Differential modification of the C-terminal tails of different α-tubulins and their importance for microtubule function in vivo
Figures
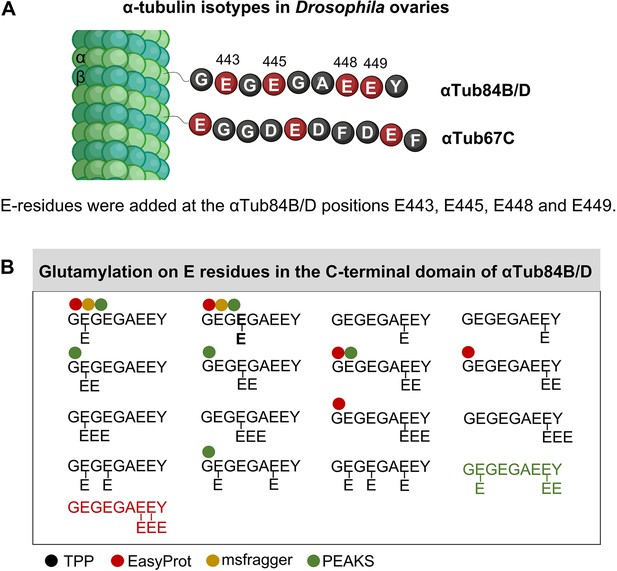
Mass spectrometry analysis of glutamylation of the C-terminal domains of Drosophila α-tubulins.
(A) Schematic representation showing how the C-terminal regions of αTub84B/D can be modified by glutamic acid residues added to the sidechains of E443, E445, E448, and E449 in Drosophila ovaries. (B) The C-terminal peptides containing the complete primary sequence were analyzed. The structure of the different C-terminal tail peptides of αTub84B/D modified by glutamylation is shown starting with G442. Different combinations of Glu (E) sidechain modifications added at positions E443, E445, E448, and E449 of αTub84B/D were found. The glutamylation patterns were determined by the Trans-Proteomics Pipeline (TPP; Supplementary file 1), and the positions with the highest probabilities for E sidechain modification according to TPP are shown with black typeface. The ‘E’ in bold typeface indicates the most frequent position for mono-Glu modification identified in this study. Peptides identified by EasyProt, MSfragger, and PEAKS, respectively, are marked with a red, yellow, and green dot, respectively. These peptide identifications are shown in Supplementary file 1. Modifications only identified by EasyProt or PEAKS are displayed in the respective font color. Scheme was drawn by biorender.com.
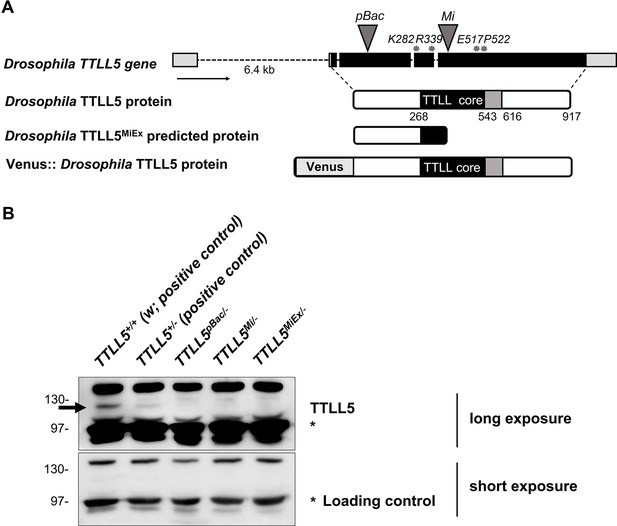
Gene structure and protein expression of the Drosophila TTLL5 alleles.
