O-GlcNAc signaling increases neuron regeneration through one-carbon metabolism in Caenorhabditis elegans
Figures
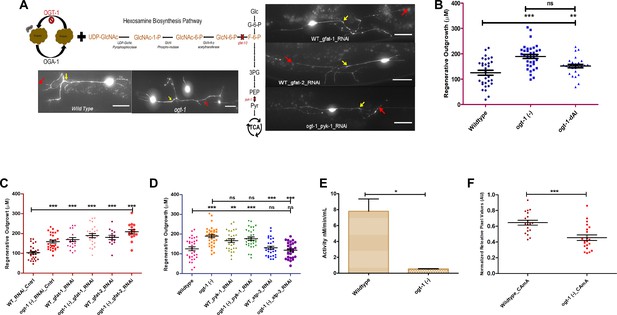
Blocking the HBS pathway is sufficient to phenocopy the neuronal regeneration of O-GlcNAc transferase (ogt-1) animals.
(A) Schematic diagram showing the hexosamine synthesis pathway linking glycolysis and ogt-1 function, and the representative image of the effect of ogt-1 mutation and gfat-1/gfat-2 and pyk-1 RNAi knockdown on regenerating neurons imaged at 24 hr (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (B) 24 hr regeneration data of wild-type (WT), ogt-1 deletion mutant (OGT-1) and ogt-1 dead allele (OGT-1-dAl, strain OG1135) worms on nematode growth media (NGM) after 24 hr. (C) 24 h regeneration data of control and gfat-1/gfat-2 RNAi experiments. (D) 24 hr regeneration data of control and RNAi experiment for pyk-1 and atp-3. (E) pyk-1 activity measured in WT and ogt-1 animal whole lysate using pyruvate kinase (PK) Assay Kit (Abcam, cat# Ab83432). (F) Relative amount of ATP measured using a fluorescence resonance energy transfer (FRET)-based ATP sensor. OGT-1-dAl; ogt-1-dead Allele, AU; Arbitrary Unit, scale bar = ~10 μM, all data shown in ± SEM, analytical methods student t-test and one-way ANOVA, *p <0.05, **p <0.01, ***p <0.001.
-
Figure 1—source data 1
Regeneration lengths measured with ImageJ/FIJI and ATP levels measured using ATP sensor data for Figure 1.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig1-data1-v1.xlsx

CeNGEN pyk expression pattern in the neurons of C. elegans, ATP levels and ATP utilization in Wildtype and ogt-1 animals.
(A) pyk-1 expression analysis in neuronal cell, single cell neuronal RNA sequencing (RNA-seq) data from worm base (https://wormbase.org/species/c_elegans/gene/WBGene00009126#0-9fce6b37d81-10) was used to generate the image. (B) pyk-2 expression analysis in neuronal cell, as for pyk-1. (C) Relative amount of ATP measured using ATP assay kit (Abcam, cat# Ab83355) in whole worm lysate. (D) Relative amount of pyrophosphate (PPi) measured using pyrophosphate assay kit (Abcam, cat# Ab112155) in whole worm lysate. All data shown in ± SEM, analytical methods, student t-test was used *p <0.05, **p <0.01, ***p <0.001.
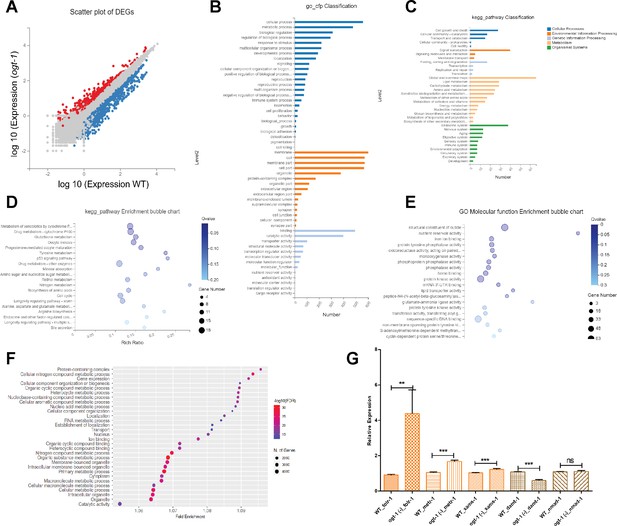
RNA sequencing (RNA-seq) data analysis suggests important role of one-carbon metabolism (OCM) and related pathways in O-GlcNAc transferase (ogt-1)-mediated neuronal regeneration.
