α-syn and DNAJC5. (E) Quantification of the membrane fractionation results in (D). (F) Proteinase K protection assay of 25k membrane-containing α-syn and DNAJC5. (G) Quantification of the proteinase K protection assay in (F). (H) Quantification of the ratio of α-syn-containing endosomes in control cells (no-DNAJC5 transfection) or cells co-transfected with DNAJC5. More than 100 enlarged endosomes were counted in each group. Error bars represent standard deviations. P value<0.0001, two-tailed t test.
-
Figure 4—source data 1
Uncropped immunoblot corresponding to Figure 4.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig4-data1-v2.zip
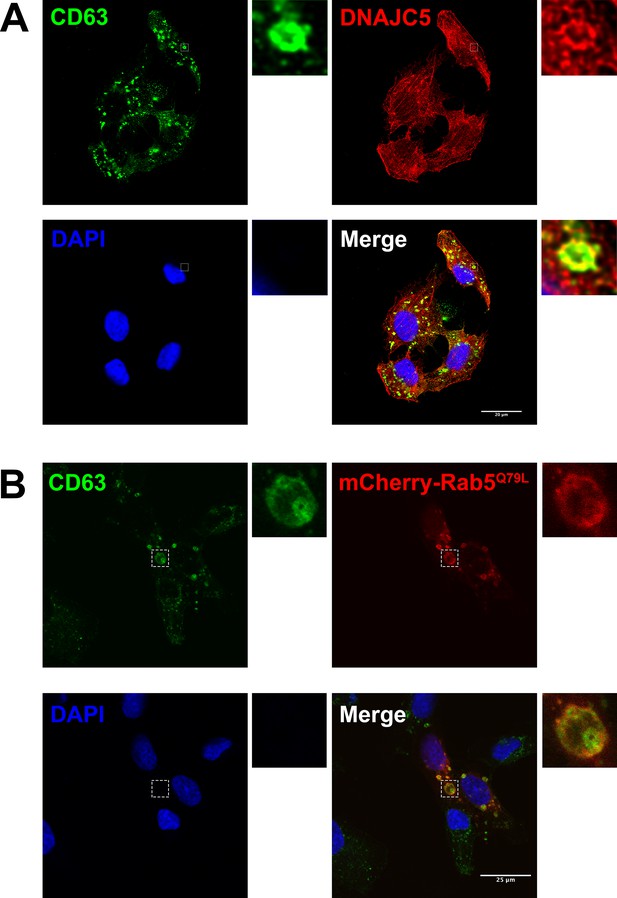
Immunofluorescence (IF) images of endogenous DNAJC5 and enlarged endosomes.
(A) Colocalization between endogenous CD63 (red) and DNAJC5 (green). U2OS cells were cultured and fixed, incubated with corresponding antibodies for IF detection of endogenous CD63 and DNAJC5. Representative colocalized region is shown in magnified insets. Scale bar: 20 μm. (B) CD63 (green) was inside the enlarged endosomes labeled by peripheral mCherry-Rab5Q79L (red). U2OS expressing mCherry-Rab5Q79L were fixed, followed by IF using anti-CD63 antibody. Scale bar: 25 μm.
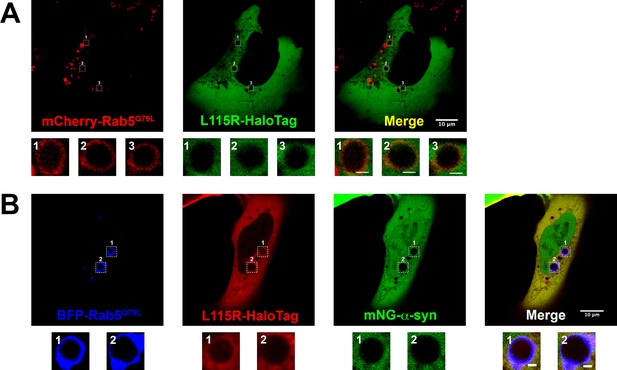
Live-cell images of U2OS cells expressing DNAJC5 L115R mutant and α-syn.
(A) Live-cell imaging of U2OS cells transfected with DNAJC5 (L115R)-HaloTag and mCherry-Rab5Q79L. DNAJC5 (L115R)-HaloTag (green) is diffuse in cytosol and outside of the enlarged endosomes labeled by peripheral mCherry-Rab5Q79L (red). Scale bar: 10 μm in overviews and 1 μm in magnified insets. (B) Live-cell imaging of U2OS cells transfected with mNeonGreen (mNG)-α-syn, DNAJC5 (L115R)-HaloTag, and mCherry-Rab5Q79L. In the condition of coexpression with DNAJC5 (L115R)-HaloTag (red), no mNG-α-syn (green) was found inside of enlarged endosomes labeled by peripheral BFP-Rab5Q79L (blue). Scale bar: 10 μm in overviews and 1 μm in magnified insets.
Time lapse of movement of internalized α-syn and DNAJC5 in the enlarged endosomes.
DNAJC5-HaloTag (red) and mNG-α-syn (green) were coexpressed in U2OS cells carrying BFP-Rab5Q79L (blue) mutant and imaged. Scale bar: 10 μm.
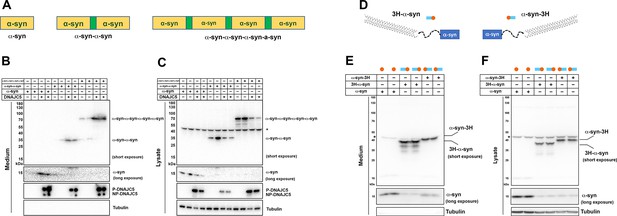
Secretion of tandem α-syn oligomers and α-syn fused with thermostable helix-bundle protein.
