Natural variation in the Caenorhabditis elegans egg-laying circuit modulates an intergenerational fitness trade-off
Figures
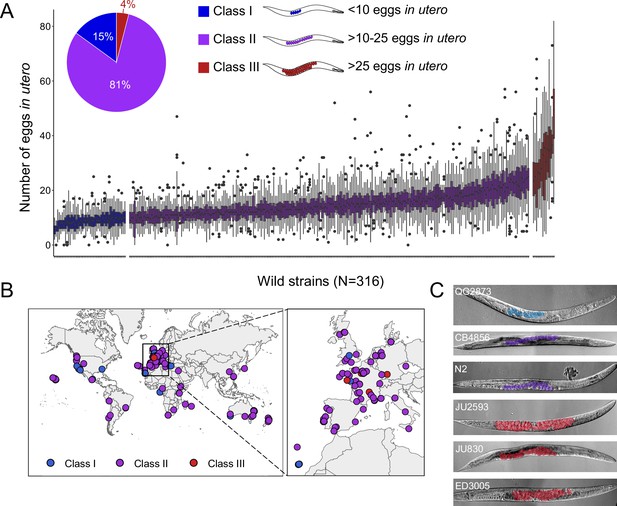
Natural variation in C. elegans egg retention.
(A) The number of eggs in utero in hermaphrodites (mid-L4 +48 hr) of 316 genetically distinct strains (isotypes) often strongly deviated from values observed in the laboratory strain N2 (~15 eggs in utero). We defined three classes of strains with distinct levels of egg retention: Class I weak:<10 eggs in utero (N=34), Class II canonical: 10–25 eggs in utero (N=230), Class III strong:>25 eggs in utero (N=14). N=18–150 individuals per strain were scored. (B) Geographic distribution of 316 C. elegans wild strains. Strains with different degrees of egg retention are labelled in different colours. For a detailed comparison of geographic distribution of the three phenotypic Classes, see Figure 1—figure supplement 1A. (C) Nomarski microscopy images of adult hermaphrodites (mid-L4 +48 hr) in wild strains with divergent egg retention. Eggs (coloured) contain embryos at different stages of development.
-
Figure 1—source data 1
Excel file containing source data for Figure 1.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig1-data1-v1.xlsx
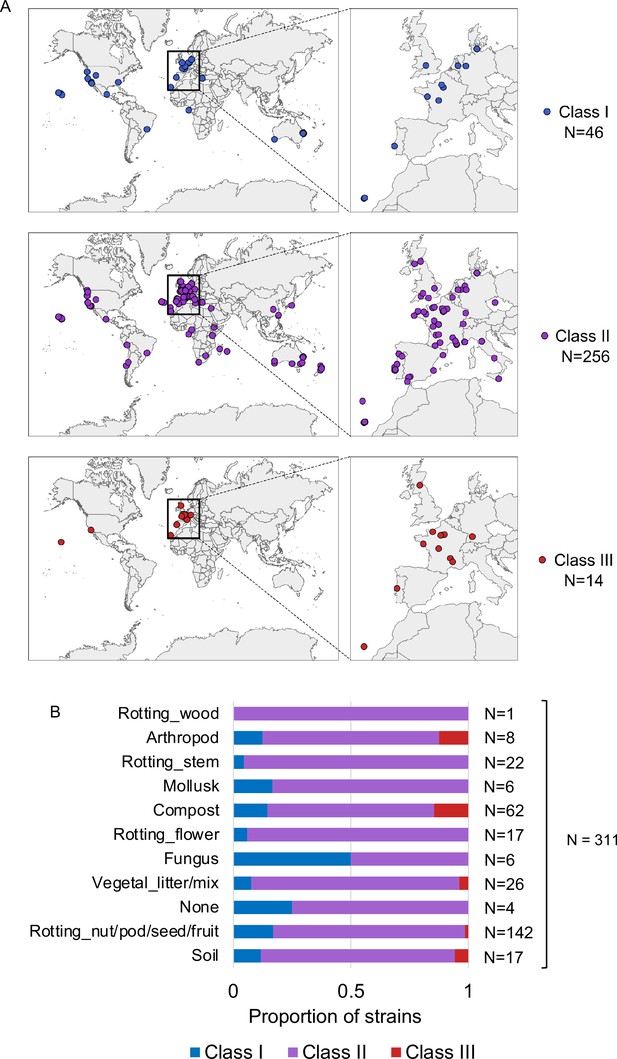
Natural variation of C. elegans egg number in utero.
(A) Geographic distribution of Class I strains:<10 eggs in utero, low retention (N=34); Class II strains: 10–25 eggs in utero, canonical retention (N=230); Class III strains:>25 eggs in utero, high retention (N=14). (B) Frequency of strains with different egg retention phenotypes (Class I to III) in each substrate category. Strain information was obtained from the CaeNDR website: caendr.org. N=311 (for five strains there was no substrate information available).
-
Figure 1—figure supplement 1—source data 1
Excel file containing source data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig1-figsupp1-data1-v1.xlsx
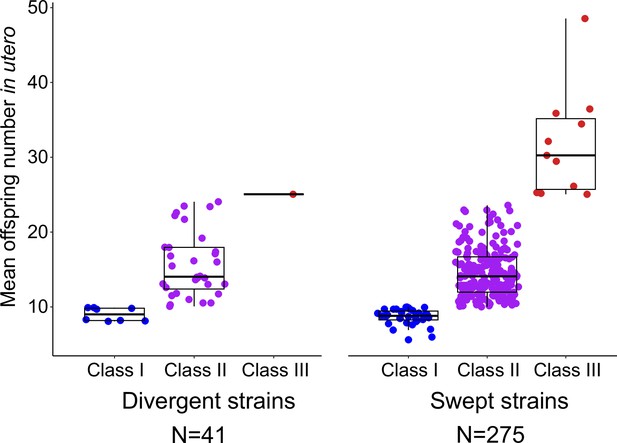
Egg number in utero in swept versus divergent C. elegans strains.
Strains, scored for egg number in utero at L4 +48 hr (Figure 1A), were categorized as swept (N=275) or as divergent strains (N=41) following previous classifications (Gilbert et al., 2022). In brief, strains are categorized as swept if any of chromosomes I, IV, V, or X contained greater than or equal to 30% of the same haplotype; strains not among the swept strains are classified as divergent (Andersen et al., 2012; Lee et al., 2021; Gilbert et al., 2022). Thirteen out of the 14 Class III strains exhibited a swept haplotype.
-
Figure 1—figure supplement 2—source data 1
Excel file containing source data for Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig1-figsupp2-data1-v1.xlsx
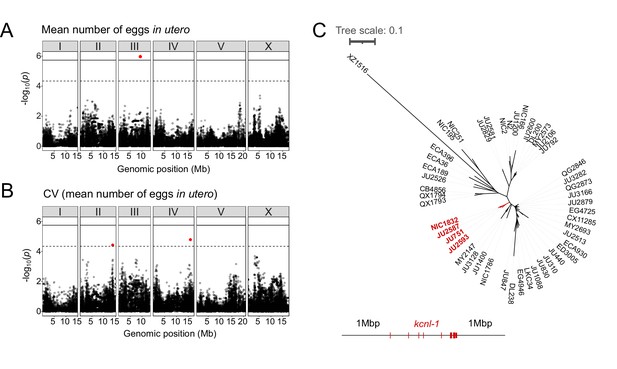
Common and rare genetic variants underlie natural differences in egg retention.