(A) A scatter plot of differentially expressed genes (DEGs) identified in RNA-seq between wild-type (WT) and ogt-1 mutants. (B) Gene Ontology (GO) classification of DEGs in WT-vs-ogt-1. (C) KEGG pathway classification of DEGs in WT-vs-ogt-1. (D) KEGG pathway enrichment bubble plot of DEGs. (E) Enrichment bubble plot of GO molecular function analysis DEGs. (F) GO analysis of DEGs identified in neuron-specific RNA-seq between WT and ogt-1 mutant (FDR0.1). (G) qRT-PCR of selected genes involved in one-carbon metabolism (OCM) (folr-1, metr-1, and sams-1) and nucleic acid methyltransferases and demethylases (damt-1 and nmad-2). All data shown ± SEM, Student t-test; *p<0.05, **p<0.01, ***p<0.001.
-
Figure 2—source data 1
List of DEGs, GO and KEGG enrichment analysis of from whole body RNAseq data.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig2-data1-v1.xls
-
Figure 2—source data 2
List of DEGs, genes showing 2 fold expression changes and GO enrichment analysis form neuronal RNAseq Data.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig2-data2-v1.xlsx
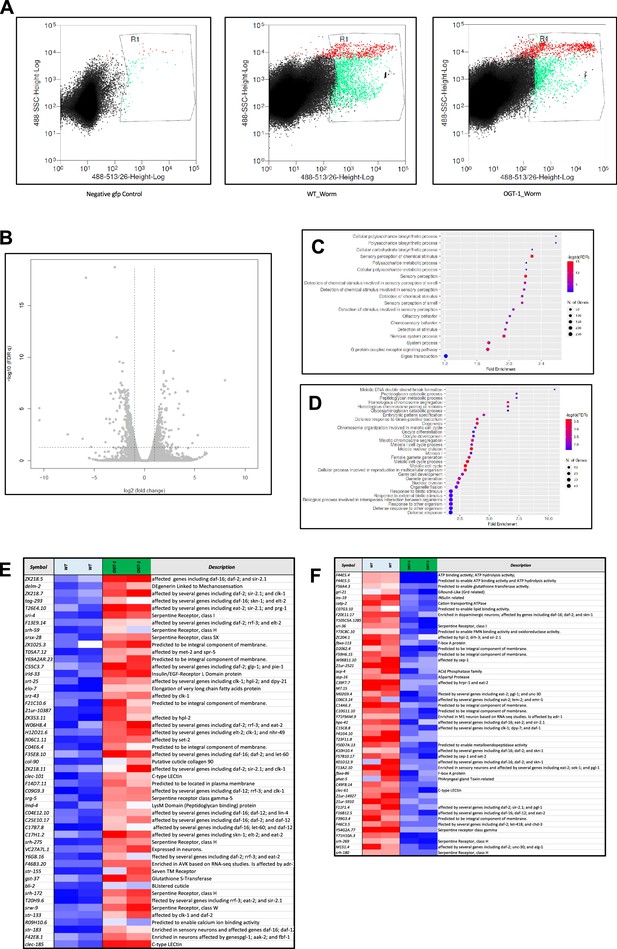
FACs shorting, Neuronal RNAseq DEGs, Gene Ontology (GO) pathways analysis and list of top 50 DEGs.
(A) Representative image of fluorescence-activated cell sorting (FACs) sorting for GFP tagged neuronal cells used for RNA isolation and RNA sequencing (RNA-seq) analysis. GFP control (left), wild type (middle), and ogt-1 mutant (right) worms, respectively. (B) Volcano plot for differentially expressed genes (DEGs) FDR0.05. (C) Gene Ontology (GO) analysis of twofold up-regulated DEGs in WT-vs-ogt-1 (FDR0.1) (D) GO analysis of twofold down regulated DEGs in WT-vs-ogt-1 (FDR0.1). (E) List of top 50 up-regulated genes, and (F) top 50 down-regulated genes and their function, in ogt-1 animals, identified in neuron-specific RNA-seq.