(A) The TTLL5 gene structure is based on the FlyBase data for the CG31108-RA transcript. The positions of the loss-of-function mutations are marked on the TTLL5 gene. Gray triangles: transposon insertions. Gray asterisks: codons targeted for generating InDels and point mutations. All TTLL5 alleles were analyzed as hemizygous animals over Df(3R)BSC679, which removes the region of the TTLL5 gene. (B) Western blot from the soluble fraction of ovarian extracts. w (TTLL5+/+) contains two wild-type copies of TTLL5 and expressed the highest levels of TTLL5. The TTLL5 signal is strongly reduced or abolished in the null mutants TTLL5pBac/-, TTLL5Mi/-, and TTLL5MiEx/-. The hemizygous TTLL5+ ovaries (TTLL5+/-) expressed less TTLL5 than w. The signal of an unspecific band (labeled with *), produced after a short exposure, was used as the loading control. In the following, we will refer to the genotypes as w for the control (TTLL5+/+) and TTLL5allele for the hemizygous mutants (TTLL5allele/-).
-
Figure 2—source data 1
Source data for Figure 2.
- https://cdn.elifesciences.org/articles/87125/elife-87125-fig2-data1-v2.zip
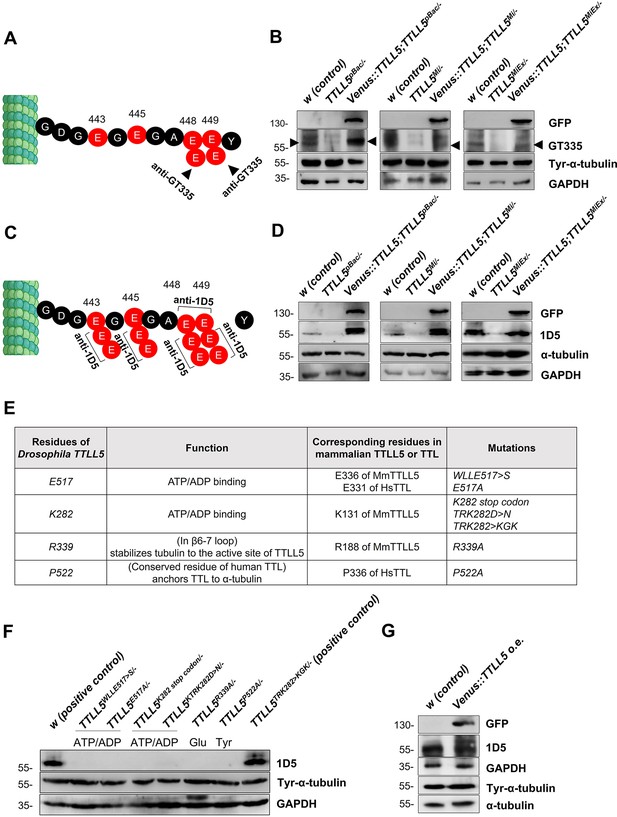
TTLL5 is required for the glutamylation of α-tubulin in ovaries.
(A, C) Sites on αTub84B/D expected to be recognized by the antibody against monoglutamylated α-Tubulin (GT335; A) and by the 1D5 antibody recognizing polyglutamylated αTub (C). (B, D) The glutamylation signal, produced by western blotting with the GT335 and 1D5 antibodies, respectively, is lower in TTLL5pBac/-, TTLL5Mi/-, and TTLL5MiEx/- than in the w control but is restored and even elevated in mutant ovaries that express Venus::TTLL5 under MattubGal4 control. The upshifted bands recognized by 1D5 can be seen in the rescued TTLL5pBac/- and TTLL5Mi/- mutants. The total tyrosinated α-tubulin levels seemed unaffected by the TTLL5 levels. α-tubulin and GAPDH served as loading controls. (E) The genotypes and rationale of the TTLL5 alleles generated by CRISPR/Cas9 are listed. The selected residues are predicted to be either important for the glutamylation or tyrosination function based on the alignment shown in Tables 1 and 3. (F) Western blotting shows that the polyglutamylation signal is absent in all point mutants. w with two copies of TTLL5+is the wild-type control and the hemizygous TTLL5TRK282>KGK282/- still contains the crucial K282 residue and behaves like the control. Relative to the loading controls, 1D5 signals decrease slightly in the hemizygous situation with only one TTLL5+ copy. (G) Stronger upshifted bands were observed with the polyglutamylation antibody (1D5) upon Venus::TTLL5 overexpression in wild-type ovaries. Total α-tubulin levels were similar in samples with excessive expression of TTLL5. GAPDH was a loading control. All mutant TTLL5 alleles were analyzed as hemizygous animals over Df(3)BSC679. The genotypes of the rescued animals were MattubGal4>UAS-Venus::TTLL5;TTLL5alleles/Df(3R)BSC679. The genotype of the Venus::TTLL5 overexpressing animals was MattubGal4>UAS-Venus::TTLL5.
