Parallel processing of quickly and slowly mobilized reserve vesicles in hippocampal synapses
Figures
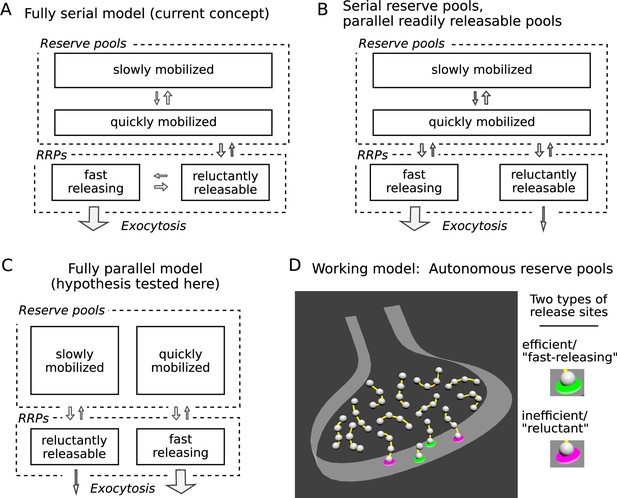
Three possible organizations for synaptic vesicle pools.
(A) The predominant view currently seems to be that all pools are connected in series as depicted; RRP signifies readily releasable pool. (B and C) However, recent evidence indicates that vesicles within reluctant and fast-releasing subdivisions of the RRP can be released in parallel. (D) And, a separate line of evidence suggested that each vesicle within any subdivision of the RRP is associated with an autonomous reserve, implying that slowly and quickly mobilized reserves may also be processed in parallel, as depicted in Panel C.
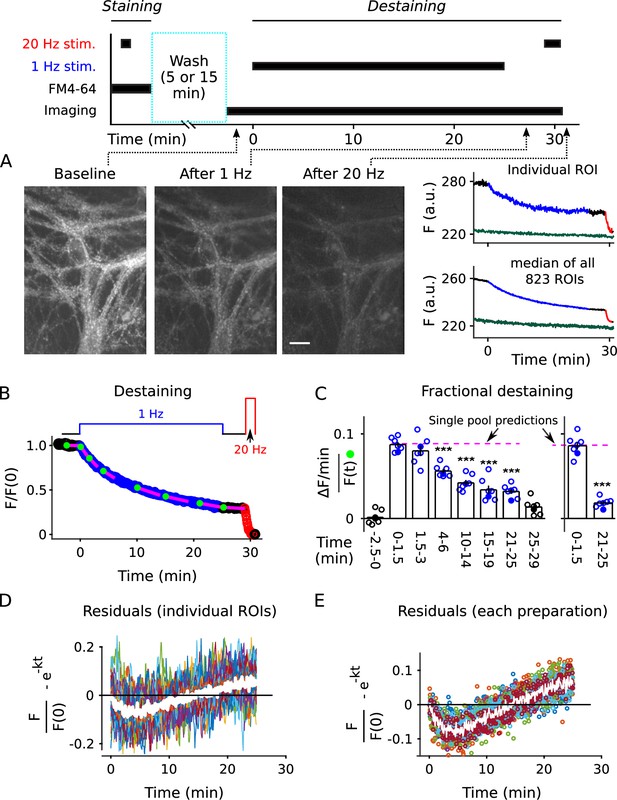
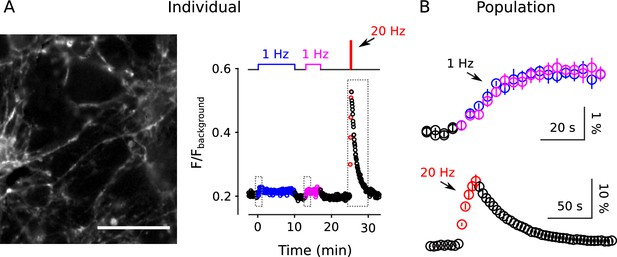
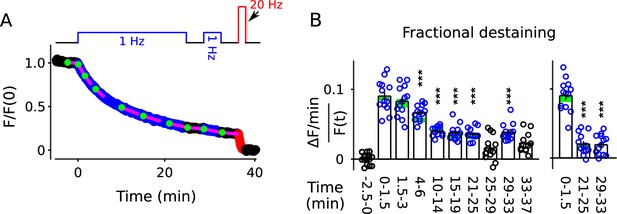
Analysis of FM4-64 destaining during 25 min of 1 Hz stimulation.
(A) Each image is the mean of 20 sequential raw images; scale is 20 μm. The traces pertain to 1 of the 7 experiments; the lower trace (dark green) in each plot is background; F (a.u.) signifies arbitrary units of fluorescence. Destaining is color coded in blue for 1 Hz stimulation and red for 20 Hz here and throughout, except where indicated. (B) Mean ± s.e.m. of median values versus time for n = 7 preparations; error bars are smaller than the symbols. See Materials and methods for automatic detection of ROIs and formula for averaging across preparations. (C) Fractional destaining for a variety of time intervals; the values were calculated by dividing the slopes of the magenta lines by the values of the preceding green circles in Panel B. Filled symbols indicate measurements from example in Panel A. Rightmost two bars are after subtracting the baseline value measured either immediately before (minute -2.5 - 0) or immediately after (minute 25 - 29) 1 Hz stimulation. The dashed magenta line is the value expected from models with a single, quickly mixing reserve pool (*** is p < E - 4 compared to the first 1.5 min interval; paired t-test; Figure 2—figure supplement 5 shows that the duration of the intervals did not affect the overall result). (D) Representative residual values for individual ROIs after subtracting the best fitting single exponential. For clarity, values for only 100 of 6252 ROIs are plotted, but were chosen at random after excluding outliers with maximum deviation from zero of > 0.25 (outliers were 23% of total). Plots are moving averages of the raw residuals with a sliding window of 5. The white line is the mean of the entire data set, including outliers. (E) Residual values for all seven preparations.
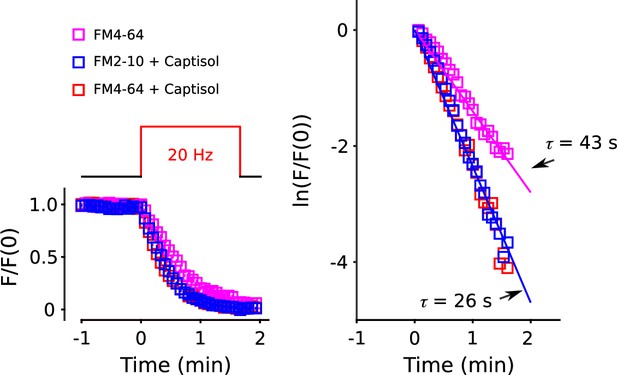
Equivalent destaining for FM4-64 and FM2-10 in presence of Captisol.