(A) Schematic diagrams of tandem α-syn oligomers. α-syn protomers (yellow) were linked head to tail by flexible linker (green) to mimic increased size of α-syn oligomers. (B) Secretion of tandem α-syn oligomers in medium. Secretion assay was performed with media from HEK293T cells transfected with indicated tandem α-syn oligomers. Tandem α-syn oligomers are more sensitively detected by immunoblot which were exposed for shorter time compared with WT α-syn. (C) Expression of tandem α-syn oligomers in HEK293T cells. *a non-specific band. (D) Schematic diagrams of N-terminal fused and C-terminal fused thermostable three helix-bundle (3H-) α-syn. 3H shown as three blue dashes, α-syn shown as orange circle. (E) Secretion of 3H-α-syn and α-syn-3H in medium. Secretion assay was performed with media from HEK293T cells transfected with indicated 3H-fused α-syn constructs. *a non-specific band. (F) Expression of 3H-α-syn and α-syn-3H in HEK293T cells. *a non-specific band.
-
Figure 5—source data 1
Uncropped immunoblot corresponding to Figure 5.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig5-data1-v2.zip
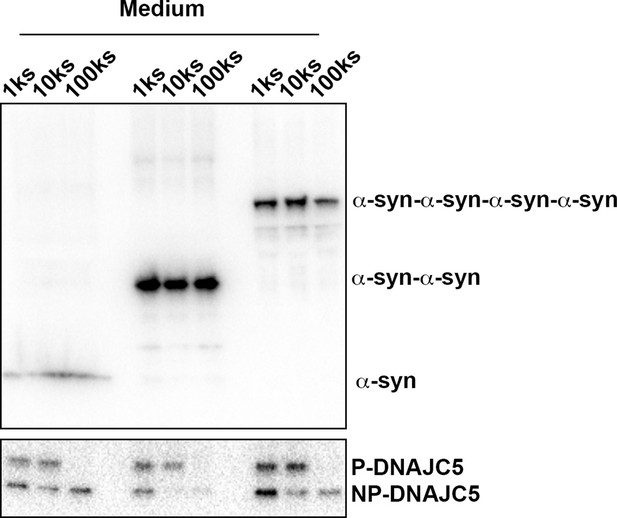
Medium fractionation of secreted tandem α-syn oligomers.
Medium fractionation of secreted tandem α-syn oligomers. P-DNAJC5 was depleted in the supernatant after centrifugation at 100,000 (100k)×g. However, no significant decrease of tandem α-syn oligomers in the supernatant was observed.
-
Figure 5—figure supplement 1—source data 1
Uncropped immunoblot corresponding to Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig5-figsupp1-data1-v2.zip
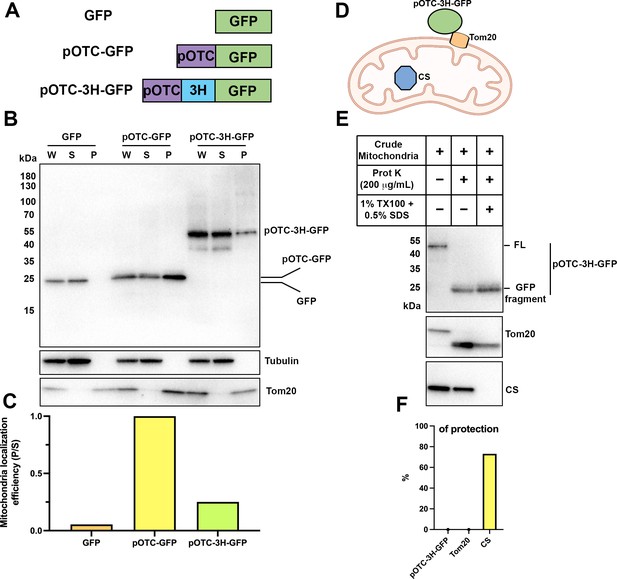
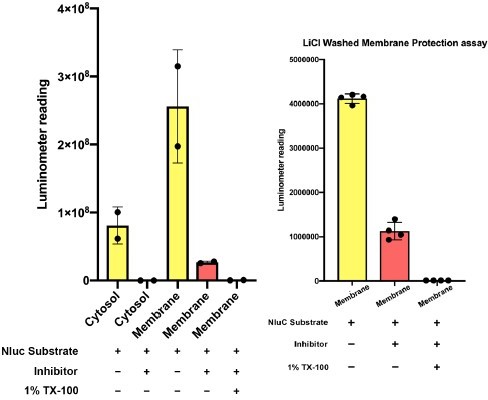
Thermostable three helix bundle blocked pOTC-mediated mitochondrial import of GFP.
(A) Schematic diagrams of constructs used in mitochondrial import assay. GFP, GFP alone. pOTC-GFP, GFP with leader peptide from ornithine ranscarbamylase (OTC) fused at amino-terminus. pOTC-3H-GFP, the thermostable three helix bundle was inserted between the leader peptide pOTC and GFP. (B) Immunoblot analysis of proteins in crude mitochondria fraction. Whole-cell lysate (W) was prepared by mixing homogenized cells with equal volume of 2.3 M sucrose buffer and centrifugation at 1200×g to remove large debris. Soluble fraction (S) and particulate fraction (P) were separated by centrifuging W fraction at 7000×g for 10 min. Mitochondria were enriched in P fraction. Tom20, mitochondrial marker. Tubulin, soluble marker. (C) The mitochondrial localization efficiency was calculated by quantifying the protein amount in P fraction divided by the amount in S fraction. (D) Cartoon depicting relative localization of proteins inside or outside of mitochondria. Tom20, a mitochondrial outer membrane protein. Citrate synthase (CS), a mitochondrial matrix protein. The import of pOTC-3H-GFP is blocked by 3H. (E) Proteinase K protection assay of crude mitochondria. SDS (0.5%) was added in addition to 1% TX-100 to sensitize protease treatment of well-folded protein, for example, CS. The protease accessibility of pOTC-3H-GFP was revealed by the production of GFP fragment and disappearance of the full-length (FL) band. (F) Quantification of percentage of protection of proteins in (E).