-
Figure 3—source data 1
Source data for Figure 3.
- https://cdn.elifesciences.org/articles/87125/elife-87125-fig3-data1-v2.zip
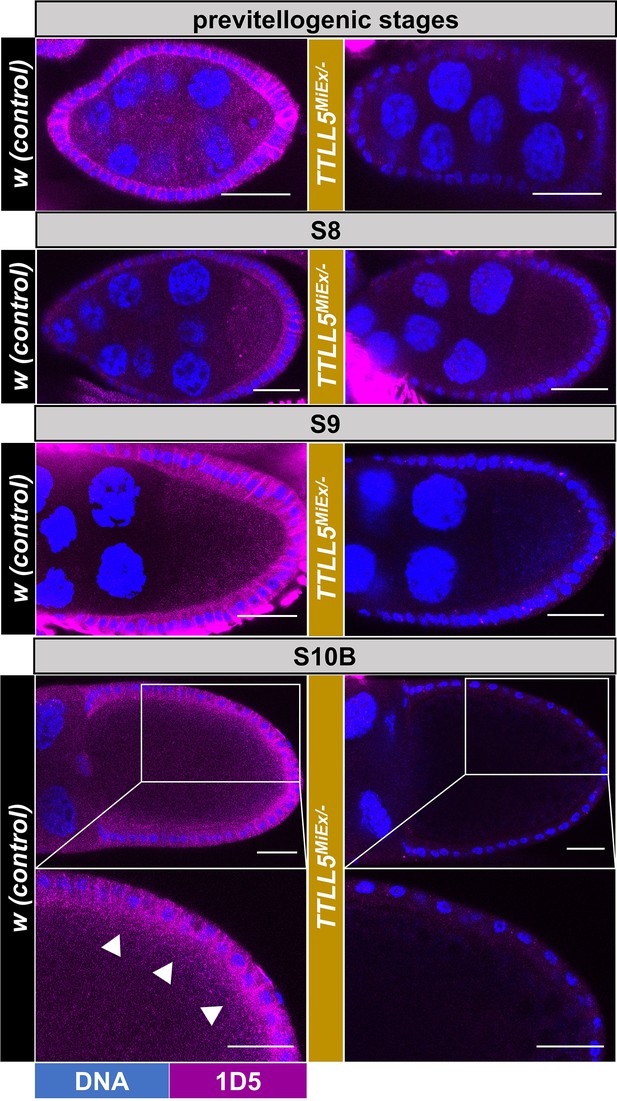
Spatial distribution of polyglutamylated microtubules in oocytes.
Confocal micrographs showing representative oocytes and follicle cells from early to late stages of w controls and TTLL5MiEx/- mutants. Polyglutamylation (1D5) signals are shown in magenta and Hoechst (blue) stains the DNA. A 1D5 signal is seen in follicle cells and oocytes from previtellogenic to late-stage oocytes. For the S10B oocytes, a high-power magnification of the posterior half is shown, too. At this stage, a slightly biased 1D5 signal intensity was seen in the cortical region of the oocyte (marked by white arrowheads). The 1D5 signal is virtually absent in the TTLL5 mutants. Imaging conditions and confocal microscope settings were identical for the two genotypes. The genotype of the control was w. Genotypes for TTLL5MiEx/- were TTLL5MiEx/Df(3R)BSC679.

Effect of TTLL5 on Staufen localization refinement in oocytes.