Synapses were stained with 60 s of 20 Hz electrical stimulation in the presence of FM4-64 or FM2-10, washed, and then destained with 100 s of 20 Hz stimulation in the presence or absence of Captisol as diagrammed above plot to the left. The plot to the right is the semi-log plot of the same destaining time course. This experiment shows that Captisol accelerated destaining of FM4-64, but no additional acceleration was seen when the dye was FM2-10, which dissociates from membranes 30-fold faster. The result indicates that FM4-64 is cleared completely from vesicular membrane after a single round of exocytosis in the presence of Captisol.
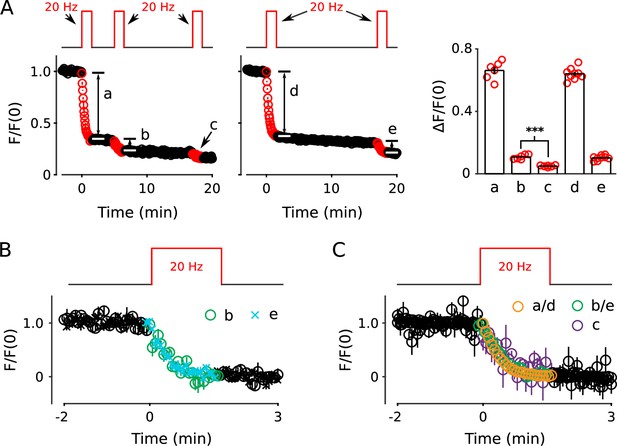
Analysis of FM4-64 signal remaining after 20 Hz stimulation for 100 s.
(A) Unlike elsewhere throughout this report, the remaining signal was not subtracted before normalizing; the value of zero indicates the background signal from non-neuronal areas of the cell culture that did not destain during electrical stimulation. The plots show that 100 s of 20 Hz stimulation likely does not completely destain all recycling vesicles because a small amount of additional destaining could be induced with additional 20 Hz stimulation (b and e). The amount was not altered by extending the rest interval between the two trains of stimulation from 3.3 to 15.3 min (b vs e in bar graph). And, destaining during a third train (c) was even less (*** is p < 0.001, paired t-test). (B) Overlay of destaining time course of second 20Hz trains begun either 3.3 min after the first train (i.e., b), or 15.3 min after (e). Unlike for Panel A the remaining signal was subtracted, and both time courses were re-normalized so that the baseline was 1.0. The plot shows that the two time courses were similar, indicating that 12 min of additional rest did not alter the time course of destaining. (C) Overlay of destaining time courses during the first, second and third 20 Hz trains showing that the time course was essentially the same under all conditions.
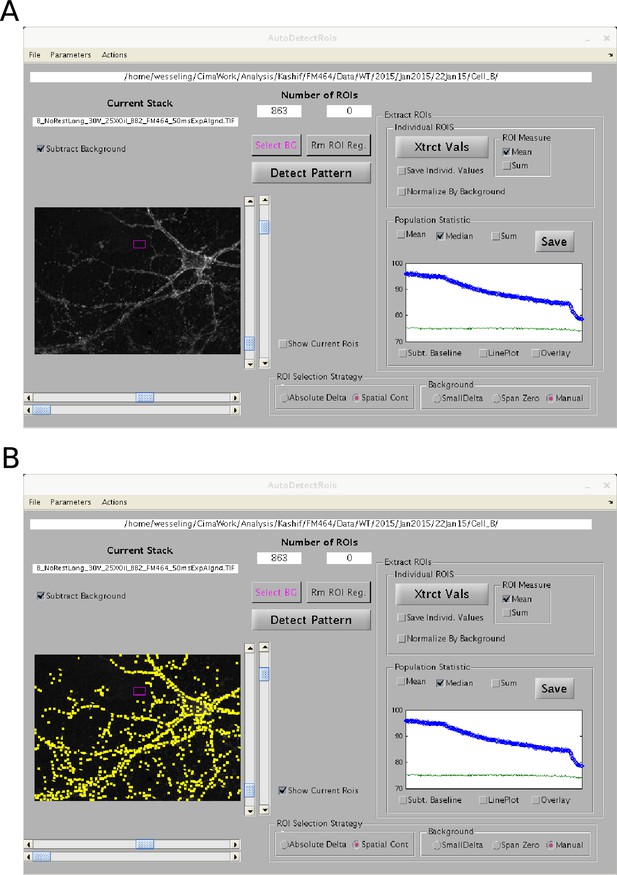
Graphical user interface for semi-automatic ROI detection.
A value for contrast for each 2X2 pixel region of each image was calculated by subtracting the mean value of surrounding pixels from the mean value of the pixels within the region. Regions were then sorted by the change in contrast during the experiment, and regions overlapping with regions with greater change in contrast were eliminated. A threshold for the minimum change in contrast for evaluation as a region of interest (ROI) was then set subjectively using the upper horizontal scroll bar beneath the image within the graphical user interface. The image is the difference calculated by subtracting the mean of destained images from the mean of images before destaining. The software has the capability of selecting background regions automatically, but, for the present study, background regions were always selected by hand (magenta box). The blue circles in the plot in the “Population Statistic” box are the median values of the ROIs vs time, and the green line is background. (A) Without displaying locations of ROIs. (B) ROIs are demarcated by yellow boxes.
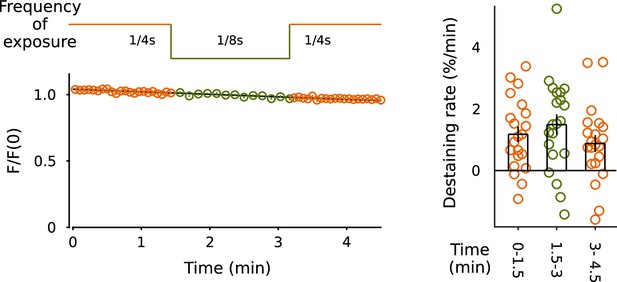
No evidence for photobleaching.
Baseline destaining did not decrease when exposure to light was decreased to half by decreasing the acquisition rate from 1/4 to 1/8 s (n = 21 preparations).
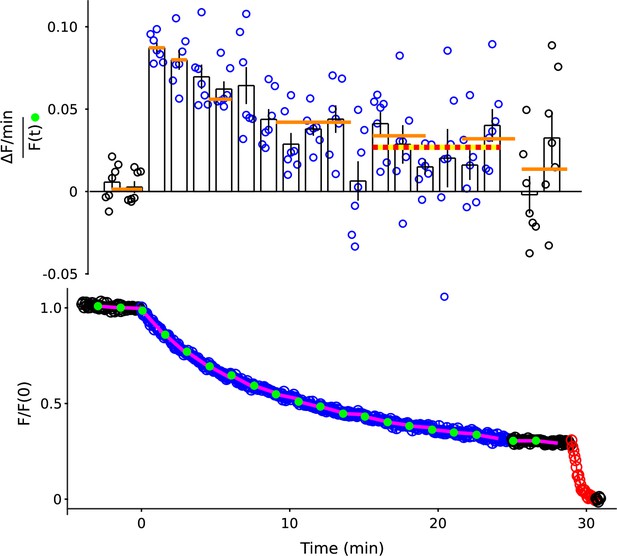
Replot of Figure 2B–C except with 1.5 min intervals throughout.