-
Figure 5—figure supplement 2—source data 1
Uncropped immunoblot corresponding to Figure 5—figure supplement 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig5-figsupp2-data1-v2.zip
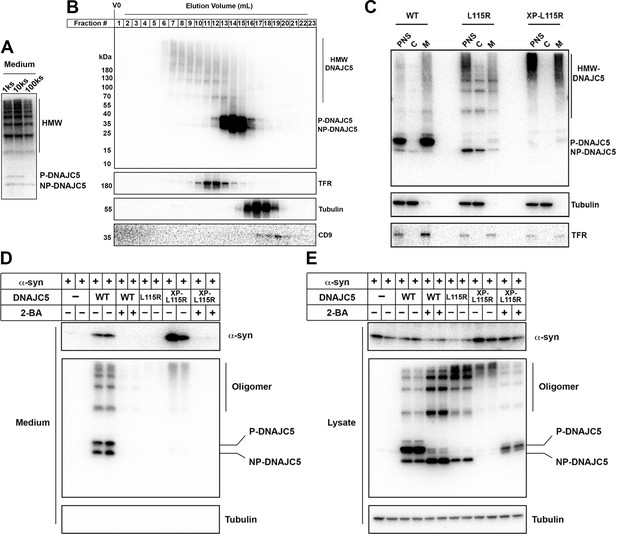
XPACK (XP)-induced DNAJC5 L115R oligomerization rescued α-syn secretion.
(A) Ladder pattern of higher molecular weight (HMW) DNAJC5 oligomers in the medium. Medium from HEK293T cell culture transfected with DNAJC5 was centrifuged at 1000 (1k)×g, 10,000 (10k)×g, and 100,000 (100k)×g, followed by SDS-PAGE and immunoblot of supernatant (s) fractions at each centrifugation step. (B) Fractionation of HMW-DNAJC5 with gel filtration. HEK293T cells transfected with DNAJC5 were lysed, clarified, and subjected to gel filtration. HMW-DNAJC5 of different sizes were separated based on their corresponding molecular weight. (C) XP-DNAJC5 L115R mutant forms a membrane-bound oligomer. Cellular fractionation was performed with HEK293T cells transfected with indicated DNAJC5 variants. Note the substantial change of electrophoretic mobility of XP-DNAJC5 L115R on SDS-PAGE. (D) α-syn secretion induced by XP-DNAJC5 L115R. Secretion assay was performed with HEK293T cells transfected with indicated plasmids. About 10 μm 2-BA was used to block induced α-syn. (E) Expression of α-syn and DNAJC5 variants in HEK293T cells. Note the substantial change in electrophoretic mobility of 2-BA-treated XP-DNAJC5 L115R on SDS-PAGE.
-
Figure 6—source data 1
Uncropped immunoblot corresponding to Figure 6.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig6-data1-v2.zip
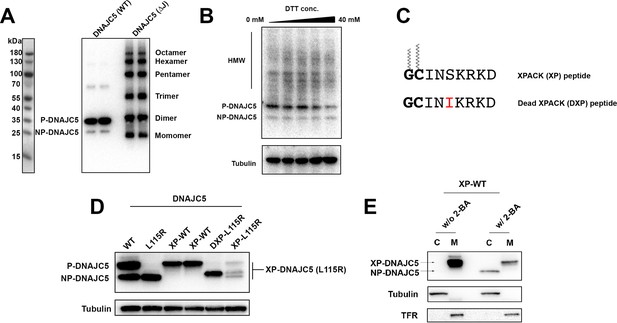
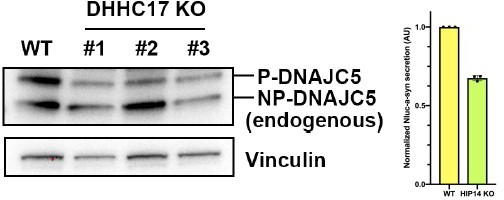
Characterization of HMW-DNAJC5 and XPACK fusion.
(A) DNAJC5 (ΔJ) forms a series of SDS-resistant oligomers. HEK293T cells transfected with WT DNAJC5 or DNAJC5 (ΔJ) were lysed and evaluated by immunoblot using anti-DNAJC5 antibody. (B) HMW-DNAJC5 is not formed by non-specific disulfide bonds. HEK293T cells transfected with DNAJC5 were lysed for SDS-PAGE followed by immunoblot. The loading samples for SDS-PAGE were prepared with increasing amount of DTT up to 40 mM. (C) Schematic diagram of XPACK. XPACK is myristoylated on the first glycine (G) and palmitoylated on the second cystine (C). Replacement of Serine (S) at position 5 with a hydrophobic isoleucine (I) abolishes normal lipidation of XPACK. (D) Assessment of different XPACK fusion constructs. Cell lysate containing different DNAJC5 constructs were evaluated by anti-DNAJC5 immunoblot. (E) Membrane association of XP-DNAJC5 dependent on palmitoylation. HEK293T cells transfected with XP-DNAJC5 were treated with DMSO or 10 μM 2-BA. After cell culture for 24 hr, cellular fractionation was performed with homogenized cells. Distribution of XP-DNAJC5 in cytosol (C) and membrane (M) fractions were evaluated with immunoblot.