(A–B) Manhattan plots of single-marker based GWA mappings for C. elegans egg retention phenotypes (N=316). Each dot represents a SNV that is present in at least 5% of the assayed population. The genomic location of each single-nucleotide variant (SNV) is plotted on the X-axis against its log10(p) value on the y-axis. SNVs that pass the genome-wide EIGEN threshold (dotted line) or the Bonferroni threshold (solid line) are marked in red. (A) Manhattan plot of single-marker based GWA mapping region for mean number of eggs in utero. (B) Manhattan plot of single-marker-based GWA mapping region for the coefficient of variation (CV) (mean number of eggs in utero). (C) Neighbour-joining tree based on the 2 Mb region surrounding the kcnl-1 genomic region using a subset of 48 C. elegans wild strains. The four strains with the KCNL-1 V530L variant are shown in red.
-
Figure 2—source data 1
Excel file containing source data for Figure 2.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig2-data1-v1.xlsx
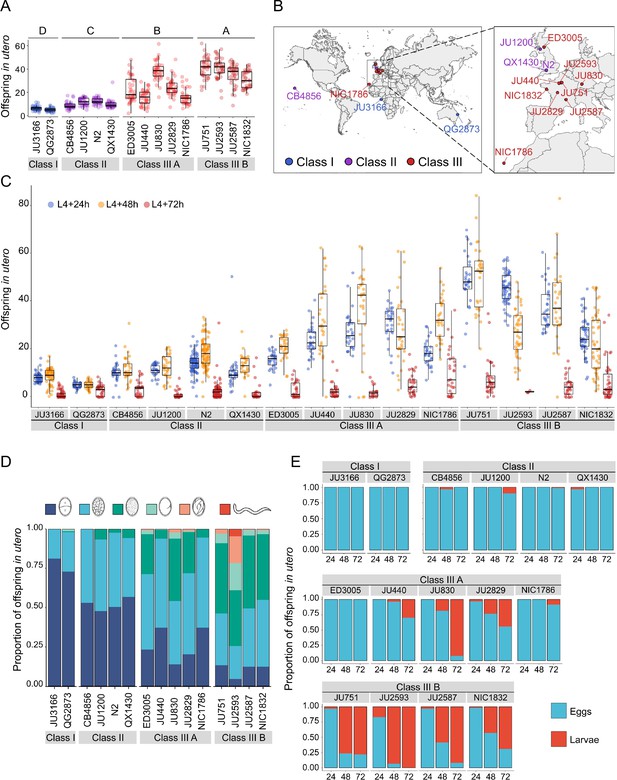
Temporal progression of egg retention and internal hatching.
(A) Egg retention in a subset of 15 strains with divergent egg retention, divided into the three phenotypic classes. Class I weak:<10 eggs in utero (N=34), Class II canonical: 10–25 eggs in utero (N=230), Class III strong:>25 eggs in utero (N=14). Class III strains were further distinguished depending on the absence (Class IIIA) or presence (Class IIIB) of the KCNL-1 V530L variant explaining strong egg retention. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05) based on results of a Two-Way ANOVA, fixed effect Class: F3,577=710.38, p<0.0001, fixed effect Strain(nested in Class): F11,577=33.58, p<0.0001. (B) Geographic distribution of the 15 focal strains with divergent egg retention. (C) Temporal dynamics of offspring number in utero in the 15 focal strains. Number of eggs and larvae in utero at three stages covering the reproductive span of self-fertilizing hermaphrodites. N=28–96 individuals per strain per time point (except for JU2593: at mid-L4 +72 hr: only four individuals were scored as most animals were dead by this time point). (D) Age distribution of embryos retained in utero of hermaphrodites (mid-L4 +30 hr) in the 15 focal strains. Embryonic stages were divided into five age groups according to the following characteristics using Nomarski microscopy (Hall and Altun, 2007): 1–2 cell stage, 4–26 cell stage, 44 cell to gastrula stage, bean to two-fold stage, three-fold stage, L1 larva. (Data from same cohort of animals used for experiment shown in A). (E) Frequency of internal hatching across three time points of the reproductive span of self-fertilizing hermaphrodites (extracted from data shown in D). Red bars indicate the proportion of individuals carrying at least one L1 larva in the uterus; blue bars indicate the proportion of individuals carrying only embryos in the uterus. Dead mothers were excluded from analyses.
-
Figure 3—source data 1
Excel file containing source data for Figure 3, Figure 3—figure supplement 1A.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig3-data1-v1.xlsx
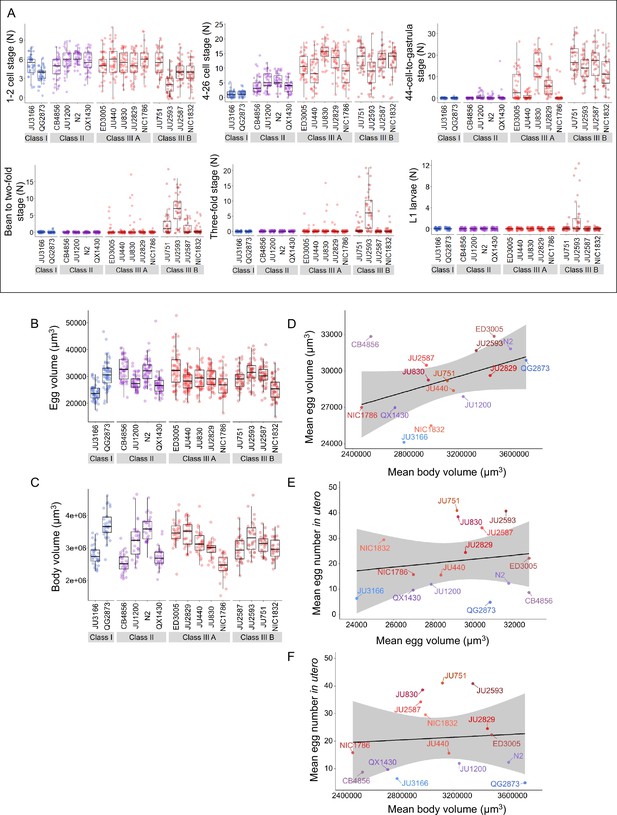
Temporal progression of egg retention and internal hatching.
(A) Age distribution of embryos within eggs retained in utero of hermaphrodites (L4 +30 hr) in the 15 focal strains, including the N2-derived strain QX1430 (Andersen et al., 2015). Embryonic stages were divided into five age groups according to the following characteristics using Nomarski microscopy (Hall and Altun, 2007): 1–2 cell stage, 4–26 cell stage, 44 cell to gastrula stage, bean to two-fold stage, three-fold stage, L1 larva. N=36–40 individuals were scored per strain; data obtained from same individuals scored for number of eggs in utero (Figure 3A). (B) Egg size (volume) in the 15 focal strains with variable egg retention, measured on laid eggs of mixed-age adult populations. Strains differed significantly in egg size (Kruskal-Wallis Test, χ2=223.97, df = 14, p<0.0001). N=50–69 eggs per strain. (C) Body size (volume) measured in the 15 focal strains; selfing hermaphrodites at first fertilization (1–2 eggs in utero). Strains differed significantly in body size (Kruskal-Wallis Test, χ2=227.45, df = 14, p<0.0001). N=47–51 individuals per strain. (D) Marginally significant positive correlation between mean egg size and mean body size (volume; at first fertilization, 1–2 eggs in utero) across the 15 focal strains with divergent egg retention (ρSpearman=0.50, p=0.06). (E) No correlation between mean egg size and mean egg retention (at L4 +30 hr) across the 15 focal strains with divergent egg retention (ρSpearman=0.01, p=0.98). (F) No correlation between mean body size (volume; at first fertilization, 1–2 eggs in utero) and mean egg retention (at L4 +30 hr) across the 15 focal strains with divergent egg retention (ρSpearman=0.1, p=0.92).