Orange lines are the means from Figure 2C. Red/yellow line is the mean of all experiments/all intervals between minutes 15 and 24.
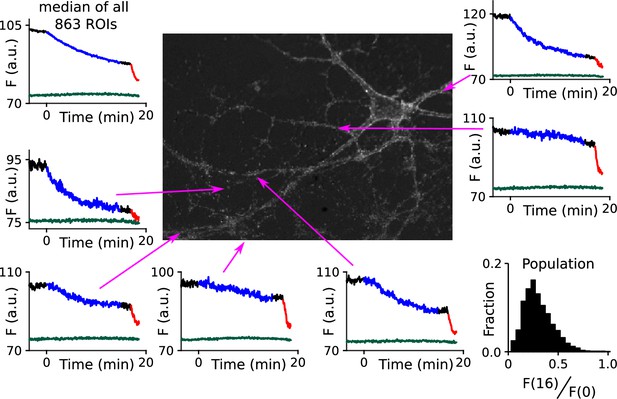
Extensive heterogeneity among synapses.
Time courses are FM4- 64 destaining at individual ROIs indicated in the central image during 15 min of 1 Hz stimulation (blue portions of traces) followed by 100 s at 20 Hz (red); the lower trace in each plot (green/blue) is background measured at a nearby region. The amount of destaining during 1 Hz stimulation could be quantified, with a single parameter, by dividing the signal remaining during the interval after 1 Hz stimulation and before 20 Hz - i.e., F(16) - by the signal before 1 Hz stimulation - i.e., F(0). The heterogeneity among synapses could then be summarized with the histogram in the lower right. The scale bar in the central image is 20 µm.
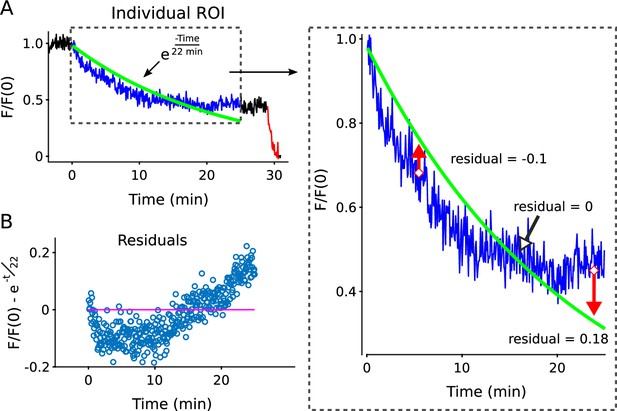
Procedure for calculating residuals after fitting with a single exponential.
Illustration of procedure for calculating residuals after fitting witha single exponential. (A) Blue trace is the destaining time course during 25 min of 1Hz stimulation, green line is the best fitting single exponentials (i.e., Equation 2; see Materials and methods for fitting procedure). The best fitting single exponential appears to come close to the destaining time courses in the linear plot, but this is illusory. The absence of a good fit is more evident when replotted on a semi-log plot (Figure 2—figure supplement 8B), or in the large changes over time observed in the fractional destaining measurement used to quantify deviations from Equation 1 throughout themanuscript. (B) The residual values are calculated by subtracting the best fitting instantiation of Equation 2 from the destaining time course. A mathematically adequate fit would imply that residual values are scattered at random about zero (magenta line), but this almost never occurred. For a statistical test of the adequacy of each fit, we compared the residual values during the first 12.5 min of 1 Hz stimulation to the values during minutes 12.5 - 25 using the Wilcoxon rank sum method. Rejection of adequacy of fit was statistically significant for > 90 % of individual ROIs, and typically highly significant for ROIs where signal/noise was good; the statistical significance for the individual analyzed here was p < E − 47.
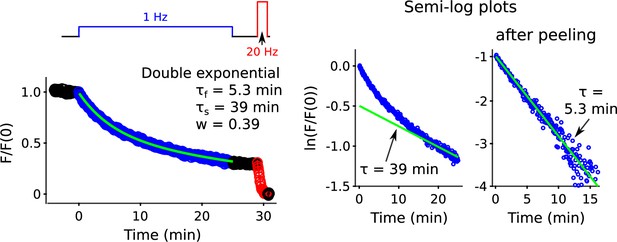
Double exponential fit during 1 Hz stimulation.
Best fitting double exponential during destaining driven by 25 min of 1 Hz stimulation. The green line in the linear plot is: . The green line in the semi-log plot before peeling is: . Peeling was accomplished by subtracting from the destaining time course. The green line in the semi-log plot after peeling is then: .
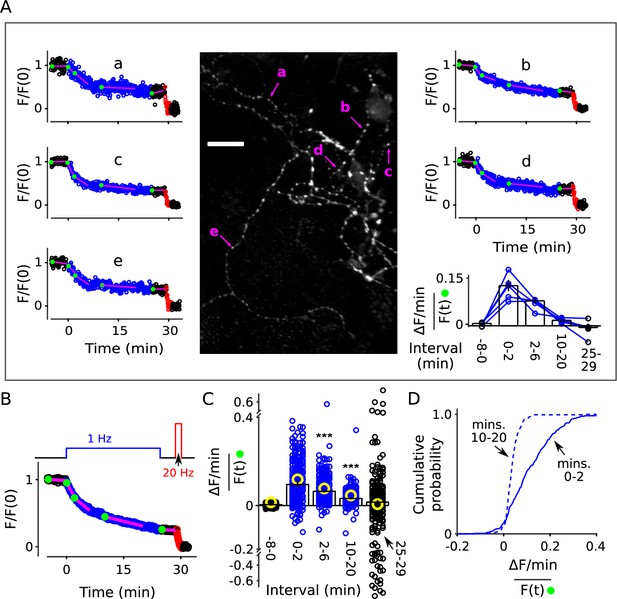
Decrease in fractional destaining during 1 Hz stimulation at individual synapses.
Neurons grown at lower density were stained and destained as for Figure 2. For some experiments we increased the exposure time for individual images from 50 to 200 ms, which improved the signal/noise ratio, but did not result in noticeable photobleaching. Individual ROIs were limited to punctae sized less than 1.5 × 1.5 μm and clearly separate from neighbors. (A) Example: Image is the mean of the time-lapse during 8 min of baseline; exposures were 200 ms; scale bar is 20 μm. Plots a-e are time courses of destaining at punctae indicated by magenta arrows, after normalizing for brightness as described in Materials and methods. The magenta lines and green circles plotted on top of the time courses are analogous to Figure 2B, and values were used to calculate fractional destaining in the bar graph. Fractional destaining between minutes 10 and 20 was lower for the punctae in this experiment compared to the average of others reflecting extensive heterogeneity among preparations in addition to heterogeneity among punctae within individual preparations; see Figure 2—figure supplement 6 and Figure 3—figure supplement 2. (B) Mean time course of 235 individual punctae from eight preparations where slope of baseline was ≤ 1.5%/min. (C–D) Fractional destaining. (C) Small circles (black and blue) are measurements from the 235 punctae. Yellow circles were calculated from the mean of the time courses of the 170 punctae where the slope of baseline was > 1.5% min. Scatter was greater for measurements for the 25-29 min interval because of lower signal than during the 2-6 min interval and fewer data points than during the 10-20 min interval. Bars are median ± 95% CI (*** is p < E - 9, Wilcoxon signed-rank). (D) Cumulative histogram of the fractional destaining values corresponding to the 0-2 and 10-20 min intervals.