-
Figure 6—figure supplement 1—source data 1
Uncropped immunoblot corresponding to Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig6-figsupp1-data1-v2.zip
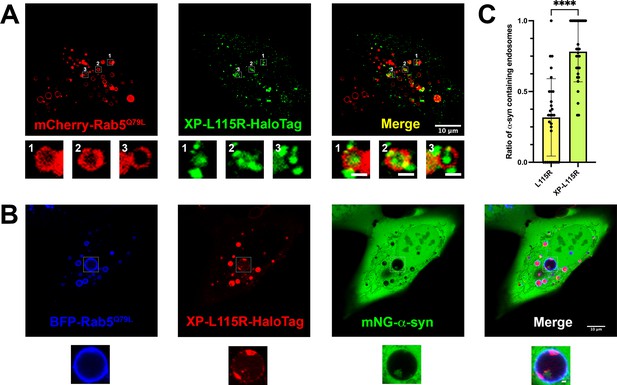
Live-cell images of U2OS cells expressing XP-DNAJC5 L115R mutant and α-syn.
(A) Internalization of punctate XP-DNAJC5 (green) into enlarged endosomes labeled by mCherry-Rab5Q79L (red). U2OS cells were transfected with XP-DNAJC5 and mCherry-Rab5Q79L. Live-cell imaging was performed 24 hr after transfection. Representative enlarged endosomes with XP-DNAJC5 are shown in magnified inset. Scale bar: 10 μm in overviews and 1 μm in magnified insets. (B) α-syn enters into enlarged endosomes in the presence of XP-DNAJC5. XP-DNAJC5-HaloTag (red) and mNG-α-syn (green) were coexpressed in U2OS cells carrying BFP-Rab5Q79L (blue) mutant and imaged. Representative enlarged endosome with both XP-DNAJC5-HaloTag and mNG-α-syn inside is shown in magnified inset. Scale bar: 10 μm in overviews and 1 μm in magnified insets. (C) Quantification of the ratio of α-syn-containing endosomes in cells co-transfect with DNAJC5 L115R or cells co-transfected with DNAJC5 XP-L115R. More than 100 enlarged endosomes were counted in each group. Error bars represent standard deviations. P value<0.0001, two-tailed t test.
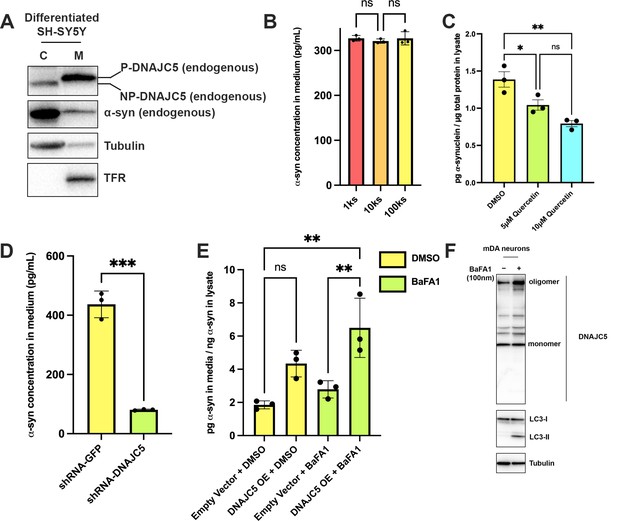
Recapitulation of endogenous DNAJC5-mediated α-syn secretion in various neuronal cell cultures.
(A) Membrane and cytosol fractionation of differentiated SH-SY5Y neuroblastoma cells. The fractionation was performed as depicted in Figure 1B. C, cytosol; M, membrane. The distribution of endogenous DNAJC5 and α-syn was evaluated by immunoblot. Transferrin receptor (TFR) was used as a membrane marker. Tubulin was used as a cytosol marker. (B) Quantification of α-syn level in the supernatant of centrifuged media with ELISA. Conditioned media were collected and sequentially centrifuged at 1000 (1k)×g, 10,000 (10k)×g, and 100,000 (100k)×g. The supernatant from each centrifugation step (1ks, 10ks, and 100ks) was collected and measured by LEGEND MAX Human α-synuclein (Colorimetric) ELISA Kit. One-way ANOVA showed no significant (ns) difference of α-syn level between fractions. (C) Quercetin inhibited endogenous α-syn secretion in hiPSC-derived midbrain dopamine neurons. hiPSC-dopamine neurons carrying the GBA-N370S mutation were treated with quercetin (5 μM or 10 μM) at day 35. Culture media samples were harvested after 3 days treatment at day 38 and α-syn levels in the media were analyzed by electro-chemiluminescent immunoassay. Data points represent individual cell lines derived from different donors and are normalised to total protein in the corresponding cell lysates. One-way ANOVA followed by Tukey’s post hoc test shows a significant reduction in α-syn secretion with increasing quercetin concentration (*p<0.05, **p<0.01). (D) Depletion of endogenous DNAJC5 in SH-SY5Y cells decreased basal α-syn secretion. After 3 days of culture, the media from differentiated SH-SY5Y cells transduced with shRNA targeting GFP (shRNA-GFP) or shRNA targeting DNAJC5 (shRNA-DNAJC5) were collected and the extracellular α-syn was quantified with ELISA. P value<0.0002, two-tailed t test. (E) Expression of exogenous human DNAJC5 in mouse mDA stimulated basal α-syn secretion. WT mDA and mDA expressing hDNAJC5 were treated with DMSO or 100 nM BaFA1. Quantification of α-syn in conditioned media was performed with Mouse α-synuclein ELISA Kit (Abcam). α-syn secretion was normalized by dividing the α-syn in media (pg/ml) by the α-syn in cell lysates (ng/ml). P value<0.01, one-way ANOVA. (F) BaFA1 increased DNAJC5 oligomerization in mouse mDA neurons.