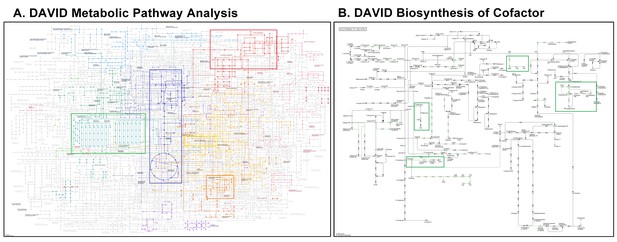
Metabolic Pathway Analysis using DAVID from neuronal differentially expressed genes from neuronal RNAseq.
(A) Visualization of metabolic pathway enriched in differentially expressed genes (FDR0.1) identified in neuron-specific RNA sequencing (RNA-seq) analysis using ‘DAVID Metabolic Pathway Analysis’ tool. Top highlighted pathways are glycolysis (blue); lipid metabolism (green); nucleotide metabolism (red); serine synthesis pathway (light yellow); and one-carbon metabolism (OCM) and related pathways (dark yellow), respectively. (B) Pathway analysis of co-factor mediated biosynthesis of differentially expressed genes (FDR0.1) identified in neuron-specific RNA-seq analysis using the ‘DAVID Biosynthesis of Cofactors Analysis’ tool. Most affected pathways (green highlighted) include those related to OCM (folate, methionine, and SAM metabolism); Transsulfuration pathway (Cystein and Glutathione metabolism); and ATP production.
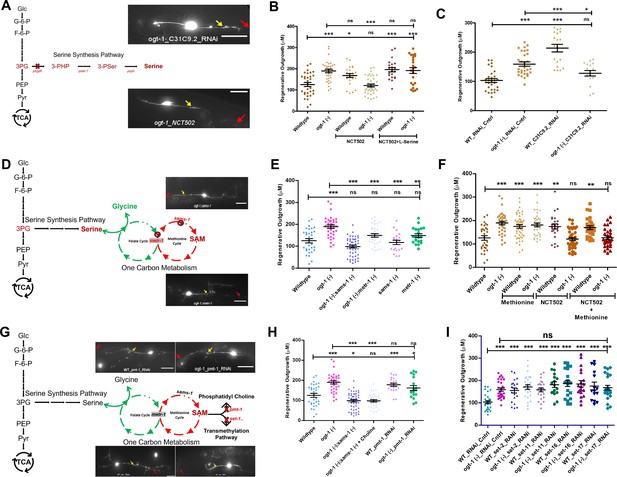
Functional one-carbon metabolism (OCM) and serine synthesis pathway (SSP) are essential for neuronal regeneration in O-GlcNAc transferase (ogt-1) worms.
(A) Schematic representation showing glycolysis and the SSP, along with representative images at 24 hr neuron regeneration in conditions blocking the SSP in ogt-1 mutants using either neuron-specific RNAi or NCT502 drug (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (B) Effect of NCT502 drug and supplementation of serine on wild-type (WT) and ogt-1 mutant 24 hr neuronal regeneration. (C) Effect of neuron-specific RNAi against C31C9.2 (ortholog of human PHGDH gene) on WT and ogt-1 mutant neuronal regeneration. (D) Schematic representation of the metabolic link between glycolysis and OCM via SSP, along with representative images of 24 hr neuron regeneration with different OCM gene mutations in ogt-1 background (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (E) Effects of metr-1 and sams-1 mutations on enhanced regeneration in ogt-1 worms. (F) Effects of methionine supplementation on regeneration in WT, ogt-1 animals, and on the phgdh-1 inhibitor drug NCT502. (G) Schematic representation of OCM metabolite SAM usage in lipogenesis and transmethylation, along with representative images of neuron regeneration when they are blocked (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (H) 24 hr neuron regeneration with choline supplementation in ogt-1/sams-1 dual mutant and neuron-specific RNAi against pmt-1. (I) 24 hr neuron regeneration when blocking methyltransferases by neuron-specific RNAi (set-2, set-11, set-16, and set-17) in WT and ogt-1 animals. scale bar = ~10 μM, all data shown in ± SEM, one-way ANOVA *p<0.05, **p<0.01, ***p<0.001.