(A–D) Confocal micrographs showing S10B oocytes of w controls (A), TTLL5pBac/-, TTLL5Mi/-, and TTLL5MiEx/- mutants (B), TTLL5pBac/-, TTLL5Mi/-, and TTLL5MiEx/- mutants rescued by Mattub4>UASP-Venus::TTLL5 (C), TTLL5E517A/-, TTLL5P522A/-, and TTLL5R339A/- mutants (D). Anti-Staufen is shown in green, anti-GFP in red, and Hoechst (DNA) in blue. (E) Quantification of the Staufen crescent length along the posterior cortex based on (A–D) ***p<0.005. z-stack: 12 μm. (F) Confocal micrographs showing previtellogenic and S9 egg chambers of w controls, TTLL5pBac/-, TTLL5MiEx/-, and TTLL5E517A/- mutants. Staufen signal is shown in green, and Hoechst in blue. Scale Bar: 25 μm. TTLL5MiEx/- and TTLL5E517A/- were hemizygous over Df(3R)BSC679. The genotype for TTLL5pBac/- was MattubGal4/+; TTLL5pBac/Df(3R)BSC679. The genotypes of the rescued animals were MattubGal4/UASP-Venus::TTLL5; TTLL5 alleles/Df(3R)BSC679.

Role of TTLL5 in fast ooplasmic streaming.
(A) Example of an S10B oocyte used for particle flow measurements based on time-lapse movies of ooplasmic streaming (Videos 1–5). Scale bar: 25 μm. (B) Kymographs were generated along a line close to the posterior cortex on one side of the ooplasm (region marked by a yellow line in A). Sample kymographs were shown based on Videos 1–5. Streaming was shown from two representative oocytes for each movie. The first and second oocytes are marked as sample 1 (#1) and sample 2 (#2), respectively. The unidirectional streaming along the posterior cortex is seen in the two control and the TTLL5 rescue samples, and in sample 1 of the TTLL5 overexpressing oocyte. Disordered streaming is seen in the second sample of the TTLL5 overexpressing line and both samples of the TTLL5 mutants. (C) Quantification of streaming patterns seen in the different genotypes. 100% control (n = 17), 71%TTLL5 rescued (n = 7), and 71% TTLL5 overexpression (n = 21) S10B oocytes showed an overall circular unidirectional streaming pattern. In contrast, 76% TTLL5MiEx/- (n = 21) and 100% TTLL5E517A/- (n = 10) oocytes showed an abnormal streaming pattern. The stronger penetrance of the TTLL5E517A/- phenotype (C) compared to the null mutant could be seen as evidence for a slight dominant effect of the point mutation. This is consistent with the stronger expressivity of the streaming defects observed in the movies (B, D), although the numbers appear a bit small to firmly conclude this. (D) Schematic representation of the different streaming patterns observed. The frequency with which each streaming pattern was observed in the different genotypes is indicated from high (left) to low (right). ‘Circular streaming pattern’ was further subdivided into ‘central streaming pattern (top)’ and ‘anterior-biased streaming pattern (below).’ The ‘central streaming’ was observed in all controls, 71% of the TTLL5 rescue, 43% of the TTLL5 overexpression, and 21% of the TTLL5MiEx/- oocytes. The ‘anterior-biased streaming’ was frequently seen in 28% of the TTLL5 overexpression ovaries, where the main circular center moved to the anterior part, leaving the posterior with a chaotic streaming flow. ‘Abnormal streaming’ included the oocytes that showed an overall chaotic flow direction (upper one) and the partially disrupted flow (below). The abnormal streaming patterns were mainly seen in the situations when TTLL5 was insufficient or inactive, and at a low frequency also in TTLL5 rescued and TTLL5 overexpressing ovaries. The genotype of the control was w or +/Df(3)BSC679. The genotypes for TTLL5MiEx/- and TTLL5E517A/- were both over Df(3)BSC679. The genotype of the rescued flies was MattubGal4/UAS-Venus::TTLL5; TTLL5MiEx/Df(3)BSC679. The genotype of TTLL5 overexpressing flies was MattubGal4/UAS-Venus::TTLL5.