-
Figure 7—source data 1
Uncropped immunoblot corresponding to Figure 7.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig7-data1-v2.zip
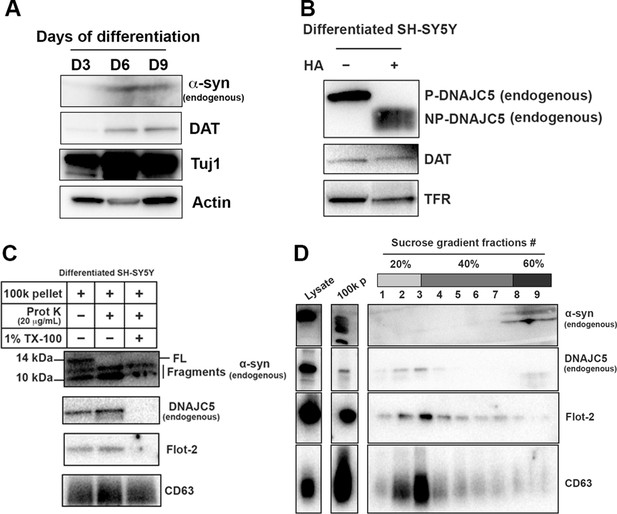
Basal α-syn secreted as a soluble form from differentiated SH-SY5Y.
(A) Differentiation of SH-SY5Y was initiated by lowering the serum concentration to 1% FBS and addition of 10 µM retinoic acid (RA). Media were replaced every 3 days and the cells were harvested at indicated time to examine the expression of neuronal marker. DAT, dopamine transporter; Tuj1, neuron-specific class III β-tubulin. (B) In vitro depalmitoylation assay of endogenous DNAJC5 in differentiated SH-SY5Y cells. The depalmitoylation assay was performed as in Figure 1—figure supplement 1B using membrane (M) fraction from SH-SY5Y cells. HA, hydroxylamine. (C) Proteinase K protection assay of 100,000 (100k) pellet fraction from the centrifuged media of differentiated SH-SY5Y culture. Flot-2 and CD63 were used as exosome markers. (D) Sedimented α-syn was not buoyant. 100k pellet from (C) was mixed with 60% sucrose in PBS and layered with 40% and 20% sucrose in PBS sequentially. After centrifuged at 150,000×g for 16 hr, fractions were collected from top to bottom.
-
Figure 7—figure supplement 1—source data 1
Uncropped immunoblot corresponding to Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig7-figsupp1-data1-v2.zip
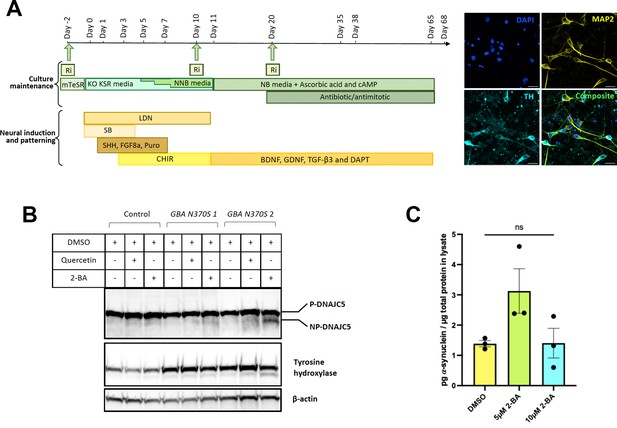
Differentiation of human induced pluripotent stem cells (hiPSCs) and palmitoylation of DNAJC5.
(A) Schematic of the differentiation protocol used to generate hiPSC-derived dopamine neurons, including a patterning phase to generate neural progenitor cells followed by differentiation into mature neurons. Green arrows represent points of replating when cells are supplemented with ROCK inhibitor (Ri) to increase survival. BDNF, brain-derived neurotrophic factor; FGF8a, fibroblast growth factor 8a; GDNF, glial cell line-derived neurotrophic factor; Puro, puromorphamine; SHH, sonic hedgehog; TGF-β3, transforming growth factor beta 3 (see Kriks et al., 2011; Beevers et al., 2017; Lang et al., 2019 for full details). Neurons are mature and harvested between 35 and 68 days for analysis. Immunocytochemical fluorescent images of mature neurons at day 50 stained for neuronal marker microtubule-associated protein (MAP2), the dopaminergic marker tyrosine hydroxylase (TH), and DAPI. Scale bar: 20 µm. (B) DNAJC5 is palmitoylated in iPSC-derived dopamine neurons. Immunoblot analysis of DNAJC5 palmitoylation in hiPSC-derived dopamine neurons treated with DMSO, 7.5 μM Quercetin or 10 μM 2-BA on day 65 and harvested on day 68. TH was used as a marker of dopaminergic identity. (C) Partial depalmitoylation of DNAJC5 by 2-BA in iPSC-derived dopamine neurons does not reduce α-syn secretion. hiPSC-derived dopamine neurons carrying the GBA-N370S mutation were treated with palmitoylation inhibitor 2-BA (5 μM or 10 μM) at day 35. Culture media samples were harvested after 3 days treatment at day 38 and α-syn levels in the media were analyzed by electro-chemiluminescent immunoassay. Data points represent individual cell lines derived from different donors and are normalized to total protein in the corresponding cell lysates. One-way ANOVA shows no effect of 2-BA on α-syn secretion.