-
Figure 3—source data 1
Regeneration lengths measured with ImageJ/FIJI data for Figure 3.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig3-data1-v1.xlsx

Effect of Serine, NCT502 and methionine supplementation on Neuronal regeneration and pyk-1 activity, systemic RNAi of C31C9.2 and Expression patter important OCM genes from Neuronal RNAseq.
(A) The effects of NCT502 mediated inhibition of the serine synthesis pathway and serine supplementation on regeneration in akt-1 (gain of function) and akt-1 (loss of function) mutations in the O-GlcNAc transferase (ogt-1) background. (B) 24 hr neuron regeneration with systemic RNAi knockdown against C31C9.2 (ortholog of human PHGDH). (C) pyk-1 activity in wild-type (WT) worms grown with and without NCT502 treatment. (D) pyk-1 activity in WT and ogt-1 worms grown with NCT502 treatment. (E) The effect of different doses of methionine supplementation on 24 hr neuron regeneration in WT worms. (F) Expression, patterns of selected genes involved in OCM (sams-1, metr-1, folr-1, and mthf-1), Transmethylation (damt-1 and nmad-1), and lipogenesis (pmt-1 and pmt-2) in neuronal cell RNA sequencing (RNA-seq) analysis which passed FDR 0.1. All data shown in ± SEM, analytical methods; student t-test and one-way ANOVA were used; ns, no significance; *p <0.05, **p <0.01, ***p<0.001.
-
Figure 3—figure supplement 1—source data 1
Regeneration lengths measured with ImageJ/FIJI data for Figure 3—figure supplement 1A.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig3-figsupp1-data1-v1.xlsx
-
Figure 3—figure supplement 1—source data 2
Regeneration lengths measured with ImageJ/FIJI data for Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig3-figsupp1-data2-v1.xlsx
-
Figure 3—figure supplement 1—source data 3
Regeneration lengths measured with ImageJ/FIJI data for Figure 3—figure supplement 1E.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig3-figsupp1-data3-v1.xlsx
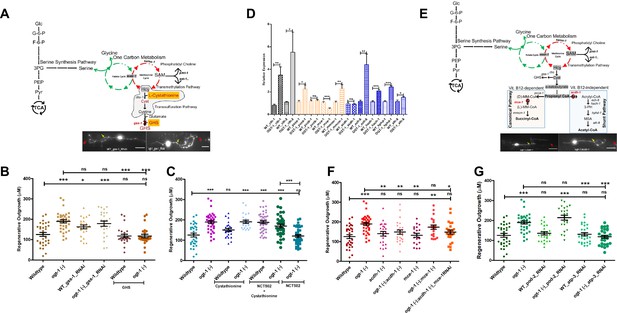
The transsulfuration pathway (TSP) leading to acetyl-CoA production mediates enhanced regeneration in O-GlcNAc transferase (ogt-1) animals.
(A) Schematic representation of the transsulfuration pathway (TSP; shaded area) branch of OCM, along with supplementation with TSP metabolites L-cystathionine, Glutathione, and neuron-specific RNAi against Glutathione synthetase (gss-1) with its effect on 24 hr neuron regenerating neuron (representative images) (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (B) Effects of GHS supplementation and neuronal RNAi knockdown against gss-1 on neuronal regeneration in wild-type (WT) and ogt-1 worms. (C) Effects of L-cystathionine supplementation on neuronal regeneration in WT and ogt-1 worms, with or without SSP blocking by NCT502. (D) qRT-PCR of selected genes involved in transsulfuration (cth-1 and cth-2), as well as the related downstream vitamin B12 dependent canonical pathways (pcca-1, pccb-1, mce-1, and mmcm-1) and the vitamin B12 independent Shunt pathway (acdh-1, ech-6, hach-1, hphd-1, and alh-8). (E) Schematic representation of the TSP metabolites L-Cystathionine metabolism into succinyl-CoA and acetyl-CoA via canonical and shunt pathway, respectively and genes involved with indicated mutants (acdh-1 and mce-1) used in the study, along with a representative regenerating neuron image (yellow arrow indicates the proximal and red arrow indicated distal point of injury). (F) Effect of acdh-1 and mce-1 mutation in WT and ogt-1 background on neuronal regeneration. (G) Effect of blocking lipid synthesis from acetyl CoA and ATP production on regeneration in WT and ogt-1. scale bar = ~10 μM, all data shown in ± SEM, analytical methods student t-test and one-way ANOVA were used *p <0.05, **p <0.01, ***p <0.001 .