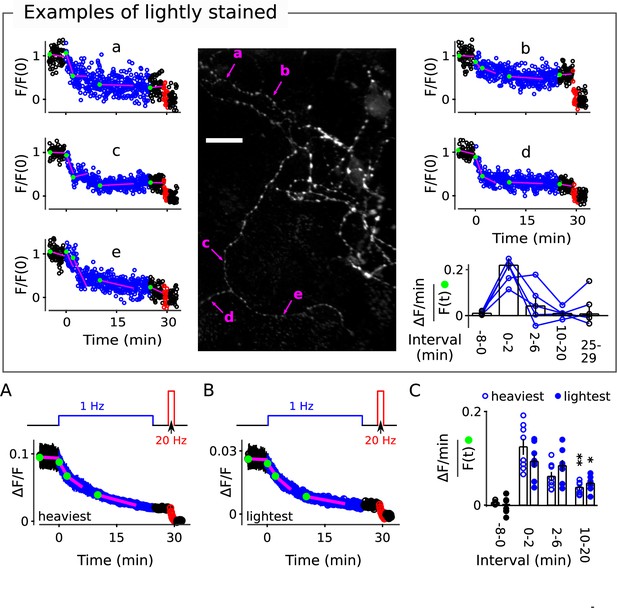
Examples of destaining at lightly stained punctae.
Image is identical to Figure 3, but a different set of punctae are indicated by arrows to illustrate destaining at synapses that were initially stained more lightly. Overall, staining intensity (Δ F/F) before destaining at 1 Hz was 0.035 ± 0.004 for the 5 examples selected here compared to 0.086 ± 0.008 for the 5 selected in Figure 3. (A) Mean of median time courses of the most heavily stained quintile of individual punctae from each of the same 8 preparations documented in Figure 3; individuals for each preparation were from the entire data set, including punctae where the slope of baseline was >1.5%/min. (B) Mean of median time courses of least heavily stained quintile. (C) Fractional destaining of the median time courses from the most and least heavily stained quintiles of each preparation (* is p<0.05 and ** is p<0.01, both compared to fractional destaining during the first two minutes of 1Hz stimulation; Wilcoxon signed-rank).
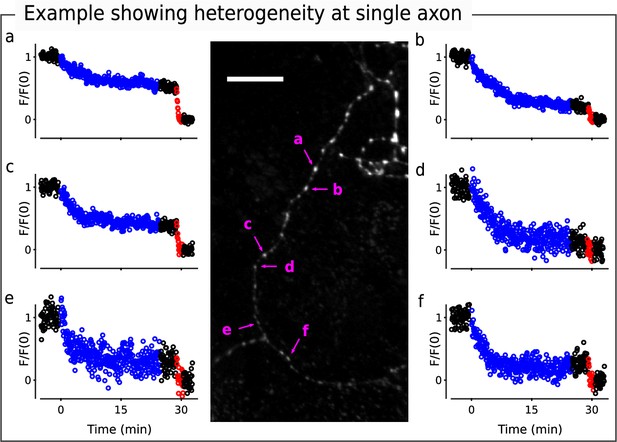
Examples showing heterogeneity among synapses.
Image is identical to the lower left of image in Figure 3; scale bar is 20μm. Individual punctae were chosen to highlight heterogeneity between individuals along what appears to be a single axon.
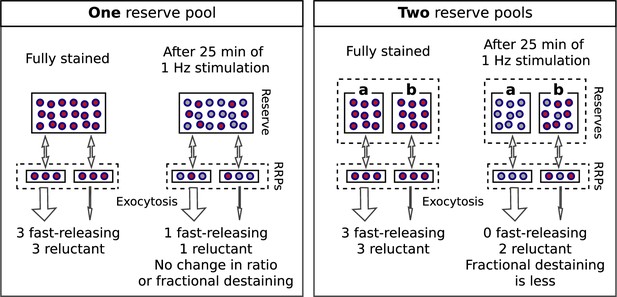
Single reserve versus two in parallel.
Comparison of schemes with one reserve pool (left) and with two parallel reserve pools that feed separate subdivisions of the readily releasable pool (right); wide arrows signify fast-releasing and narrow arrows signify reluctant readily releasable vesicles. For both schemes, the fraction of vesicles within the readily releasable pools (RRPs) and that are stained decreases over time of stimulation, accounting for the decrease in the absolute amount of destaining for each unit of time. However, with a single reserve pool, the ratio of stained fast-releasing to stained reluctant vesicles never changes, and, as a consequence, the fractional destaining at any point in time does not change. In contrast, with two reserves, vesicle mobilization in path a is faster than in path b - i.e. because the readily releasable vesicles in path a are fast-releasing - causing path a to become exhausted sooner. As a consequence, the ratio of stained fast-releasing to stained reluctant vesicles decreases over time, resulting in decreased fractional destaining.
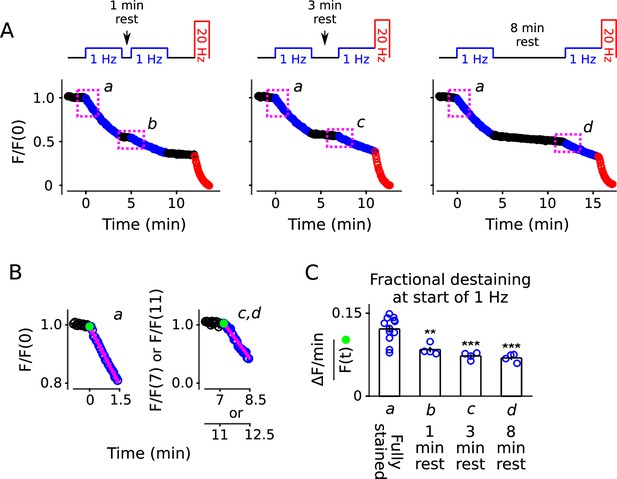
Decrease in fractional destaining induced by 1 Hz stimulation persists during long rest intervals.