-
Figure 7—figure supplement 2—source data 1
Uncropped immunoblot corresponding to Figure 7—figure supplement 2.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig7-figsupp2-data1-v2.zip
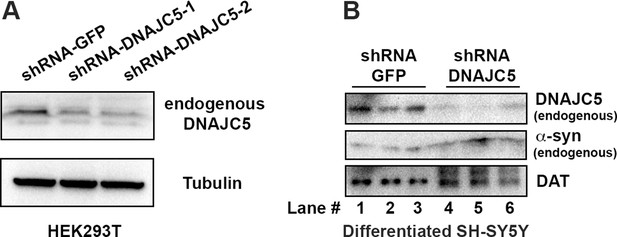
shRNA-mediated DNAJC5 knockdown in differentiated SH-SY5Y cells.
(A) Examination of knockdown efficiency by shRNA targeting DNAJC5 in HEK293T cells. (B) Endogenous DNAJC5 expression decreased in SH-SY5Y cells transduced with shRNA targeting DNAJC5.
-
Figure 7—figure supplement 3—source data 1
Uncropped immunoblot corresponding to Figure 7—figure supplement 3.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig7-figsupp3-data1-v2.zip
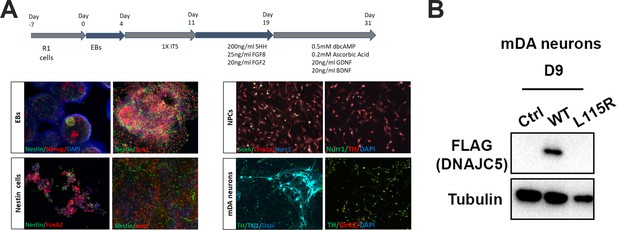
Differentiation of mouse embryonic stem cells (mESCs) and expression of human DNAJC5.
(A) Schematic overview of protocol used for differentiation of mESCs into middle-brain dopaminergic (mDA) neuronal cultures. Immunocytochemical staining using stem cell markers (Nanog, Sox2), neuronal precursor marker (Nestin), mDA markers [FoxA2, Nurr1, Lmx1a, Sox6 (selective marker of substantia nigra pars compacta lineage), TH (tyrosine hydroxylase), Girk2 (G-protein-regulated inward-rectifier potassium channel 2, expressed in DA neurons)], neuronal marker (Tuj1) and nuclear marker (dapi). (B) Expression of human DNAJC5 with C-terminal FLAG tag in mDA neurons.
-
Figure 7—figure supplement 4—source data 1
Uncropped immunoblot corresponding to Figure 7—figure supplement 4.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig7-figsupp4-data1-v2.zip
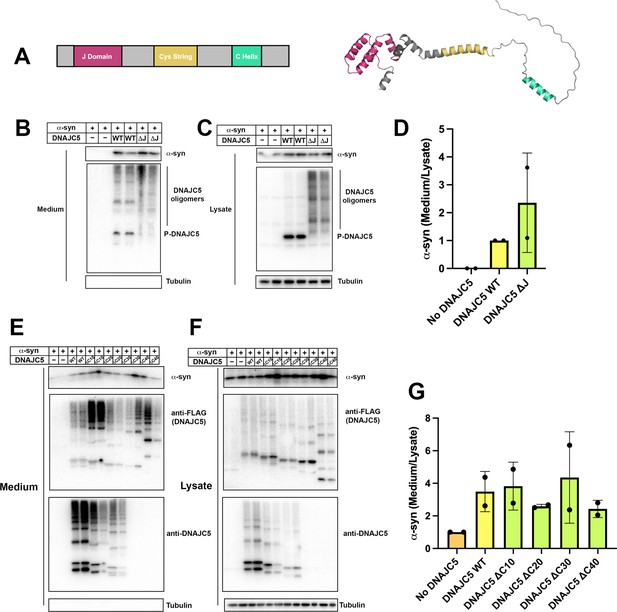
Domain mapping of secretion-competent DNAJC5.
(A) Predicted structure of DNAJC5 by AlphaFold. Color scheme: J domain (magenta), Cys string domain (yellow) and C-terminal helix (green). (B) DNAJC5 (ΔJ) was competent to induce α-syn secretion into the medium. HEK293T cells were transfected with indicated plasmids. Media were collected after 36 hr and evaluated with immunoblot. (C) DNAJC5 (ΔJ) formed oligomers in HEK293T cells. (D) Quantification of normalized α-syn secretion in HEK293T cells transfected with WT DNAJC5 or DNAJC5 (ΔJ). Quantification was based on immunoblot in (B) and (C). The α-syn secretion was calculated as the amount of α-syn in media divided by the amount in lysate. α-syn secretion in cells transfected with WT DNAJC5 was normalized as 1. (E) C-terminal truncated DNAJC5 constructs were competent to induce α-syn secretion in the medium. HEK293T cells were transfected with C-terminal truncated DNAJC5 and α-syn. DNAJC5 antibodies cannot recognize DNAJC5 (ΔC30) and DNAJC5 (ΔC40) because of a missing epitope in the C-terminus. Instead, DNAJC5 (ΔC30) and DNAJC5 (ΔC40) were detected by C-terminal FLAG tags. All the C-terminal truncated DNAJC5 constructs showed smear-like oligomers. (F) Expression of C-terminal truncated DNAJC5 constructs in HEK293T cells. Immunoblot of anti-FLAG antibody and anti-DNAJC5 antibody cross-validated the existence of oligomers. (G) Quantification of normalized α-syn secretion in HEK293T cells transfected with WT DNAJC5 or different C-terminal truncated DNAJC5 constructs (ΔC10, ΔC20, ΔC30, and ΔC40). Quantification was based on immunoblot in (E) and (F). The α-syn secretion was calculated as the amount of α-syn in media divided by the amount in lysate. α-syn secretion in cells without DNAJC5 transfection was normalized as 1.