Role of TTLL5 for kinesin distribution in ovaries.
(A, B) Khc levels remained unchanged between w control, TTLL5 deficient mutants, rescued TTLL5 mutants, and TTLL5 overexpressing ovaries. Anti-GFP antibodies reveal the expression of Venus:TTLL5. GAPDH served as the loading control. (C, E) Confocal micrographs showing the Khc distribution in stage 10B oocytes. (C) White arrowheads point to the posterior enrichment of Khc along the cortical region in the wild-type control and TTLL5 overexpressing oocytes. (D) Blind quantification of the posterior enrichment of Khc. The frequency of oocytes with posterior enrichment is shown graphically and numerically. N, number of oocytes evaluated. (E) A white line was drawn along the anterior–posterior axis of the oocyte using Fiji. The lines start at the nurse cell oocyte border on the left and end at the posterior follicle cells. The line width was 50 units. (G) Intensity charts were plotted based on the line drawn along the AP axis in (E). The orange arrowheads in the oocytes and the intensity charts point to the regions showing higher Khc accumulation in the inner region of the oocyte. (F) Fraction of the oocytes that showed inner hyperaccumulation of Khc were quantified. N, number of oocytes evaluated. The percentage of oocytes showing inner enrichment of Khc is shown graphically and numerically. Judging Khc distribution was done blindly by two persons. The fractions were taken from the mean values obtained from the two persons. Scale bar: 25 μm. The Khc signal is shown in green, and Hoechst in blue. The genotype for controls was w or +/Df(3R)BSC679. Genotype for TTLL5pBac/-: MattubGal4/+, TTLL5pBac/Df(3R)BSC679; TTLL5MiEx/-: TTLL5MiEx/Df(3R)BSC679. Genotype for TTLL5 overexpression: MattubGal4/UAS-Venus::TTLL5.
-
Figure 7—source data 1
Source data for Figure 7.
- https://cdn.elifesciences.org/articles/87125/elife-87125-fig7-data1-v2.zip

Role of TTLL5 in the transport of mitochondria in the L1 wing nerve.
(A) Representative still image of GFP-labeled mitochondria in axons of the L1 vein of both control and TTLL5E517A/- wings. The position of the L1 region is indicated in the picture above. (B) Scheme of the transport behavior of the mitochondria in the wing neuron. See the main text for a detailed description. The schema was drawn by biorender.com. (C–F) Mean values of run length, total run velocity, transport velocity, and pausing time ratio in the control and the TTLL5 E517A/- mutant for both anterograde and retrograde transport. (C) The run length is the sum of the distances traveled by individual mitochondria in a transporting state. (D) The total run velocity indicates the run distance divided by the run time. The run time is the sum of the total pausing time plus the total transporting time. (E) Transport velocity indicates the mean of the instant velocity of the transported mitochondria. (F) The pausing time ratio was calculated as total pausing time divided by run time. The total pausing time was the sum of the time when the mitochondria were pausing. (G) Kymographs were generated from three representative mitochondria in the control and the TTLL5E517A/- mutant. Yellow arrowheads indicate the pauses of selected mitochondria over the 3 min time window. (H) The mean values of pausing frequency. Pausing frequency indicates the number of pauses divided by run length. Mitochondria were quantified from seven control fly wings and 8 TTLL5E517A/- fly wings. The numbers of mitochondria (n) analyzed for the anterograde control, anterograde TTLL5E517A/-, retrograde control, and retrograde TTLL5E517A/- were 46, 36, 25, and 21, respectively. ***p<0.005, **p<0.01. Genotypes for controls were a mixture of ApplGal4;UAS-Mito::GFP;+/Df(3R)BSC679 and ApplGal4;UAS-Mito::GFP;TTLL5 E517A/+. Genotypes for TTLL5E517A/- mutants were ApplGal4; UAS-Mito::GFP; TTLL5E517A/Df(3R)BSC679.