-
Figure 3—figure supplement 1—source data 1
Excel file containing source data for Figure 3—figure supplement 1B–F.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig3-figsupp1-data1-v1.xlsx
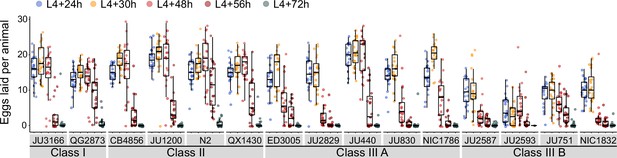
Natural variation in egg-laying activity.
Temporal dynamics of egg-laying activity in the 15 focal strains. Number of eggs laid (within a two-hour window) at five time points across the reproductive span of self-fertilizing hermaphrodites. N=20 individuals per strain per time point except for JU2593 (at mid-L4 +72 hr: only four individuals could be scored as most animals were dead by this time point). Note that several Class III strains laid eggs containing advanced-stage embryos, evidenced by L1 hatching within the two-hour window of the experiment (Figure 4—source data 1).
-
Figure 4—source data 1
Excel file containing source data for Figure 4.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig4-data1-v1.xlsx
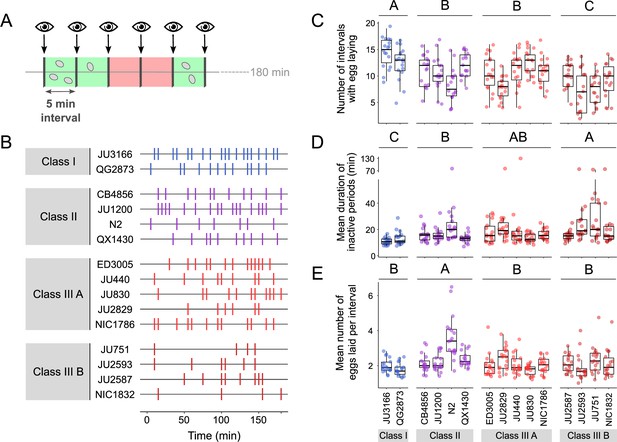
Natural variation in egg-laying behaviour.
(A) Cartoon depicting the design of our scan-sampling experiment to quantify temporal patterns of C. elegans egg-laying behaviour in the 15 focal strains (B–E). We scored the presence and number of eggs laid by isolated adults (at mid-L4 +30 hr every 5 min across a 3-hr period, resulting in a total of 36 observations [intervals] per individual [N=17–18 individuals per strain]). Intervals with and without egg laying are marked in green and red, respectively. (B) Raster plots illustrating strain variation in temporal patterns of egg-laying behaviour across 3 hr of observation. Each horizontal line represents single individual and vertical bars indicate 5-min intervals during which one or more eggs were laid. For a detailed figure of the same data, see Figure 5—figure supplement 1A. (C) The number of 5-min intervals with egg laying differed significantly between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,248=19.94, p<0.0001, fixed effect Strain(nested in Class): F11,248=5.20, p<0.0001. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p<0.05). Each dot represents the number of intervals with egg laying (out of a total of 37 intervals) per individual (N=17–18 individuals per strain). (D) The estimated mean duration of inactive periods (min) differed significantly between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,248=8.46, p<0.0001, fixed effect Strain(nested in Class): F11,248=2.93, p=0.0012. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05). For each individual, this value was estimated as the mean time (corresponding to the number of intervals without egg laying) separating successive intervals with egg laying. Each dot represents the mean duration of inactive periods (min) per individual (N=17–18 individuals per strain). (E) The mean number of eggs laid per interval with egg laying differed between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,248=12.41, p<0.0001, fixed effect Strain(nested in Class): F11,248=6.30, p<0.0001. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05). These significant effects are exclusively explained by the higher value of the N2 strain (Class II) relative to all other strains (p<0.05); none of the strains other than N2 did differ significantly from each other. Each dot represents the mean number of eggs laid per interval with egg laying per individual (N=17–18 individuals per strain). For data on total number eggs laid during the three-hour experiment, see Figure 5—figure supplement 1B.
-
Figure 5—source data 1
Excel file containing source data for Figure 5.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig5-data1-v1.xlsx
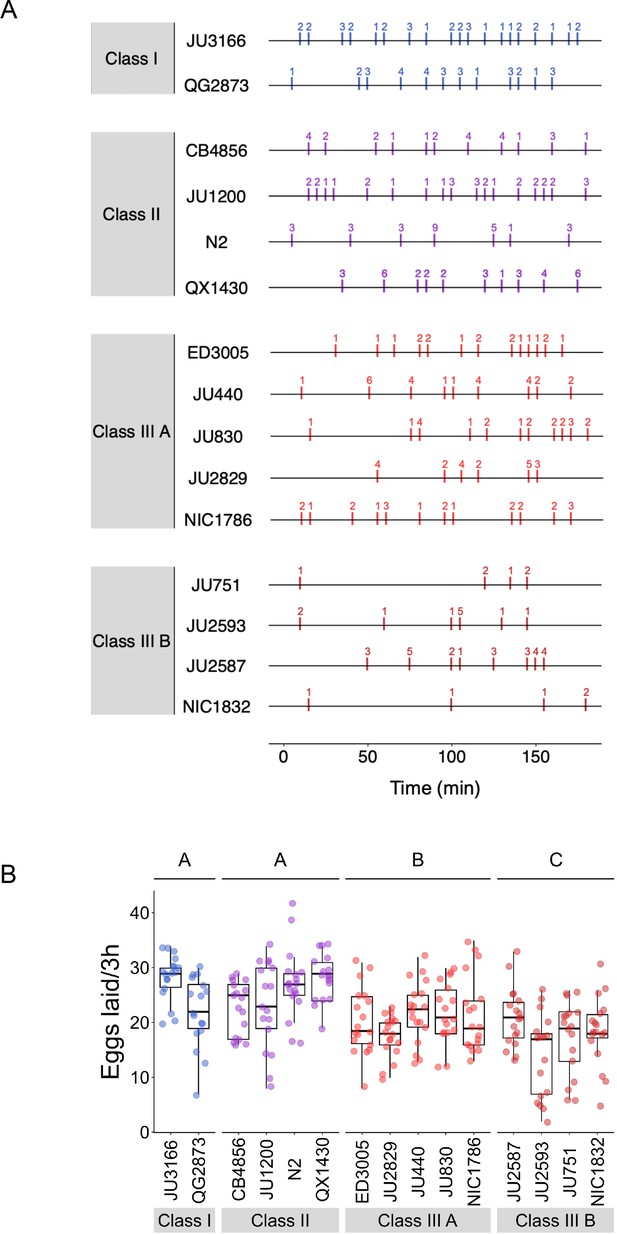
Natural variation in egg-laying behaviour.
(A) Detailed representation of data shown in Figure 5B: Representative raster plots of temporal patterns of egg-laying behaviour during a 3-hr interval, with each horizontal line representing a single individual; vertical bars indicate 5-min intervals during which one or more eggs were laid. The exact number of eggs laid in each interval is indicated above bars. (B) The total number of eggs laid during the three hours of observation differed significantly between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,248=23.07, p<0.0001, fixed effect Strain(nested in Class): F11,248=3.73, p<0.0001. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05). Data from same experiment as shown in Figure 5B-E.
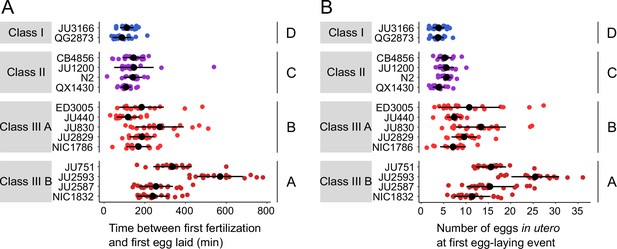
Natural variation in egg retention at the first egg-laying event.