-
Figure 8—source data 1
Uncropped immunoblot corresponding to Figure 8.
- https://cdn.elifesciences.org/articles/85837/elife-85837-fig8-data1-v2.zip
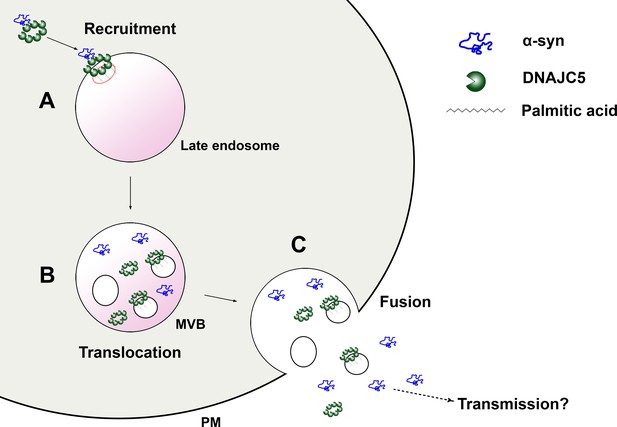
A model for palmitoylated DNAJC5 oligomer-mediated α-syn secretion.
(A) Recruitment of α-syn on the membrane by DNAJC5. DNAJC5 binds to α-syn and targets it to late endosomes by palmitoylation. DNAJC5 forms a high-order oligomer to accommodate α-syn. (B) Translocation of α-syn and DNAJC5 into the membrane compartment. Both α-syn and DNAJC5 are translocated into the endosome lumen along with intraluminal vesicles (ILVs), forming a multivesicular body (MVB). (C) Secretion of α-syn and DNAJC5. Upon fusion between MVB and plasma membrane (PM), the cargos are expelled into the extracellular space. α-syn is soluble. DNAJC5 exists in both soluble and membrane-bound forms. Further transmission potentially occurs after secretion.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Mouse monoclonal anti-α-synuclein | BD Biosciences | Cat# 610787 | (1:500) |
| Antibody | Rabbit polyclonal anti-α-synuclein | Proteintech | Cat# 10842-1-AP | (1:500) |
| Antibody | Rabbit polyclonal anti-DNAJC5 | RayBiotech | Cat# 144-10489-200 | (1:1,000) |
| Antibody | Mouse monoclonal anti-alpha tubulin | Abcam | Cat# ab7291 | (1:2,000) |
| Antibody | Mouse monoclonal anti-Alix | Santa Cruz Biotechnology | Cat# Sc-53540 | (1:1,000) |
| Antibody | Rabbit monoclonal anti-CD9 | Cell Signaling Technology | Cat# 13174S | (1:1,000) |
| Antibody | Mouse monoclonal anti-PDI | Enzo Life Sciences | Cat# ADI-SPA-891-D | (1:1,000) |
| Antibody | Mouse monoclonal anti-CD63 | Thermo Fisher Scientific | Cat# BDB556019 | (1:1,000) |
| Antibody | Mouse monoclonal anti-Flotillin-2 | BD Biosciences | Cat# 610383 | (1:1,000) |
| Antibody | Mouse monoclonal anti-Transferrin Receptor | Thermo Fisher Scientific | Cat# 13-6800 | (1:1,000) |
| Antibody | Mouse monoclonal anti-GM130 | BD Biosciences | Cat# 610823 | (1:1,000) |
| Antibody | Rabbit monoclonal anti-Tom20 | Cell Signaling Technology | Cat# 42406S | (1:1,000) |
| Antibody | Rabbit polyclonal anti-GFP | Fisher Scientific | Cat# NC9589665 | (1:1,000) |
| Antibody | Rabbit polyclonal anti-LC3B | Novus Biologicals | Cat# NB100-2220 | (1:1,000) |
| Antibody | Rabbit monoclonal anti-Citrate Synthase | Cell Signaling Technology | Cat# 14309S | (1:1,000) |
| Antibody | Rabbit polyclonal anti-Dopamine transporter | Bioss Antibodies | Cat# BS-1714R | (1:1,000) |
| Antibody | Rabbit monoclonal anti-beta III Tubulin | Abcam | Cat# ab215037 | (1:1,000) |
| Antibody | Rabbit polyclonal anti-Tyrosine hydroxylase | Millipore | Cat# AB152 | (1:1,000) |
| Antibody | Chicken polyclonal Microtubule-associated protein 2 | Abcam | Cat# ab92434 | (1:1,000) |
| Antibody | Mouse monoclonal anti-FLAG | Sigma-Aldrich | Cat# F9291 | (1:1,000) |
| Strain, strain background (Escherichia coli) | XL1-Blue competent cells | MacroLab Berkeley | N/A | |
| Strain, strain background (E. coli) | Rossetta (DE3) pLysS competent cells | MacroLab Berkeley | N/A | |
| Chemical compound, drug | Anti-FLAG M2 Affinity Gel | Sigma-Aldrich | Cat# A2220-5ML | |
| Chemical compound, drug | Dimethyl sulfoxide (DMSO) | Thermo Fisher Scientific | Cat# BP231-100 | |
| Chemical compound, drug | Quercetin | Sigma-Aldrich | Cat# Q4951-10G | |
| Chemical compound, drug | 2-Bromopalmitic acid | Millipore Sigma | Cat# 21604-1G | |
| Chemical compound, drug | Balfilomycin A1 | Cayman Chemical | Cat# 11038 | |
| Chemical compound, drug | Retinoic acid | Sigma-Aldrich | Cat# R2625-100MG | |
| Chemical compound, drug | HaloTag Oregon Green Ligand | Promega | Cat# G2802 | |
| Chemical compound, drug | HaloTag TMR Ligand | Promega | Cat# G8251 | |
| Chemical compound, drug | Prolong Gold with DAPI | Thermo Fisher Scientific | Cat# P36931 | |
| Chemical compound, drug | Proteinase K | Sigma-Aldrich | Cat# P2308 | |
| Peptide, recombinant protein | α-syn tandem repeats protein | This paper | N/A | |
| Commercial assay or kit | Nano-Glo Luciferase Assay System | Promega | Cat# N1150 | |