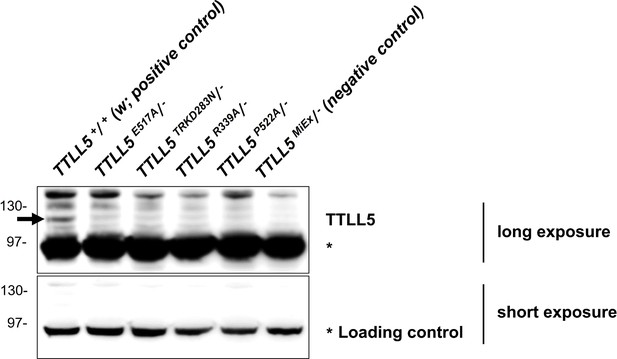
TTLL5 was stably expressed in all Crispr/Cas9 generated TTLL5 mutants.
All Crispr/Cas9 generated TTLL5 mutations were analyzed hemizygously over Df(3R)BSC679. TRKD283N refers to a more complex mutation in which the four codons TRKD283 were replaced by a single N. We expect extracts from these animals to contain at most half the normal amount of TTLL5. w (TTLL5+/TTLL5+) was the positive control and the null mutation TTLL5MiEx/Df(3R)BSC679 was the negative control. The signal of an unspecific band (labeled with *) under short exposure was treated as a loading control. w, which contains two copies of the TTLL5+ allele expressed the highest levels of TTLL5. Crispr/Cas9 generated TTLL5 mutants with a single allele of TTLL5* expressed less TTLL5 compared to w, but more compared to the null mutant TTLL5MiEx/Df(3R)BSC679. The TTLL5 levels of the hemizygous point mutants were similar to the ones of the hemizygous TTLL5+ (see Figure 2B). The faint band seen in TTLL5MiEx/Df(3R)BSC679 is probably a background band.
-
Appendix 1—figure 1—source data 1
Source data for Appendix 1—figure 1.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig1-data1-v2.zip
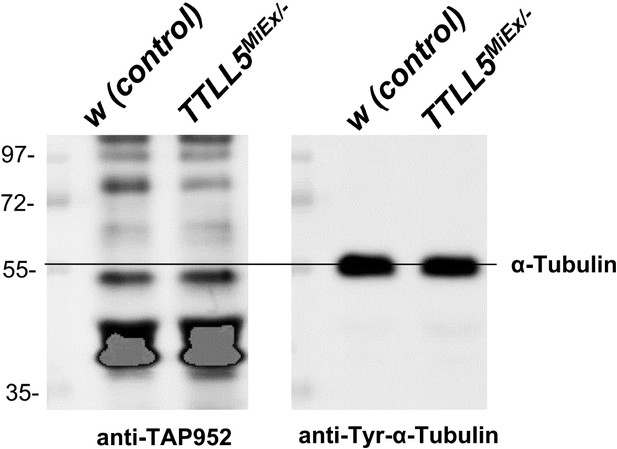
Glycylation of α-tubulin was not detected by western blotting from ovarian extracts.
The glycylation of α-tubulin was evaluated by western blotting with the anti-monoglycylated Tubulin antibody TAP952. The anti-Tyr-α-Tubulin was a loading and size control. TAP952 did not detect a clear band in the α-Tubulin region in the wild type and TTLL5 mutant.
-
Appendix 1—figure 2—source data 1
Source data for Appendix 1—figure 2.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig2-data1-v2.zip

Venus::TTLL5 expression in S10 oocytes.
Confocal micrographs showing the S10 oocyte with or without anti-GFP staining. (A) w oocyte shows the autofluorescence background of the oocyte under the microscope. (B) The oocyte overexpressing Venus:TTLL5 shows the live fluorescence of Venus (green). (C) The oocyte overexpressing Venus:TTLL5 shows the Venus::TTLL5 protein after staining with an anti-GFP antibody (red). Hoechst was in blue. The cortical signal of Venus::TTLL5 in the oocytes is pointed out with white arrowheads. The genotype for Venus:TTLL5 overexpression was MattubGal4/UAS-Venus::TTLL5.