-
Figure 4—source data 1
Regeneration lengths measured with ImageJ/FIJI data for Figure 4.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig4-data1-v1.xlsx
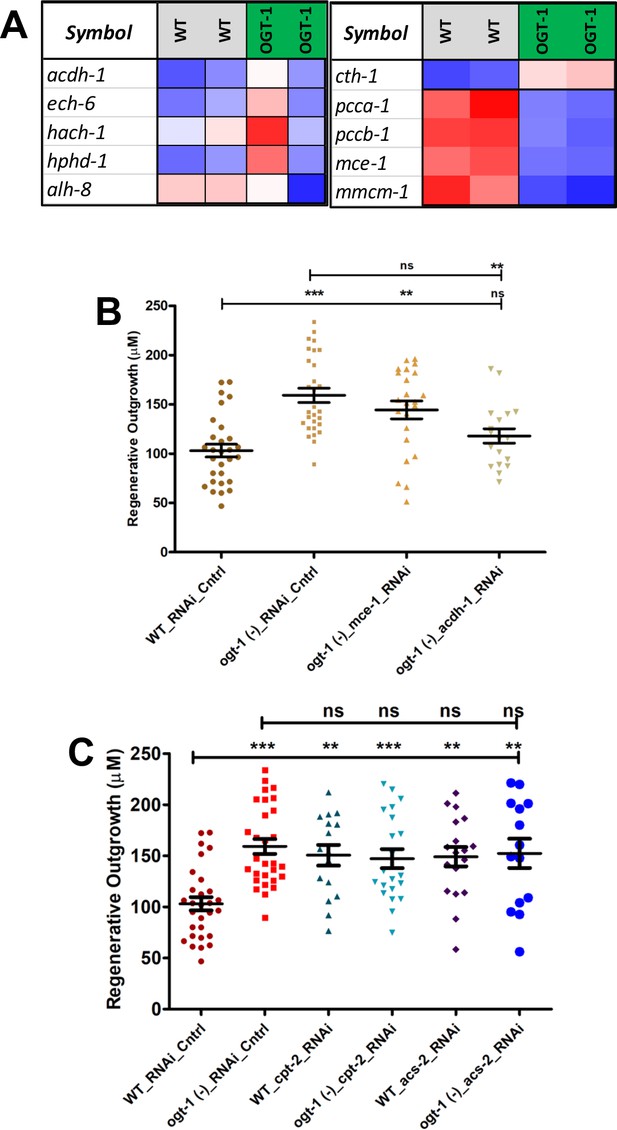
Expression patter important genes from TSP in Neuronal RNAseq data and effect of gene knock down of TSP and lipid metabolism.
(A) Expression patterns, of selected genes involved in vitamin B12 independent shunt pathway (acdh-1, each-6, hach-1, hphd-1, and alh-8) and vitamin B12 dependent canonical pathway (cth-1, pcca-1, pccb-1, mce-1, and mmc-1) downstream to transsulfuration pathway (TSP), in neuronal cell RNA sequencing (RNA-seq) analysis which passed FDR 0.1. (B) The effect on 24 hr neuron regeneration from neuron-specific RNAi knockdown of acdh-1 and mce-1 in ogt-1 and wild-type (WT) worms. (C) The effect on 24 hr neuron regeneration from neuron-specific RNAi knockdown of cpt-2 and acs-2 in ogt-1 and WT worms. All data shown in ± SEM, analytical methods; one-way ANOVA was used; ns, no significance; *p <0.05, **p <0.01, ***p <0.001.