| Commercial assay or kit | LEGEND MAX Human α-synuclein (Colorimetric) ELISA Kit | BioLegend | Cat# 448607 | |
| Commercial assay or kit | Mouse α-synulcein ELISA Kit | Abcam | Cat# ab282865 | |
| Cell line (Homo sapiens) | HEK293T cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | HEK293-lenti-X cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | MDA-MB-231 cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | Hela cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | SH-SY5Y cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | U2OS cells | Cell Culture Facility, UC Berkeley | N/A | |
| Cell line (H. sapiens) | HEK293T-DNAJC5-CRISPR KO cells | This study | N/A | |
| Cell line (H. sapiens) | SH-SY5Y-DNAJC5-shRNA KD cells | This study | N/A | |
| Cell line (Mus musculus) | Mouse Embryonic Stem Cells | |||
| Cell line (M. musculus) | Mouse Embryonic Stem Cells-hDNAJC5-OE cells | This study | N/A | |
| Cell line (H. sapiens) | Human-Induced Pluripotent Stem Cells | University of Oxford; EBiSC repository | N/A | |
| Recombinant DNA reagent | mCherry-Rab5CA (Q79L) | Addgene | Cat# 35138 | |
| Recombinant DNA reagent | BFP-Rab5CA (Q79L) | This study | N/A | |
| Recombinant DNA reagent | SNCA (Myc-DDK-tagged)-Human synuclein, alpha | OriGene Technoogy | Cat# RC221446 | |
| Recombinant DNA reagent | CSP (DNAJC5) (NM_025219) Human Tagged ORF Clone | OriGene Technology | Cat# RC208826 | |
| Recombinant DNA reagent | TCH1003-MGC premier cDNA clone for USP19 | transOMIC | Cat# TCH1003 | |
| Recombinant DNA reagent | pCMV-α-synuclein-A30P | Gift of Dr. Thomas Südhof lab | N/A | |
| Recombinant DNA reagent | pCMV-α-synuclein-E46K | Gift of Dr. Thomas Südhof lab | N/A | |
| Recombinant DNA reagent | pCMV-α-synuclein-A53T | Gift of Dr. Thomas Südhof lab | N/A | |
| Recombinant DNA reagent | αS-2 (α-syn-α-syn) tandem dimer | Gift of Michael Woodside lab | N/A | |
| Recombinant DNA reagent | αS-4 (α-syn-α-syn-α-syn-α-syn) tandem Tetramer | Gift of Michael Woodside lab | N/A | |
| Recombinant DNA reagent | pCMV-αS-2 | This study | N/A | |
| Recombinant DNA reagent | pCMV-αS-4 | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-L115R | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-L116Δ | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5 (WT)-HaloTag | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5 (L115R)-HaloTag | This study | N/A | |
| Recombinant DNA reagent | pCMV-XPACK-DNAJC5 (L115R)-HaloTag | This study | N/A | |
| Recombinant DNA reagent | mNeonGreen-α-synuclein | This study | N/A | |
| Recombinant DNA reagent | 3H-α-synuclein | This study | N/A | |
| Recombinant DNA reagent | α-synuclein-3H | This study | N/A | |
| Recombinant DNA reagent | pCMV-XPACK-DNAJC5 (WT) | This study | N/A | |
| Recombinant DNA reagent | pCMV-XPACK-DNAJC5 (L115R) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DEAD XPACK-DNAJC5 (L115R) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-ΔJ (Δ14–82) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-ΔC10 (D189–198) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-ΔC20 (D179–198) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-ΔC30 (D169–198) | This study | N/A | |
| Recombinant DNA reagent | pCMV-DNAJC5-ΔC40 (D151–198) | This study | N/A | |
| Recombinant DNA reagent | pCMV-pOTC-GFP | This study | N/A | |
| Recombinant DNA reagent | pCMV-pOTC-3H-GFP | This study | N/A | |
| Recombinant DNA reagent | pX330-Venus-DNAJC5-Exon 4-gRNA | This study | N/A | |
| Recombinant DNA reagent | pLenti-CMV-DNAJC5 | This study | N/A | |
| Recombinant DNA reagent | pLenti-CMV-DNAJC5 (L115R) | This study | N/A | |
| Recombinant DNA reagent | plKO.1-DNAJC5-ShRNA | This study | N/A | |
| Software, algorithm | Fiji (ImageJ) | NIH | https://imagej.nih.gov/ij/ | |
| Software, algorithm | PyMOL | Schrödinger | https://pymol.org/2/ | |
| Software, algorithm | Prism 8 | Graphpad | https://www.graphpad.com/scientific-software/prism/ | |
| Software, algorithm | AlphaFold Protein Structure Database | DeepMind | https://alphafold.ebi.ac.uk/ |