(A) The time (min) between first fertilization and first egg-laying event differed between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,261=102.38, p<0.0001, fixed effect Strain(nested in Class): F11,261=9.40, p<0.0001. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05). N=16–22 individuals per strain. (B) The number of eggs in utero at first egg-laying event differed between strains and Classes. Two-Way ANOVA, fixed effect Class: F3,261=198.73, p<0.0001, fixed effect Strain(nested in Class): F11,261=13.00, p<0.0001. Estimates of Class effects labelled with the same letter are not significantly different from each other (Tukey’s honestly significant difference, p>0.05). N=16–22 individuals per strain; measured in the same individuals as shown in (A).
-
Figure 6—source data 1
Excel file containing source data for Figure 6.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig6-data1-v1.xlsx
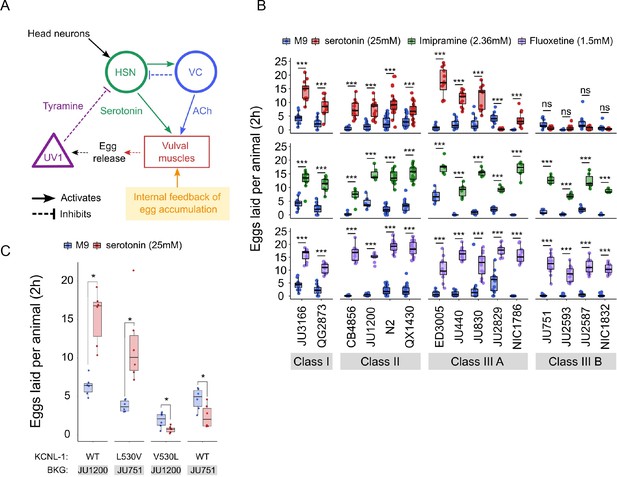
Natural variation in C. elegans egg-laying in response to neuromodulatory agents.
(A) Cartoon of the neural circuit controlling C. elegans egg laying (Collins et al., 2016; Kopchock et al., 2021). The structure of the C. elegans egg-laying circuit is simple, containing two classes of motoneurons, the two serotonergic hermaphrodite-specific motoneurons (HSN) and the six ventral cord motoneurons (VC), which provide synaptic input to the egg-laying muscles (VM). Serotonin from HSN act through vulval muscle receptors to increase muscle excitability, which together with rhythmic signals from motor neurons, causes contractions of VM during egg laying (Collins et al., 2016; Kopchock et al., 2021). Mechanical feedback in response to egg accumulation favours exit from the inactive state (Collins et al., 2016; Medrano and Collins, 2023). Muscles are indicated by rectangles, neurons by circles, and neurosecretory cells (uv1) by triangles. Principal neurotransmitters released by neurons are indicated next to neurons (ACh: Acetylcholine). (B) Natural variation in egg-laying activity in response to exogenous serotonin, fluoxetine, and imipramine. Adult hermaphrodites (mid-L4 +30 hr) were placed in M9 buffer without food (control) or in M9 containing the indicated concentrations of serotonin, fluoxetine, and imipramine. The number of eggs laid were scored after two hours. Assays for each of the three treatments were carried out independently; the 15 strains were scored in parallel in both control and treatment conditions for each of the three assays. Serotonin: for each strain, 11–24 replicates (each containing 3.73±0.36 individuals on average) were scored for serotonin and control (M9 buffer) conditions. Align Rank Transform ANOVA, fixed effect Treatment: F1,390=432.62, p<0.0001, fixed effect Strain: F14,390=42.94, p<0.0001; interaction Treatment x Strain: F14,390=34.40, p<0.0001. Serotonin stimulated egg-laying in Class, II and III A strains but had no effect on egg laying in Class III B and JU2829 (Class III A) (Tukey’s honestly significant difference, ***p<0.0001; ns: not significant). Imipramine: for each strain, 6–18 replicates/wells (each containing 4.62±0.50 individuals on average) were scored for serotonin and control (M9 buffer) conditions. Align Rank Transform ANOVA, fixed effect Treatment: F1,222=562, p<0.0001, fixed effect Strain: F14,222=23.86, p<0.0001; interaction Treatment x Strain: F14,222=8.52, p<0.0001. Imipramine stimulated egg laying in strains from the 4 Class (Tukey’s honestly significant difference, ***p<0.0001; ns: not significant). Fluoxetine: for each strain, 12–24 replicates/wells (each containing 3.31±0.37 individuals on average) were scored for serotonin and control (M9) conditions. Align Rank Transform ANOVA, fixed effect Treatment: F1,378=1005, p<0.0001, fixed effect Strain: F14,378=30.09, p<0.0001; interaction Treatment x Strain: F14,378=16.35, p<0.0001. Imipramine stimulated egg-laying in strains from the 4 Class (Tukey’s honestly significant difference, ***p<0.0001; ns: not significant). For detailed statistical results, see Figure 7—source data 2. (C) Effects of exogenous serotonin (25 mM) on egg laying activity in strains with strongly divergent egg retention due to variation in a single amino acid residue of KCNL-1. Strains JU1200WT (canonical egg retention), JU751KCNL-1 L530V (CRISPR-Cas9-engineered, weak egg retention), JU1200KCNL-1 V530L (CRISPR-Cas9-engineered, strong egg retention) and JU751WT (strong egg retention). Adult hermaphrodites (mid-L4 +30 hr) were placed into M9 buffer without food (control) or M9 with serotonin (25 mM). Serotonin stimulated egg-laying in JU751KCNL-1 L530V and JU1200WT but inhibited egg laying in JU751WT and JU1200KCNL-1 V530L (Kruskal-Wallis Tests were performed separately for each strain to test for the effect of serotonin on the number of eggs laid; *p<0.05). For each strain, six replicates (each containing 5.50±0.92 individuals on average) were scored for serotonin and control conditions. For additional data, see Figure 7—figure supplement 1.
-
Figure 7—source data 1
Excel file containing source data for Figure 7B and C.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig7-data1-v1.xlsx
-
Figure 7—source data 2
Excel file containing statistical results for analyses of data shown in Figure 7B and C, Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig7-data2-v1.xlsx
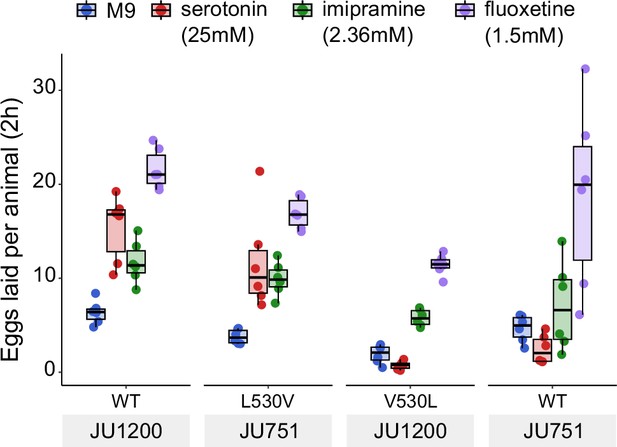
Effects of exogenous serotonin, Imipramine and Fluoxetine on egg laying activity in strains with strongly divergent egg retention due to variation in a single amino acid residue of KCNL-1.
Effects of exogenous serotonin, fluoxetine and imipramine on egg laying activity in strains with strongly divergent egg retention due to variation in a single amino acid residue of KCNL-1. Strains JU1200WT (canonical egg retention), JU1200KCNL-1 V530L (CRISPR-engineered, strong egg retention), JU751WT (strong egg retention) and JU751KCNL-1 L530V (CRISPR-engineered, low egg retention). Adult hermaphrodites (L4 +30 hr) were placed into M9 buffer without food (control) or M9 with serotonin (25 mM). In contrast to serotonin, imipramine and fluoxetine stimulated egg-laying activity in all four strains. For each strain, six replicates (each containing 5.50±0.92 individuals on average) were scored for each treatment and control condition. (For clarity, Figure 7C only shows the serotonin versus control conditions of this experiment).