(A) Comparison of destaining during two 4 min-long trains of 1 Hz stimulation separated by 1 min, 3 min, and 8 min. (B) Replots of the first 1.5 min of destaining (magenta dashed boxes in Panel A) during 1 Hz stimulation at fully stained synapses (a); or after ≥ 3 min of rest following 4 min of 1 Hz stimulation (c, d). The fragments of the destaining time courses for c and d were renormalized by the immediately preceding rest interval to illustrate that fractional destaining was substantially less during the second 1 Hz trains. Magenta lines and green circles are slope and initial intensity as in Figure 2B. (C) Fractional destaining estimated for the first 1.5 min interval at the start of each 1 Hz train, calculated by dividing the slopes of the magenta lines by the values of the preceding green circles in Panel B, matching the calculation in Figure 2C; ** is p < 0.01 and *** is p < 0.001 compared to bar a (two-sample t-test).
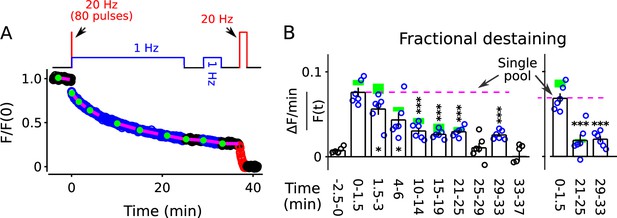
FM4-64 destaining during 25 min of 1 Hz stimulation immediately following 4 s of 20 Hz stimulation.
Decrease in fractional destaining over time in Panel B is similar to when the initial 4 s of 20 Hz stimulation is omitted - green rectangles are mean ± s.e.m. from Figure 2C - ruling out selective depletion of the readily releasable pool as the cause of the decrease. Rightmost three bars are after subtracting the baseline value measured either immediately before (minute -2.5 - 0) or immediately after (minute 25 - 29) or both immediately before and after (minute 25 - 29 and minute 33 - 37) 1 Hz stimulation. The dashed magenta line is the value expected from models with a single reserve pool where fractional destaining is constant (* is p < 0.05 and *** is p < 0.001 compared to the measurement during the first 1.5 min of 1 Hz stimulation; paired t-test).
No long-term depression during 1 Hz stimulation when measured with vGlut1-SynaptopHluorin.
Synapses were stimulated with two trains of 1 Hz electrical stimulation followed by 20 s of 20 Hz. (A) Example from a single preparation. The image is the mean of 20 sequential raw images starting with the start of 20 Hz stimulation; scale bar is 20 μm. The plot is the corresponding fluorescence signal from the entire experiment. (B) Mean changes in fluorescence intensity from n = 6 fields of view at the start of the two 1 Hz trains showing no difference (upper, blue is first train, magenta is second), and during the 20 Hz train (lower, red). For these experiments, fluorescence intensity was only measured over short intervals corresponding to the dashed boxes in Panel A - rather than during the entire experiments - to avoid photobleaching. Baseline intensity values immediately before the onset of stimulation were subtracted before combining across experiments. was 1.7 ± 0.2% at the start of the first and 1.9 ± 0.2% at the start of the second 1 Hz trains. Scale bars are versus time. The individual in Panel A was acquired using more light (see Materials and methods), and was part of a larger data set where a small amount of photobleaching (∼ 15%) did occur over the 10 min of 1 Hz stimulation.
FM2-10 destaining; similar to Figure 2B–C except synapses were stained with FM2-10 instead of FM4-64 (n = 13 preparations).
(A) Time course. (B) Analogous to Figure 2C. Green rectangles are mean ± s.e.m. from Figure 2C. Values for rightmost 3 bars were calculated by subtracting background fractional destaining values as in Figure 5B (*** is p < 0.001 compared to the measurement during the first 1.5 min of 1 Hz stimulation; paired t-test).
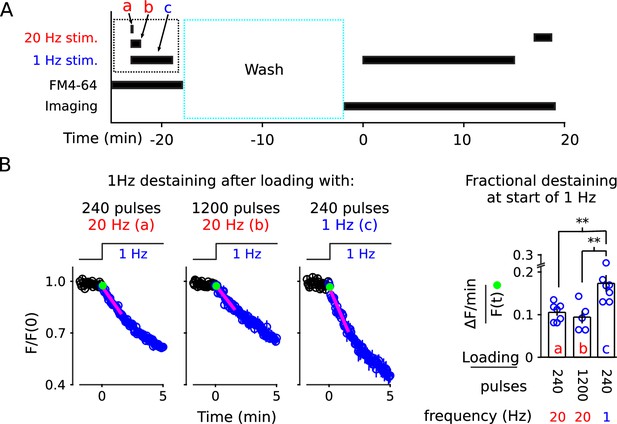
1 Hz destaining is faster after loading at 1 Hz compared to after loading at 20 Hz.
(A) Experimental protocol. The experimental variables are the frequency and duration of stimulation during the staining phase of the experiment (i.e., a, b, and c), which is different from the previous experiments. (B) FM4-64 destaining during the first 5 min of 1 Hz stimulation after loading with 1 Hz or 20 Hz stimulation; see Figure 8—figure supplement 1 for full destaining time courses. Magenta lines and green circles are slope and initial intensity as in Figure 2B (n ≥ 5; ** is p < 0.02, two-sample t-test).
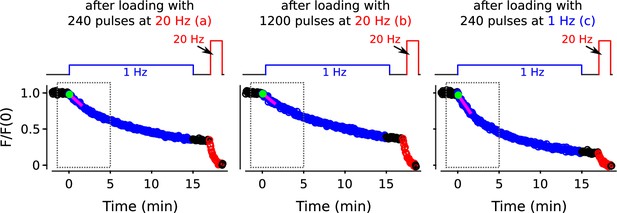
Full destaining time courses for experiments in Figure 8.
Values within gray dashed boxes are already plotted in Figure 8B. The letters a, b, and c correspond to the same letters in Figure 8A, B.
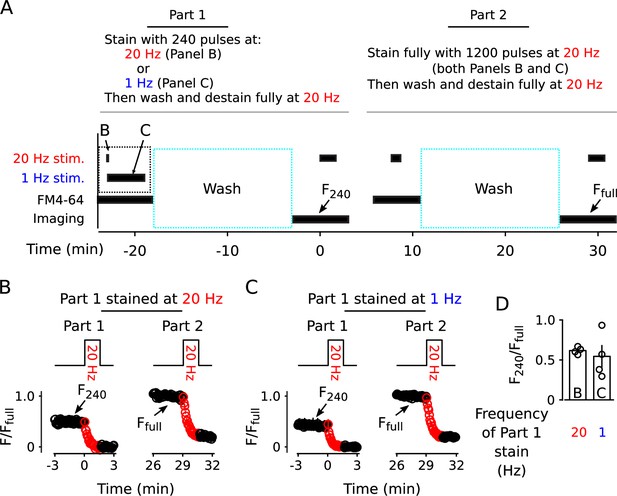
Similar amount of staining induced by 240 pulses at 1 vs 20 Hz.
(A) Experimental protocol. (B) Destaining (2000 pulses at 20 Hz) after staining with 240 pulses at 20 Hz (Part 1) and, subsequently, after staining with 1200 pulses at 20 Hz (Part 2); (n = 4). (C) Same as Panel B except frequency of stimulation during staining in Part 1 was 1 instead of 20 Hz (n = 4). (D) Quantification of fractional staining induced by 240 pulses in Panels B and C.