-
Figure 4—figure supplement 1—source data 1
Regeneration lengths measured with ImageJ/FIJI data for Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig4-figsupp1-data1-v1.xlsx
-
Figure 4—figure supplement 1—source data 2
Regeneration lengths measured with ImageJ/FIJI data for Figure 4—figure supplement 1C.
- https://cdn.elifesciences.org/articles/86478/elife-86478-fig4-figsupp1-data2-v1.xlsx
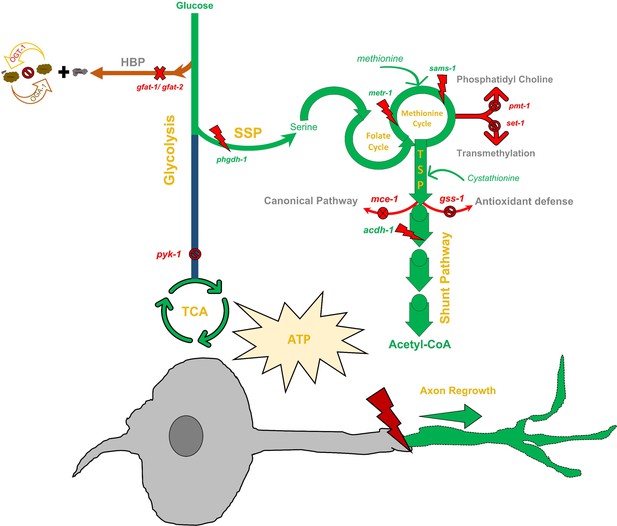
The metabolic pathway essential for enhanced neuronal regeneration in O-GlcNAc transferase (ogt-1) animals.
A detailed schematic of the metabolic pathway essential for the enhanced regeneration in ogt-1 animals with the tested genes, metabolite supplementations, and pharmacological treatments indicated. As highlighted in green, ogt-1 mutations required metabolic pathways apart from glycolysis including one-carbon metabolism (OCM), the SPP, the transsulfuration pathway (TSP), and the vitamin B12 independent shunt pathway to support enhanced regeneration. Dispensable metabolic branches are shown in red. Different genetic manipulations (RNAi and mutants) in respective pathways are mentioned at respective places along with methionine and cystathionine metabolites supplementation. Details are mentioned in the main manuscript text.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Escherichia coli) | Caenorhabditis Genetics Centre | Wormbase, E. coli OP50 | WBStrain00041969 | Other names: CBb1 |
| Recombinant DNA reagent | Source Bioscience | Vidal and Ahringer RNAi Libraries in HT115 (D3) E. coli | RNAi Library | |
| Chemical compound, drug | L-methionine | Fisher Scientific | Cat#: AC166160025 CAS: 63-68-3 | |
| Chemical compound, drug | L-Cystathionine | Sigma | Cat#: C7505-10MG | |
| Chemical compound, drug | L-Methionine | Sigma | Cat#: M-9625 | |
| Chemical compound, drug | L-Serine | Sigma | Cat#: S-4500 | |
| Chemical compound, drug | Choline Chloride | Millipore Sigma | Cat#: C7017-5G, CAS:67-48-1 | |
| Chemical compound, drug | Sodium Chloride (NaCl) | Fisher Bioreagents | Cat#: BP358-1 CAS:7647-14-5 | |
| Chemical compound, drug | β-meracaptoethanol | Fisher Scientific | Cat#: AC125470100 CAS: 60-24-2 | |
| Chemical compound, drug | Trizol | ThermoFisher Scientific | Cat#: 15596018 | |
| Chemical compound, drug | PowerUp SYBR Green Master Mix | Applied Biosystems | Cat#: A25741 | |
| Chemical compound, drug | Agarose | Fisher Bioreagents | Cat#: BP160-500, CAS: 9012-36-6 | |
| Chemical compound, drug | Pronase | Sigma- Aldrich | SKU# 10165921001 | |
| Chemical compound, drug | DMSO (Dimethyl Sulfoxide) | ThermoFisher Scientific | Cat#: 85190, Cas:67-68-5 | |
| Chemical compound, drug | Water, Molecular Grade, Sterile, DEPC Free | Fisher Scientific | CAS: 7732-18-5 Cat#: R91450001G, | |