Khc distribution in S10B oocytes.
Intensity charts from six samples of each genotype were plotted based on the line drawn along the AP axis of S10B oocytes (see Figure 7E). The orange triangles in the control and o.e. Venus::TTLL5 point out the inner regions accumulating higher levels of Khc. The control was w or +/Df(3R)BSC679. Genotypes for TTLL5 mutants: MattubGal4/+;TTLL5pBac/Df(3R)BSC679 and TTLL5MiEx/Df(3R)BSC679, respectively. Genotype for TTLL5 overexpression: MattubGal4/UAS-Venus::TTLL5.

TTLL5 has little or no effects on BicD expression and distribution in ovaries.
(A) BicD levels remained unchanged between the w control, a TTLL5 deficiency mutant, and the TTLL5 rescue strain. GAPDH served as the loading control. (B) Confocal micrographs show BicD localizing preferentially at the posterior of the previtellogenic oocytes of w controls and TTLL5 mutants. Scale bar: 25 μm. BicD protein in red, and Hoechst in blue. (C) 97%, 93.5%, and 95.8% of the oocytes showed posteriorly localized BicD in the w control, TTLL5MiEx and TTLL5E517A mutants, respectively. The total numbers of early oocytes are indicated as n = in the chart. Genotype for control: w or +/Df(3R)BSC679. Genotypes for TTLL5 mutants: TTLL5MiEx/Df(3R)BSC679 and TTLL5E517A/Df(3R)BSC679, respectively.
-
Appendix 1—figure 5—source data 1
Source data for Appendix 1—figure 5.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig5-data1-v2.zip
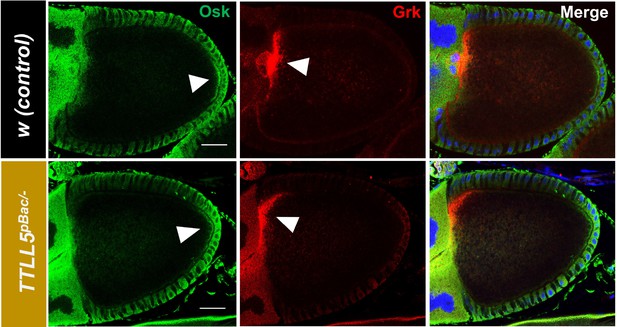
The polarity of S10B oocytes is normal in TTLL5 mutant.
In TTLL5pBac oocytes, Gurken (red) localizes properly to the dorsoanterior part of the oocyte cortex and Oskar (green) to the posterior cortex of 10B stage oocytes, indicating the polarity of MTs at this stage was normal. Blue channel: DNA. Genotypes for TTLL5pBac: TTLL5pBac /Df(3R)BSC679.
Videos
Ooplasmic streaming in w (control): two oocytes.
Ooplasmic streaming in TTLL5MiEx/-: two oocytes.
Ooplasmic streaming in TTLL5E517A/-: two oocytes.
Ooplasmic streaming in Venus::TTLL5; TTLL5MiEx/- (rescued mutant): two oocytes.
Ooplasmic streaming in Venus::TTLL5 (overexpressed): two oocytes.
Appl-Gal4>Mito::GFP transport in the L1 vein.
Top movie: control; bottom movie: TTLL5E517A/-.
Tables
Conservation between mammalian TTLL5 and Drosophila TTLL5.
Residues labeled in red are critical residues for α-tubulin glutamylation by murine TTLL5 and are conserved in human and Drosophila TTLL5 (van Dijk et al., 2007; Natarajan et al., 2017).
Mm TTLL5 Hs TTLL5 | K131 β6-7 loop (R188) R225 E366 N368 TRDR--KPVASSRGG VY---VLYVL---LLVLSP TRDR--KPVASSRGG VY---VLYVL---LLVLSP |
| Dm TTLL5 | K282 β6-7 loop (R339) R376 E517 N519 TRDR--KPAASSRGG IF---LVYVL---LLILSP |
-
The critical domain of the β6-7 loop is underlined.