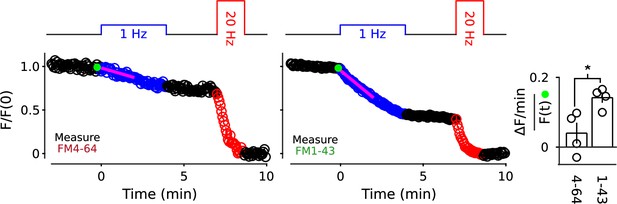
Two color separation of reserve pools.
(A) Slowly mobilized reserve is labeled with FM4-64 (red) and quickly mobilized is labeled with FM1-43 (green); n ≥ 6; *** is p < E - 4 (two-sample t-test). (B) Analogous to Panel A, except the colors are reversed. (A and B) Magenta lines and green circles are slope and initial intensity as in Figure 2B. (C) Similar amounts of stain for each dye when applied first during 20 Hz stimulation, and then partially destained with 1 Hz stimulation, or when applied second during 1 Hz stimulation.
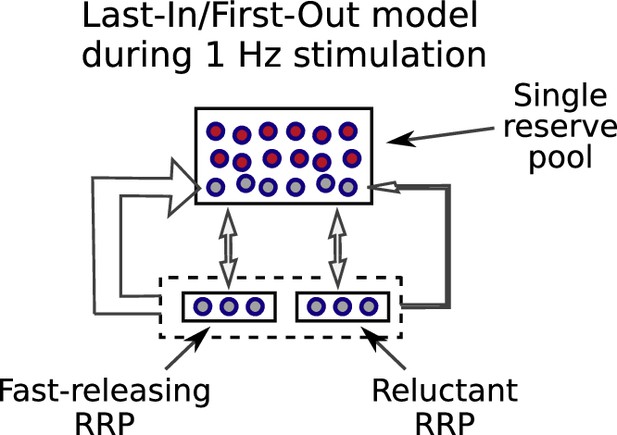
Description of last-in/first-out models.
Last-in/first-out models are a special case of serial models where reserve vesicles that have been reconstituted from recycled membrane are recruited to the readily releasable pool before vesicles that have been in the reserve pool for longer. If so, a small number of reserve vesicles might undergo multiple rounds of exocytosis during long-trains of low frequency stimulation, whereas others would not undergo exocytosis a single time. This could explain the decrease in fractional destaining seen in FM-dye destaining experiments during 1 Hz stimulation (e.g., Figure 2B, C), and other observations. However, without a mixing mechanism, the vesicles that did not undergo exocytosis during 1 Hz stimulation would be recruited to the readily releasable pool with a delay during 20 Hz stimulation, which is not compatible with the results in Figure 10B, C. And, mixing is ruled out in the analysis of results in Figure 11.
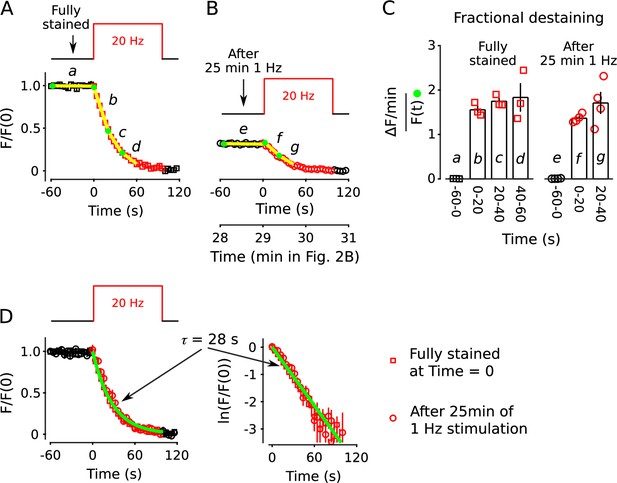
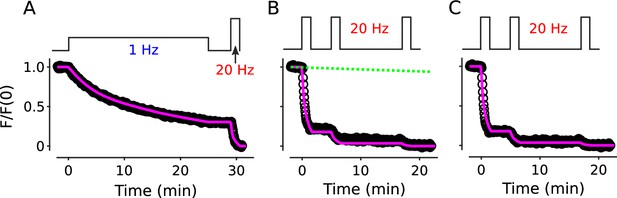
No decrease in fractional destaining when stimulation is 20 Hz.
(A) Synapses were first stained with FM4-64 by stimulating at 20 Hz for 60 s - matching Figure 2 and others - then destained with a single train of 20 Hz stimulation as diagrammed at top (n = 3). Yellow lines and green circles are slope and initial intensity, analogous to the magenta lines and green circles in Figure 2B and elsewhere. (B) Destaining time course after 25 min of 1 Hz stimulation; data are a subset of data plotted in Figure 2B; n = 4 instead of the 7 in Figure 2 because the criterion of ≤ 1.5% destaining per min before stimulation was calculated from the rest period following the 1 Hz stimulation when the remaining stain was 3-fold less. The experiments in Panels A and B were interleaved. (C) Fractional destaining during 20 Hz stimulation showing no decrease over time (e.g. compare to Figure 2C). Note that the time intervals for calculating fractional destaining are 20 s versus ≥ 1.5 min elsewhere (i.e. because elsewhere stimulation was 1 Hz and destaining was slower). (D) Linear and semi-log plots of overlaid time courses after scaling to 1.0. Green lines are the single exponential described by Equation 2 with .
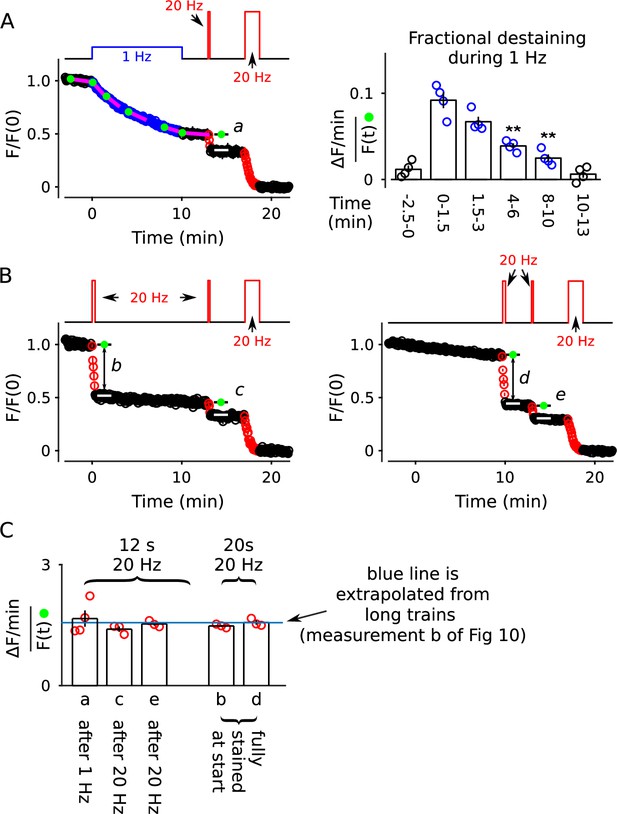
Further evidence that the decrease in fractional destaining during 1 Hz stimulation is no longer evident when subsequent stimulation is 20 Hz.