| Chemical compound, drug | NCT502 | MedChemExpress (MCE) | Cat#: HY-117240 | |
| Chemical compound, drug | Polybead polystyrene | Polysciences | Cat#08691–10 | |
| Commercial assay or kit | ATP Assay Kit (Colorometric/ Fluorometric) | Abcam | Ab83355 | |
| Commercial assay or kit | Pyrophosphate Assay Kit (Fluorometric) | Abcam | Ab112155 | |
| Commercial assay or kit | Pyruvate Kinase (PK) Assay Kit (Colorimetric) | Abcam | Ab83432 | |
| Commercial assay or kit | BCA Protein Quantification Kit | Abcam | Ab102536 | |
| Commercial assay or kit | RNAeasy columns | QIAGEN | Cat#74034 | |
| Commercial assay or kit | Direct-zol RNA Miniprep Plus Kit | Zymo Research | Cat# R2070 | |
| Strain, strain background (C. elegans) | SK4005 | Taub et. al. | WT (zdis5 pmec-4::GFP) | |
| Strain, strain background (C. elegans) | NA | Taub et. al. | ogt-1(ok1474)_zdis-5 pmec-4::GFP | |
| Strain, strain background (C. elegans) | OG1135 | This study | Ogt-1 (OG1135)_TU3568_ pmec-4::GFP | |
| Strain, strain background (C. elegans) | RB1342 | Taub et. al. | ogt-1(ok1474) | |
| Strain, strain background (C. elegans) | NA | Taub et. al. | TU3568 (sid-1(pk3321) him-5(e1490) V; lin-15B(n744) X; uIs71[(pCFJ90) pmyo-2::mCherry +pmec-18::sid-1]) | |
| Strain, strain background (C. elegans) | NA | Taub et. al. | ogt-1(ok1474)_TU3568 | |
| Strain, strain background (C. elegans) | RB2240 | CGC | sams-1(ok3033) | |
| Strain, strain background (C. elegans) | NA | in this study | sams-1_zdis-5 | |
| Strain, strain background (C. elegans) | NA | in this study | ogt-1;sams-1_zdis-5 | |
| Strain, strain background (C. elegans) | RB755 | CGC | metr-1(R03D7.1(ok521)) | |
| Strain, strain background (C. elegans) | NA | in this study | metr-1_zdis-5 | |
| Strain, strain background (C. elegans) | NA | in this study | ogt-1;metr-1_zdis-5 | |
| Strain, strain background (C. elegans) | VC1011 | CGC | acdh-1(ok1489) | |
| Strain, strain background (C. elegans) | NA | in this study | acdh-1_zdis-5 | |
| Strain, strain background (C. elegans) | NA | in this study | ogt-1;acdh-1_zdis-5 | |
| Strain, strain background (C. elegans) | RB512 | CGC | mce-1(D2030.5(ok243)) | |
| Strain, strain background (C. elegans) | NA | in this study | mce-1_zdis-5 | |
| Strain, strain background (C. elegans) | NA | in this study | mce-1_TU3568_zdis-5 | |
| Strain, strain background (C. elegans) | NA | in this study | ogt-1;mce-1_TU3568_zdis-5 | |
| Strain, strain background (C. elegans) | CG122 | Taub et. al. | ogt-1;akt-1(mg144) | |
| Strain, strain background (C. elegans) | CG125 | Taub et. al. | ogt-1;akt-1(ok525) | |
| Strain, strain background (C. elegans) | MS2495 | Soto and Rivera et. al. | irIs158 (normal ATP sensor, CAmA) | |
| Strain, strain background (C. elegans) | NA | In this study | ogt-1; CAmA | |