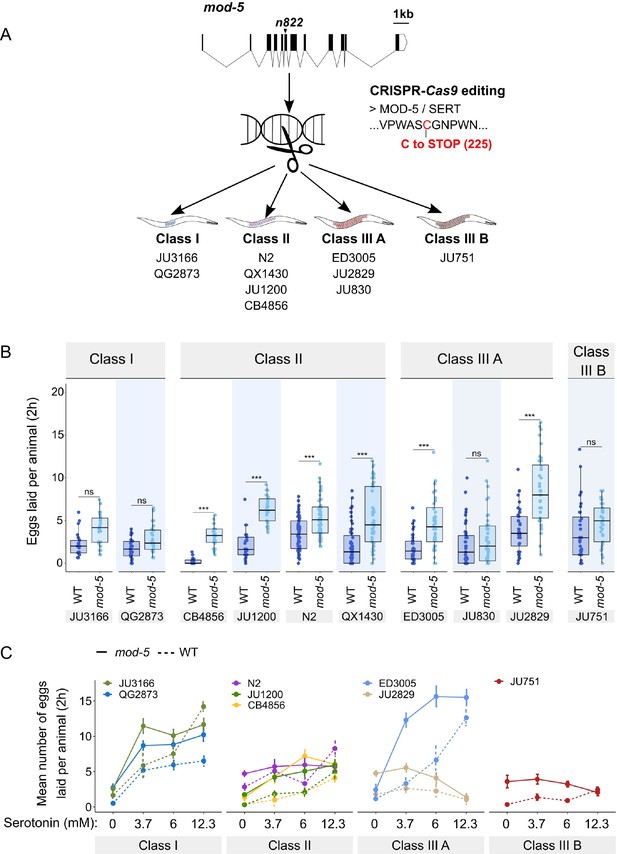
Manipulating endogenous serotonin levels uncovers natural variation in the neuromodulatory architecture of the C. elegans egg-laying system.
(A) Cartoon outlining the experimental design to introduce (by CRISPR-Cas9 technology) the loss-of-function mutation mod-5(n822) (Ranganathan et al., 2001) into 10 C. elegans strains with divergent egg-laying behaviour. (B) Natural variation in the effect of mod-5(lf) on egg laying (adult hermaphrodites, mid-L4 +30 hr). mod-5(lf) increased basal egg-laying activity relative to wild type in the absence of food (M9 buffer), except for the two Class I strains and the Class III strains JU830 and JU751. The stimulatory effect of mod-5(lf) on egg laying also varied quantitatively between strains within the same Class, that is, within Class II and Class IIIA. Align Rank Transform ANOVA, fixed effect mod-5: F1,709=175.55, p<0.0001, fixed effect Background: F9,709 = 20.98, p<0.0001; interaction mod-5 x Background: F9,709=5.34, p<0.0001. N=23–60 replicates per strain, with each replicate containing 3.15±0.18 individuals on average were scored. (C) Natural variation in egg laying in response to a gradient of low exogeneous serotonin concentrations in wild type and mod-5(n822) animals. Adult hermaphrodites (mid-L4 +30 hr) were placed into M9 buffer without food containing four different concentrations of serotonin (0.0 mM, 3.7 mM, 6.0 mM, 12.3 mM). Strains differed strongly in sensitivity to specific concentrations of exogenous serotonin and these effects of genetic background were further contingent on the presence of mod-5(lf) as indicated by the significant three-way interaction term genetic background x mod-5 allele x serotonin treatment (Table 3). For complete results of statistical analyses, see Table 3; for an alternative representation of the same data, see Figure 8—figure supplement 1B. For each concentration, 8–10 replicates (each containing 3.15±0.22 individuals on average) were scored per strain.
-
Figure 8—source data 1
Excel file containing source data for Figure 8, Figure 8—figure supplement 1A and B.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig8-data1-v1.xlsx
-
Figure 8—source data 2
Excel file containing statistical results for analyses of data shown in Figure 8B and Figure 8—figure supplement 1A.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig8-data2-v1.xlsx
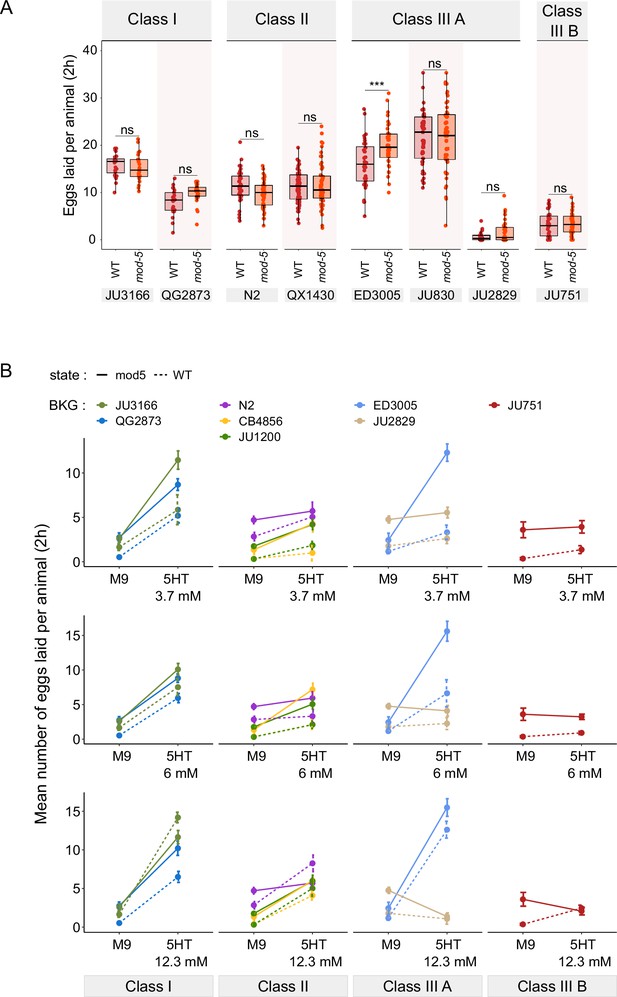
Manipulating endogenous serotonin levels uncovers natural variation in the neuromodulatory architecture of the C. elegans egg-laying system.
(A) Natural variation in serotonin sensitivity of mod-5(lf) on egg laying (adult hermaphrodites, L4 +30 hr). Align Rank Transform ANOVA, fixed effect mod-5: F1,582=0.29, p=0.59, fixed effect Background: F7,582 = 277.73, p<0.0001; interaction mod-5 x Background: F7,582=3.57, p<0.0001. mod-5(lf) did not increase egg-laying when exposed to a high dose of exogenous serotonin (25 mM), except for a slight increase observed in ED3005 (Tukey’s honestly significant difference, ***p<0.0001; ns: not significant). For each strain, 24–60 replicates (each containing 3.08±0.14 individuals on average) were scored. For complete results of statistical analyses, see Figure 8—source data 2. (B) Alternative representation of data shown in Figure 8C: Natural variation in egg laying in response to a gradient of low exogeneous serotonin concentrations in wild type and mod-5(n822) animals. Adult hermaphrodites (L4 +30 hr) were placed into M9 buffer without food containing four different concentrations of serotonin (0.0 mM, 3.7 mM, 6.0 mM, 12.3 mM). Strains differed strongly in sensitivity to specific concentrations of exogenous serotonin and these effects of genetic background were further contingent on the presence of mod-5(lf) as indicated by the significant 3-way interaction term genetic background x mod-5 allele x serotonin treatment (see Table 3 for statistical results). For complete results of statistical analyses, see Table 3. For each concentration, 8–10 replicates (each containing 3.15±0.22 individuals on average) were scored per strain. For detailed results of statistical analyses, see Figure 8—source data 1.