For all experiments, synapses were first stained with FM4-64 with the standard protocol of 20 Hz stimulation for 60 s. (A) Destaining during 10 min of 1 Hz stimulation followed by 12 s at 20 Hz and then 100 s at 20 Hz (n = 4). The bar graph confirms that 10 min of 1 Hz stimulation drives fractional destaining to a low rate when measured during continued 1 Hz stimulation (** is p < 0.01, paired t-test). The calculation of fractional destaining is analogous to Figure 2C. (B) Similar to destaining in Panel A, except the first train was 20 s at 20 Hz instead of 10 min at 1 Hz; 20 s at 20 Hz was chosen because it destains the synapses to the same level as 10 min at 1 Hz (n = 3 for both types of experiments). (C) Quantification of fractional destaining: during 12 s of 20 Hz stimulation after partial destaining with 1 Hz or 20 Hz stimulation from Panels A-B (a, c, e); and during 20 s of 20 Hz stimulation at fully stained synapses from Panel B (bars b, d). The blue line is the mean fractional destaining during the 1st 20s of 20 Hz stimulation during long trains (b in Figure 10A); the match to fractional destaining during short trains confirms that FM4-64 is cleared from membranes quickly compared to the overall timing of exocytosis during 20 Hz stimulation (i.e. in the presence of Advasep-7/Captisol used throughout the present study).

Comparison of rate-limiting mechanisms at 1 and 20 Hz.
Wide arrows signify fast-releasing and narrow arrows signify reluctant subdivisions of the readily releasable pool (RRP).The absence of rundown in fractional destaining when stimulating at 20 Hz (right panel) is in-line with parallel models because: (1) 20 Hz is intense enough to quickly drive the entire RRP - including both fast-releasing and reluctantly-releasing subdivisions - to a near-empty steady state; after which, (2) exocytosis is rate-limited by the timing of recruitment of reserve vesicles to vacant space within the subdivisions (Wesseling and Lo, 2002; Garcia-Perez and Wesseling, 2008; Garcia-Perez et al., 2008; Raja et al., 2019) instead of by mechanisms that determine probability of release of already releasable vesicles; and, finally (3) the timing of recruitment is the same for fast- and reluctantly-releasing subdivisions (Garcia-Perez and Wesseling, 2008; Mahfooz et al., 2016; see Wesseling, 2019 for a discussion of discrepancies reported for other synapse types).
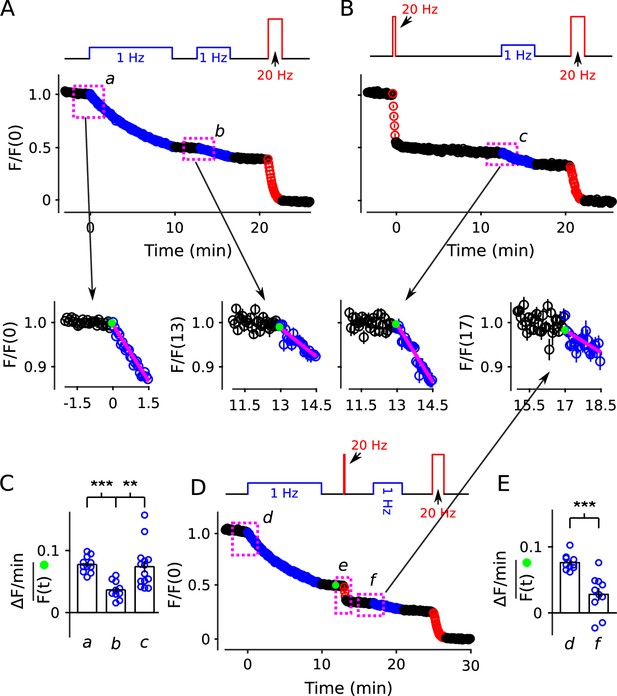
20 Hz stimulation does not influence fractional destaining measured during 1 Hz stimulation.
Synapses were first stained with FM4-64 by stimulating at 20 Hz for 60 s throughout. (A and B) Synapses were destained 50% by stimulating either at 1 Hz (Panel A, n = 11) or at 20 Hz (Panel B, n = 8), followed by a second train at 1 Hz. Insets are destaining during the first 1.5 min of the 1 Hz trains after normalizing by during the preceding rest interval. Magenta lines and green circles are slope and initial intensity as in Figure 2B and elsewhere. The inset corresponding to magenta box c in Panel B summarizes results from n = 14 preparations, including the n = 8 in Panel B and n = 6 more from Figure 11—figure supplement 1A where the first 20 Hz train was delayed by 10 min. (C) Quantification of fractional destaining - calculated as in Figure 2C - during the first 1.5 min of 1 Hz stimulation in Panels A and B. The graph shows that fractional destaining during the second train was reduced when the preceding train was at 1 Hz (a vs b), but not when at 20 Hz (a vs c). The result confirms that 20 Hz stimulation depletes dye from quickly and slowly mobilized reserves with equivalent timing. ** is p < 0.01 and *** is p < E−4 (two-sample t-test). (D) Synapses were destained with 10 min of 1 Hz stimulation followed by 12 s of 20 Hz stimulation, then another 4 min of 1 Hz stimulation (n = 10). (E) Quantification of fractional destaining during the first 1.5 min of the two 1 Hz trains in Panel D showing that the interleaved 20 Hz stimulation did not reverse the decrease in fractional destaining induced by the first 1 Hz train. Fractional destaining in box marked e in Panel D is quantified in Figure 11—figure supplement 2. *** is p < 0.005 (paired t-test).
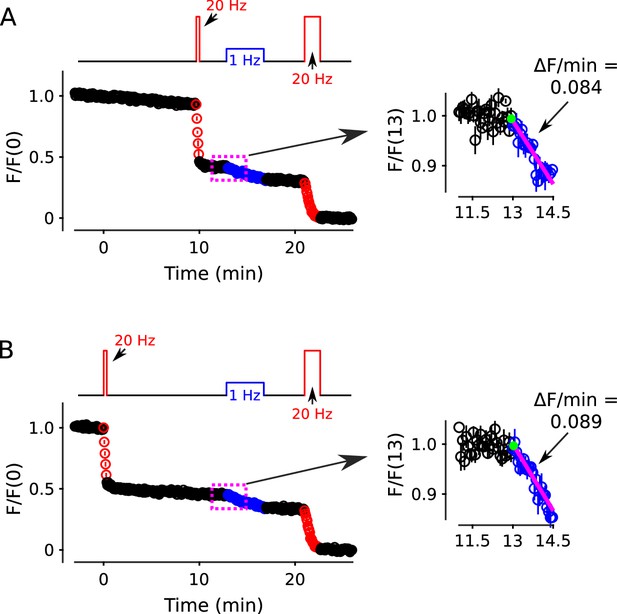
Formal control for matching Figure 11 Panels A and B.