| Sequence-based reagent | folr-1_F | C17G1.1 | RT-qPCR | GGCTTCCATTGCCGTCATAA |
| Sequence-based reagent | folr-1_R | C17G1.1 | RT-qPCR | GCTAACCACTGGCTCACGAT |
| Sequence-based reagent | metr-1_F | R03D7.1 | RT-qPCR | CCCGAATCGCAGTTATCCGA |
| Sequence-based reagent | metr-1_R | R03D7.1 | RT-qPCR | GAAGCAGCTGGGAGGAATGA |
| Sequence-based reagent | sams-1_F | C49F5.1 | RT-qPCR | CACTCACCGACGAAGAGCTT |
| Sequence-based reagent | sams-1_R | C49F5.1 | RT-qPCR | GTGACCGAAGTGACCGTTCT |
| Sequence-based reagent | dmat-1_F | C18A3.1 | RT-qPCR | ATTGCCGATCCACCATGGTT |
| Sequence-based reagent | dmat-1_R | C18A3.1 | RT-qPCR | CCGATTTGTGATCCAGAAAGCA |
| Sequence-based reagent | nmad-1_F | F09F7.7 | RT-qPCR | GCACAGTCACAAAGTGGTCG |
| Sequence-based reagent | nmad-1_R | F09F7.7 | RT-qPCR | CGTACTCTGGCATTCCGACA |
| Sequence-based reagent | cth-1_F | F22B8.6 | RT-qPCR | TCTGATATTATTATGGGAGCCGC |
| Sequence-based reagent | cth-1_R | F22B8.6 | RT-qPCR | TGCAGTCATGAGCTTCAAGGA |
| Sequence-based reagent | cth-2_F | ZK1127.10 | RT-qPCR | TTGGAGCGGATGTTGTCGTT |
| Sequence-based reagent | cth-2_R | ZK1127.10 | RT-qPCR | AGTGAGCTCTCATTCTGATGTGA |
| Sequence-based reagent | pcca-1_F | F27D9.5 | RT-qPCR | AAATGGGAGAACAGGCCGTT |
| Sequence-based reagent | pcca-1_R | F27D9.5 | RT-qPCR | TGGGTGATTGGAAGTGGGTG |
| Sequence-based reagent | pccb-1_F | F52E4.1 | RT-qPCR | AAAGTTTGCTGCTGGATGCC |
| Sequence-based reagent | pccb-1_R | F52E4.1 | RT-qPCR | AATCTTTGGAACGGTGGCCT |
| Sequence-based reagent | mce-1_F | D2030.5 | RT-qPCR | TGTCCACAAGAACCATGGCT |
| Sequence-based reagent | mce-1_R | D2030.5 | RT-qPCR | CGCCGAATGGATGAAGAAGC |
| Sequence-based reagent | mmcm-1_F | ZK1058.1 | RT-qPCR | CAATGTTGCCGATCCTTGGG |
| Sequence-based reagent | mmcm-1_R | ZK1058.1 | RT-qPCR | TCCAACAATCACATCTTTTCCAGC |
| Sequence-based reagent | acdh-1_F | C55B7.4 | RT-qPCR | TCCGAGCTTCATCCACTTGT |
| Sequence-based reagent | acdh-1_R | C55B7.4 | RT-qPCR | CTGACCGAACTGTTCTCTCTGT |
| Sequence-based reagent | ech-6_F | T05G5.6 | RT-qPCR | AGGTGGAAACGAGTTGGCAA |
| Sequence-based reagent | ech-6_R | T05G5.6 | RT-qPCR | GCTCACAATACCGTGCTCCT |
| Sequence-based reagent | hach-1_F | F09F7.4 | RT-qPCR | AGTCATCAGATCGTTCGAGCC |
| Sequence-based reagent | hach-1_R | F09F7.4 | RT-qPCR | TCGGTGATTTGTCGGTGAGT |
| Sequence-based reagent | hphd-1_F | Y38F1A.6 | RT-qPCR | CAAAGATCTCCACGCCCTGA |
| Sequence-based reagent | hphd-1_R | Y38F1A.6 | RT-qPCR | GGAGAGTCCGTGGCAAAGAT |
| Sequence-based reagent | alh-8_F | F13D12.4 | RT-qPCR | GGGAGCTCAGGTTCCACTTG |
| Sequence-based reagent | alh-8_R | F13D12.4 | RT-qPCR | TGAAGATGGCCGTTCCGTTT |
| Sequence-based reagent | act-1_F | T04C12.6 | RT-qPCR | TCGGTATGGGACAGAAGGAC |
| Sequence-based reagent | act-1_R | T04C12.6 | RT-qPCR | CATCCCAGTTGGTGACGATA |