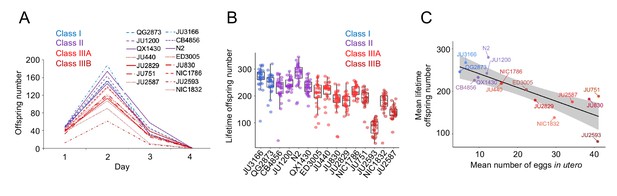
Strains with stronger egg retention show reduced lifetime self-fertility and survival.
(A) Dynamics of lifetime offspring production in self-fertilizing hermaphrodites of the 15 focal strains with divergent egg retention. Hermaphrodites at the mid-L4 stage were isolated to individual NGM plates and their offspring production was scored every 24 hr until reproduction had ceased (~mid-L4+96 hr). N=28–30 individuals per strain. (B) Significant differences in total lifetime offspring number between the 15 focal strains (Kruskal-Wallis Test, χ2=290.79, df=14, p<0.0001). Same experiment as shown in (A), N=28–30 individuals per strain. (C) Significant negative correlation between mean lifetime offspring number and mean egg retention across the 15 focal strains with divergent egg retention (at mid-L4 +30 hr) (ρSpearman=-0.85, p<0.0001).
-
Figure 9—source data 1
Excel file containing source data for Figure 9.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig9-data1-v1.xlsx
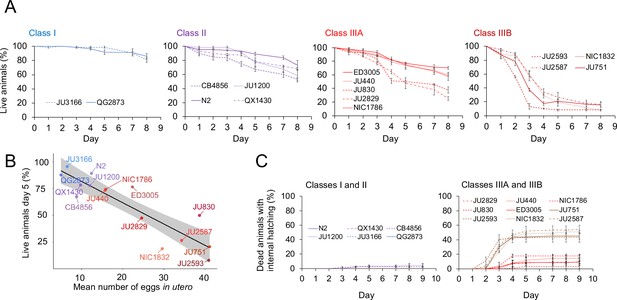
Strains with strong egg retention show reduced survival and increased internal hatching.
(A) Hermaphrodite survival in the 15 focal strains with divergent egg retention, separated by phenotypic classes. Survival was scored every 24 hr across the first eight days of adulthood. For each strain, two to three replicates were scored, with each replicate containing 30–36 individuals. On day 5, the fraction of surviving individuals was significantly different between all four Classes (Tukey’s honestly significant difference, all p<0.05) (Two-Way ANOVA, fixed effect Class: F3,29=178.49, p<0.0001, fixed effect Strain(nested in Class): F11,29=6.41, p<0.0001). (B) Significant negative correlation between mean percentage of survival (day 5) and mean egg retention across the 15 focal strains with divergent egg retention (at mid-L4 +30 hr) (ρSpearman=-0.84, p=0.00013). (C) Temporal progression of internal hatching during the survival assay (from data shown in (A)) in the 15 focal strains, measured as the cumulative percentage of dead mothers containing one or more internally hatched larva. For each strain, two to three replicates were scored, and each replicate consisted of 30–36 individuals. No individuals were censored.
-
Figure 10—source data 1
Excel file containing source data for Figure 10.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig10-data1-v1.xlsx
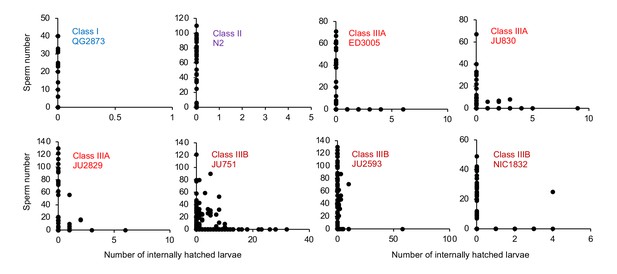
Internal hatching may occur during the reproductive span of hermaphrodites.
Quantifying hermaphrodite self-sperm numbers in select focal strains with divergent egg retention and testing for the co-occurrence of internally hatched larvae and self-sperm. Adult hermaphrodites derived from age-synchronized populations were collected across multiple time points of their reproductive span, then stained with DAPI to visualize and count spermatids (N=46–175 individuals per strain). Sperm and internally hatched larvae were found to co-occur in multiple Class III strains but not in strains of Class I or II.
-
Figure 11—source data 1
Excel file containing source data for Figure 11.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig11-data1-v1.xlsx
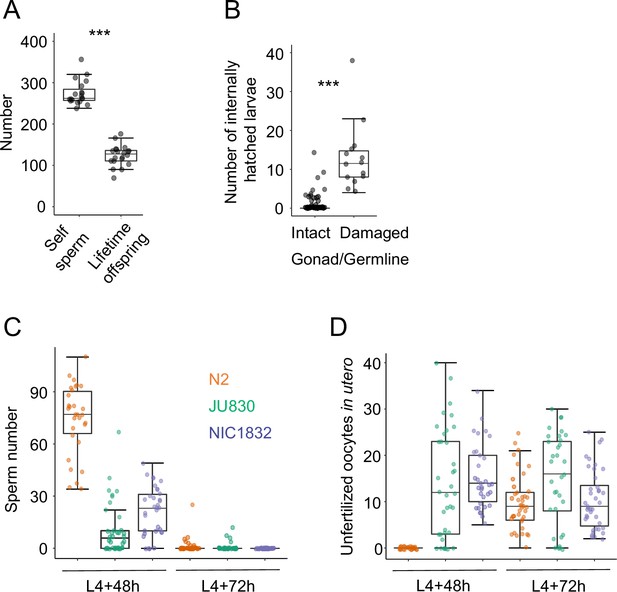
Internal hatching may have deleterious effects on germline integrity and reproduction.
(A) The Class IIIB strain JU2593 shows a strong discordance between total self-sperm number (N=20 individuals) and lifetime offspring production (N=19 individuals (ANOVA, F1,37=27.66, p<0.0001)). Self-sperm was counted in DAPI-stained worms that had just reached reproductive maturity (0–5 eggs in the uterus). (B) Internal larval hatching increases the incidence of gonad/germline damage in the Class IIIB strain JU2593 (hermaphrodites at midL4 +40 hr and midL4 +48 hr were fixed and stained with DAPI to visualize germlines, N=105). The number of larvae in utero was higher in individuals with damaged gonads versus individuals with intact gonads (Kruskal-Wallis Test, χ2=41.74, df=1, p<0.0001). (C) and (D) Uterine accumulation of unfertilized oocytes precedes self-sperm depletion in the Class III strains JU830 and NIC1832 (but not in the Class II strain N2). For each strain, hermaphrodites (at L4 +48 hr and L4 +72 hr) were fixed and stained with DAPI to visualize and count remaining (C) self-sperm and (D) unfertilized oocytes in the uterus. At L4 +48 h, JU830 and NIC1832 have generated large numbers of unfertilized oocytes despite the presence of self-sperm (N2: N=30, JU830: N=37, NIC1832: N=33). At L4 +72 h, all strains have depleted their self-sperm and have unfertilized oocytes in utero (N2: N=40, JU830: N=34, NIC1832: N=40).