C-terminal peptides of the Drosophila α-tubulins analyzed by mass spectrometry (MS).
| (A) Number of C-terminal peptides of αTub84B, αTub84D, and αTub67C | |||
|---|---|---|---|
| Isotype | w | TTLL5pBac/-(PSM value*) | TTLL5MiEx/- |
| αTub84B/D | 58 | 24 | 6 |
| αTub67C | 12 | 12 | 4 |
| (B) Role of TTLL5 for the glutamylation of the C-terminal E443, 445, 448, and 449 of αTub84B/D. Frequency of glutamylated C-terminal peptides (containing the entire primary sequence) in w controls, TTLL5pBac/-, and TTLL5MiEx/- mutants containing modifications with the Glu sidechain length of 1E, 2E, or 3Es. | |||
| Sidechain length | w | TTLL5pBac/-(%E mod†) | TTLL5MiEx/- |
| +1 | 50 | 4 | 0 |
| +2 | 14 | 0 | 0 |
| +3 | 7 | 0 | 0 |
| Total Glu modifications‡ | 71 | 0 | 0 |
-
*
The total C-terminal peptide numbers were revealed by the values of total peptide spectrum match (PSM) of peptides containing the unmodified full primary C-terminal sequence (Supplementary file 1) and the C-terminal sequence modified with the Glu sidechain (Supplementary file 1).
-
†
The frequency was quantified based on the glutamylated C-terminal αTub84B/D PSM values (quantified based on Supplementary file 1) divided by total PSM for the C-terminal peptide. The total PSM for w, TTLL5pBac/-, and TTLL5MiEx/- were 58, 24, and 6, respectively.
-
‡
Total Glu modifications show the sum of the three frequencies.
Conservation between TTL and TTLL5.
Many critical residues of human TTL (red) are conserved in Drosophila, mouse, and human TTLL5 (Prota et al., 2013).
| Hs TTL | R202 R222 D318 E331 N333 P336 ISW---VLTA-----FFM---LIVGAA |
Dm TTLL5 Mm TTLL5 Hs TTLL5 | R376 R398 D504 E517 N519 P522 LVY---IVLA-----FIL---LLILSS R225 R247 D353 E366 N368 P371 VLY---LAFA-----FVL---LLVLSS R225 R247 D353 E366 N368 P371 VLY---LAFA-----FVL---LLVLSS |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87125/elife-87125-mdarchecklist1-v2.pdf
-
Supplementary file 1
Tissue specific expression of the 11 Drosophila TTLL genes.
(a) Tissue--specific expression of the 11 Drosophila TTLL genes. (b) Peptide sequence and frequency of unmodified full primary C-terminal sequence of α-tubulins identified by MS. (c) The full primary C-terminal sequence of α-tubulins modified with E sidechain modifications. The probabilities of E sidechain modifications in each peptide according to TPP are indicated for the four positions close to the C-term. (d) The full primary C-terminal sequence of α-tubulins modified by E sidechain modifications. The probabilities of E sidechain modifications in each peptide according to EasyProt, MSfragger, and PEAKS are indicated for the four positions close to the C-term. (e) Primers for cloning the sequences encoding the sgRNAs into the Drosophila transformation vector pCFD5. (f) Templates to introduce point mutations.
- https://cdn.elifesciences.org/articles/87125/elife-87125-supp1-v2.docx
-
Appendix 1—figure 1—source data 1
Source data for Appendix 1—figure 1.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig1-data1-v2.zip
-
Appendix 1—figure 2—source data 1
Source data for Appendix 1—figure 2.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig2-data1-v2.zip
-
Appendix 1—figure 5—source data 1
Source data for Appendix 1—figure 5.
- https://cdn.elifesciences.org/articles/87125/elife-87125-app1-fig5-data1-v2.zip