Control showing destaining during a 1 Hz train following a 20 Hz train is equivalent after delays of 0 and 10 min. The control is relevant to Figure 11A–C where the goal was to compare destaining during 1 Hz trains following a 10 min-long 1 Hz train to following a 20 s-long 20 Hz train. When designing the experiment, it was not clear if the 20 Hz train (i.e., in Figure 11B) should be matched in time to the beginning or to the end of the 10 min-long 1Hz train (in Figure 11A). Therefore, both types of experiments were conducted and were interleaved with each other and with the experiments documented in Figure 11A. (A) After a delay of 10 min (n = 6). (B) After no delay; the full time course is replotted from Figure 11B, but the inset here pertains only to the 8 experiments summarized by the full time course whereas the inset of Figure 11B pertains to all 14 experiments summarized here and in Panel A.
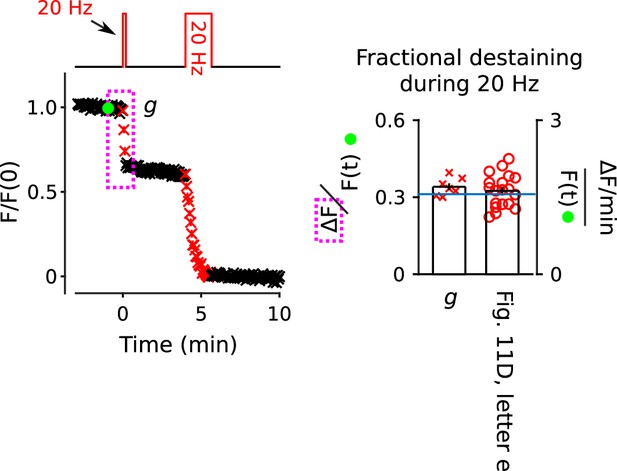
Confirmation that fractional destaining is maximal during the interleaved 20 Hz train in in Figure 11D.
Fully stained synapses were destained with 100 s of 20 Hz stimulation, followed by complete destaining with 100 s of 20 Hz stimulation (n = 7). The bar graph shows that fractional destaining during the 12 s-long train matches fractional destaining during 12 s of 20 Hz stimulation initiated after 10 min of 1 Hz stimulation in Figure 11D (magenta box e). The blue line in the bar graph is the mean fractional destaining during the 1st 20 s of 20 Hz stimulation during long trains (b in Figure 10A), and is included here to facilitate comparison to Figure 10—figure supplement 1.
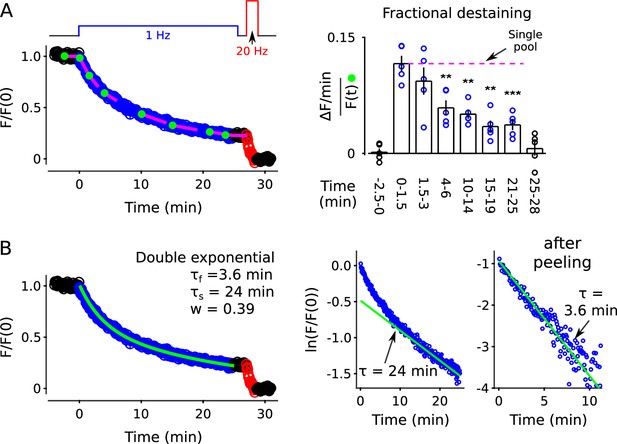
FM4-64 destaining at 35 C.
(A) Analogous to Figure 2B–C, except at 35 C (n = 5, ** is p < 0.01, *** is p < 0.001, compared to the measurement during the first 1.5 min of 1 Hz stimulation, paired t-test). (B) Double exponential fit. Analogous to Figure 2—figure supplement 8.
Two color experiment at 35 C.
Analogous to Figure 9A, except at 35 C (n = 4; * is p< 0.05, two-sample t-test).
Magenta lines are the simulation with three types of release sites with = 0.28 (7%), 0.035 (33%), and 0.0025 (60%).
(A) Replot of results in Figure 2B confirming that the model is compatible with FM-dye destaining when stimulation is 1 Hz. (B) Replot of results in the left panel of Figure 2—figure supplement 2A after re-normalizing so that the final points have a value of 0. The near-miss of the magenta line illustrates how highly constrained the model is for destaining during 20 Hz stimulation since there are essentially no free parameters. The green line is the estimated stimulation-independent rundown of 0.25 %/min. (C) Replot after correcting for the rundown, confirming that the model is compatible with FM-dye destaining when stimulation is 20 Hz.
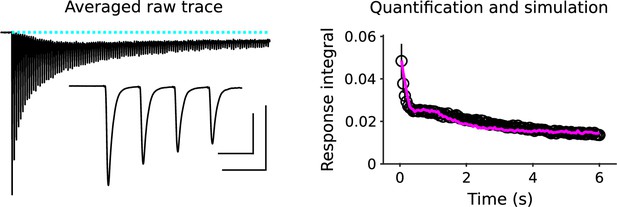
Electrophysiological recordings of synaptic responses between pairs of neurons in culture during 6 s of 20 Hz stimulation, and corresponding simulation using the model in Appendix 1—figure 1 (magenta line).
The electrophysiological trace is the average across 11 pairs, including the pairs that constituted the untreated wildtype control in Figure 5 of García-Pérez et al., 2015. The inset shows the first 4 responses on an expanded time scale. Outer scale bars pertain to the entire trace and are 500 pA by 1s; inner bars pertain to the inset and are 500 pA by 50 ms. Responses were quantified by integrating each interval after subtracting the baseline before the first response (dashed cyan line), so that later responses included a substantial component caused by asynchronous release (Hagler and Goda, 2001). Results for each cell pair were normalized by the sum of the first 40 responses (2 s) before combining across cell pairs; we normalized this way because of extreme variation between cell pairs in the short-term plasticity seen during the first few responses - the paired pulse ratio of the first two responses varied from 1.27 to 0.64 - and because 40 responses has been used elsewhere to approximate readily-releasable pool size (Murthy and Stevens, 1999). The 80 responses to trains designed to ensure exhaustion above would be an overestimate because of ongoing recruitment to vacant release sites (Wesseling and Lo, 2002).

Image to left is a reproduction of the example image in Figure 3, which was the average of 120 time lapse raw data images; scale bar is 20 µm.
The second image is a replicate except all 69 punctae that were included in the study are occluded by 1.5 µm × 1.5 µm yellow squares. The third image is another replicate except with a different brightness setting. The rightmost image is one of the raw data images with brightness matched to the third image.