-
Figure 11—figure supplement 1—source data 1
Excel file containing source data for Figure 11—figure supplement 1.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig11-figsupp1-data1-v1.xlsx
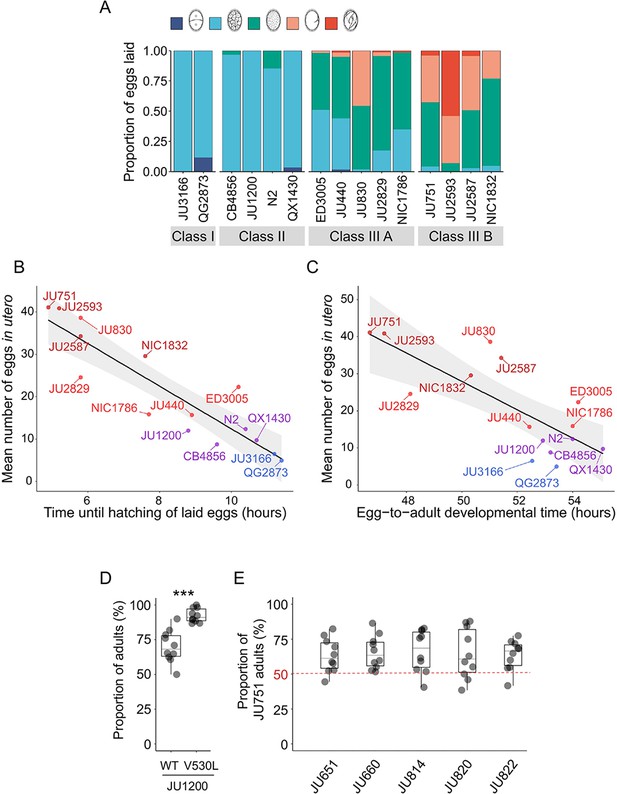
Strong egg retention may provide a competitive advantage in resource-limited environments.
(A) Age distribution of embryos contained within eggs laid by hermaphrodites (mid-L4 +40 hr) of the 15 focal strains. Embryonic stages were divided into five age groups according to the following characteristics using Nomarski microscopy (Hall and Altun, 2007): 1–2 cell stage, 4–26 cell stage, 44 cell to gastrula stage, bean to two-fold stage, three-fold stage, L1 larva. N=45–72 eggs per strain. (B) Significant negative correlation between hatching time of laid eggs and mean egg retention across the 15 focal strains with divergent egg retention (at mid-L4 +30 hr) (ρSpearman=-0.92, p<0.0001). Values are estimates of the time point at which 50% of the eggs had hatched. For each strain, 10–20 adult hermaphrodites (mid-L4 +30 hr) were allowed to lay eggs within an 1-hr window (N=48–177 eggs per strain). The fraction of hatched eggs was scored every hour until all eggs had hatched. (C) Significant negative correlation between egg-to-adult developmental time and mean egg retention across the 15 focal strains with divergent egg retention (at mid-L4 +30 hr) (ρSpearman=-0.69, p=0.0041). Values are estimates of the time point at which 50% of individuals had reached reproductive maturity (one or two eggs in utero). For each strain, 10–20 adult hermaphrodites (mid-L4 +30 hr) were allowed to lay eggs within a one-hour window (N=77–318 eggs per strain). After removal of adults, eggs were allowed to hatch and after 45 hr of development, populations were surveyed every two hours to count the fraction of adults that had reached reproductive maturity. (D) Short-term competition of JU1200WT and JU1200KCNL-1 V530L against a GFP-tester strain (myo-2::gfp) with a genotype starting frequencies of 50:50. The strain JU1200KCNL-1 V530L (strong egg retention, Class III phenotype) outperformed JU1200WT (regular egg retention, Class II phenotype). For each replicate, 20 laid eggs of either genotype were mixed with 20 laid eggs of the GFP-tester strain on a NGM plate; after 4–5 days (when food became exhausted) the fraction of GFP-positive adult individuals was determined. Relative to the GFP-tester strain, JU1200KCNL-1 V530L showed a significantly higher fraction of adults compared to JU1200WT (Kruskal-Wallis Test, χ2=11.57, df=1, p=0.0007). N=10 replicates per genotype. (E) Short- term competition of JU751 (Class IIIB, strong egg retention) against each of five wild strains (Class II, canonical egg retention) isolated from the same locality. For each of the five strains, 20 freshly laid eggs were mixed with 20 freshly laid eggs from JU751 and allowed to develop. Adult population size and genotype frequencies were determined after 4–5 days (when food became exhausted). In each of the five competition experiments, JU751 showed a significantly higher number of adults (Wilcoxon signed-rank test for matched pairs, all p<0.05). N=10 replicates per strain.
-
Figure 12—source data 1
Excel file containing source data for Figure 12.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig12-data1-v1.xlsx
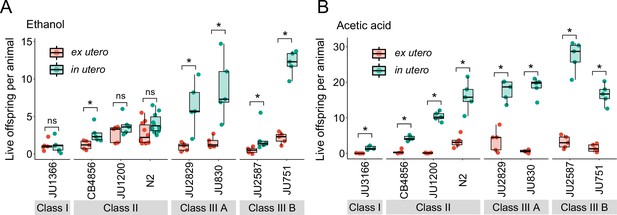
Strong egg retention improves progeny protection when facing sudden environmental stress.
(A) Differences in survival of eggs developing ex utero versus in utero when exposed to a high concentration of ethanol (96%, 10-min exposure). A subset of the 15 focal strains with divergent egg retention was selected to compare the number of surviving eggs ex utero (extracted by dissection) versus in utero (eggs retained in mothers) exposed to ethanol. Overall, the number of surviving eggs tended to be greater when exposed to ethanol in utero compared to ex utero (Kruskal-Wallis Tests performed separately for each strain to compare the number of surviving offspring in utero versus ex utero when exposed to ethanol; *p<0.05, ns: not significant). Class III strains tended to have a higher number of surviving offspring than Class I and II strains. N=5–10 replicates per genotype and treatment (10 hermaphrodites at mid-L4 +30 hr per replicate). See Figure 13—figure supplement 1A, for additional (control) data of the experiment. (B) Differences in survival of eggs developing ex utero versus in utero when exposed to a high concentration of acetic acid (10 M, 15-min exposure). A subset of the 15 focal strains with divergent egg retention was selected to compare the number of surviving eggs ex utero (extracted by dissection) versus in utero (eggs retained in mothers) exposed to acetic acid. For all strains, the number of surviving eggs was significantly greater when exposed to acetic acid in utero compared to ex utero (Kruskal-Wallis Tests performed separately for each strain to compare the number of surviving offspring in utero versus ex utero when exposed to acetic acid; *p<0.05, ns: not significant). Class III strains tended to have a higher number of surviving offspring than Class I and II strains. N=5 replicates per genotype and treatment (10 hermaphrodites at mid-L4 +30 hr per replicate). See Figure 13—figure supplement 1B for additional (control) data of the experiment.
-
Figure 13—source data 1
Excel file containing source data for Figure 13, Figure 13—figure supplement 1A and B.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig13-data1-v1.xlsx
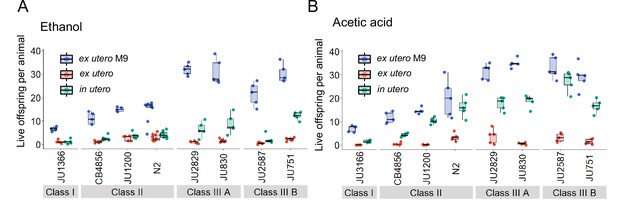
Additional data for experiment shown in Figure 13A and B, including data for control conditions (M9 buffer).
(A) Additional data for the experiment shown in Figure 13A: Differences in survival of eggs developing ex utero versus in utero when exposed to a high concentration of ethanol (96%, 10-min exposure). A subset of the 15 focal strains with divergent egg retention was selected to compare embryonic survival of eggs ex utero (extracted by dissection) versus in utero (eggs retained in mothers) exposed to ethanol. In addition to data shown in Figure 13A, this figure includes control data for survival of eggs ex utero exposed to control conditions (M9 buffer). N=5–10 replicates (each containing 10 mothers) per genotype and treatment (hermaphrodites at L4 +30 hr). (B) Additional data for the experiment shown in Figure 13B: Differences in survival of eggs developing ex utero versus in utero when exposed to a high concentration of acetic acid (10 M, 15-min exposure). A subset of the 15 focal strains with divergent egg retention was selected to compare embryonic survival of eggs ex utero (extracted by dissection) versus in utero (eggs retained in mothers) exposed to acetic acid. In addition to data shown in Figure 13B, this figure includes control data for survival of eggs ex utero exposed to control conditions (M9 buffer). N=5 replicates (each containing 10 mothers) per genotype and treatment (hermaphrodites at L4 +30 hr).
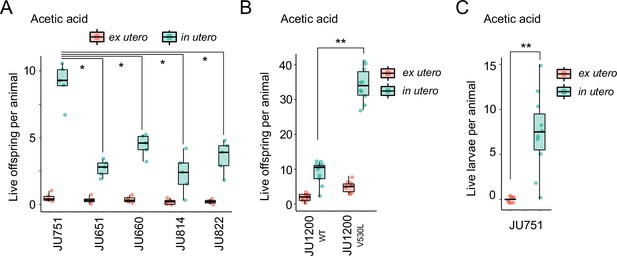
Differences in survival of eggs developing ex utero versus in utero when exposed to environmental stress.
(A) Differences in survival of eggs developing ex utero versus in utero when exposed to acetic acid (10 M, 15-min exposure). Comparison of the strain JU751 (Class IIIB, strong retention) to four strains (Class II, canonical retention) isolated from the same locality. Examining only data for eggs exposed in utero, JU751 exhibited a significantly higher number of surviving offspring compared to all other strains (ANOVA, effect Strain: F4,20=15.96, p<0.0001; Tukey’s honestly significant difference, all p<0.05). N=5 replicates per genotype and treatment (10 hermaphrodites at mid-L4 +30 hr per replicate). See Figure 14—figure supplement 1 for additional (control) data of the experiment. (B) Comparing the Class II strain JU1200WT (canonical retention) and the JU1200KCNL-1 V530L strain (strong retention): differences in the number of surviving eggs ex utero (extracted by dissection) versus in utero (eggs retained in mothers) exposed to a high concentration of acetic acid (10 M, 15-min exposure). JU1200KCNL-1 V530L exhibited a significantly higher number of surviving offspring in utero when exposed to acetic acid compared to JU1200WT (Kruskal-Wallis Test, χ2=14.35, df=1, p=0.0002). N=10 replicates per genotype per treatment. (C) Differences in the number of surviving internally hatched larvae exposed to acetic acid (10 M, 15-min exposure) using the strain JU1200KCNL-1 V530L: ex utero (extracted by dissection) versus in utero (larvae retained in mothers). The number of live larvae per mother was significantly higher in utero compared to ex utero (Kruskal-Wallis Test, χ2=13.89, df=1, p=0.0002). N=10 replicates per genotype per treatment.
-
Figure 14—source data 1
Excel file containing source data for Figure 14, Figure 14—figure supplement 1.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig14-data1-v1.xlsx
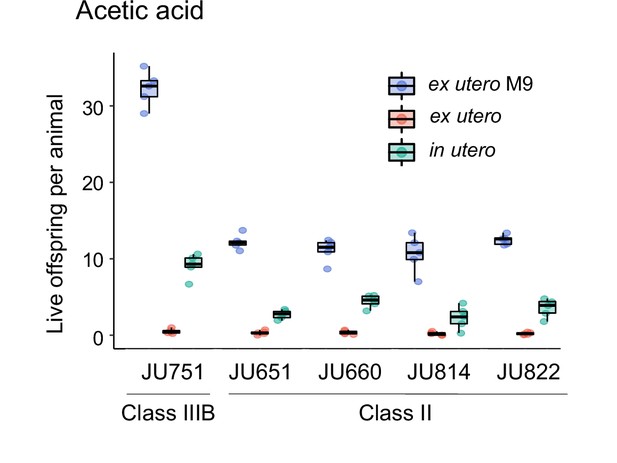
Additional data for the experiment shown in Figure 14A.
Differences in survival of eggs developing ex utero versus in utero when exposed to a high concentration of acetic acid (10 M, 15-min exposure). Comparison of the strain JU751 (Class IIIB, high retention) to four strains (Class II, canonical retention) isolated from the same locality. In addition to data shown in Figure 14A, this figure includes control data for survival of eggs ex utero exposed to control conditions (M9 buffer). N=5 replicates (each containing 10 mothers) per genotype and treatment (hermaphrodites at L4 +30 hr).
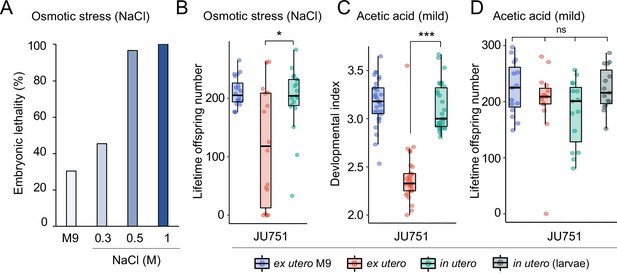
Increased egg retention protects offspring viability and fertility under mild environmental stress.
(A) Effects of osmotic stress on survival of embryos from eggs developing ex utero in the strain JU751. Eggs were extracted from adult hermaphrodites (mid-L4 +36 hr) by dissection and exposed to variable concentrations of NaCl (and M9 control condition) for 15 hr. Embryonic survival was estimated by counting the fraction of live larvae 24 hr later. N=120–288 eggs per treatment. (B) Differences in total lifetime offspring production of selfing JU751 animals derived from surviving eggs developing ex utero (dissected from adults at mid-L4 +36 hr) versus in utero when exposed to mild osmotic stress (0.3 M NaCl) for 15 hr; in parallel, ex utero eggs (dissected from adults at mid-L4 +36 hr) were exposed to a control treatment (M9 buffer) for 15 hr. 24 hr after the treatment, larvae from each of the three treatments were allowed to develop and reproduce for 3 days and scored for total offspring production. Animals derived from eggs developing in utero showed significantly higher fertility than animals derived from eggs developing ex utero when exposed to osmotic stress (Kruskal-Wallis Test, χ2=4.66, df=2, p=0.03). N=18–19 animals per treatment. (C) Differences in developmental time of JU751 animals derived from surviving eggs developing ex utero (dissected from adults at mid-L4 +36 hr) versus in utero when exposed to a low concentration of acetic acid (1 M) for 15 min; in parallel, ex utero eggs (dissected from adults at mid-L4 +36 hr) were exposed to a control treatment (M9 buffer). Eggs were then allowed to hatch and develop for 45 hr, at which time we determined their developmental stages. Each stage was assigned a score of development as follows: L3=1; early mid-L4=2; midL4=3; lateL4=4; Adult=5. Each dot represents the mean score reached by offspring produced by one single mother (N=30) mothers per treatment, producing between surviving 9–57 larval offspring. Animals derived from in utero eggs had a significantly higher mean developmental time score, that is they exhibited accelerated development compared to animals derived from ex utero eggs (Kruskal-Wallis Test, χ2=38.93, df=1, p<0.0001). (D) Differences in total lifetime offspring production of selfing JU751 animals derived from eggs developing ex utero versus eggs and L1 larvae in utero when exposed to a low concentration of acetic acid (1 M) for 15 min; in parallel, ex utero eggs (dissected from adults at mid-L4 +36 hr) were exposed to a control treatment (M9 buffer). Note that L1 larvae directly exposed to 1 M acetic acid died immediately. Twenty-four hr after the treatment, larvae from each of the four treatments were allowed to develop and reproduce for four days until cessation of reproduction; total offspring production was then scored 24 hr later. There were no significant differences in mean fertility between the four different treatment groups (Kruskal-Wallis Test, χ2=4.00, df=3, p=0.26). N=15 animals per treatment. Eggs were dissected from adults at mid-L4 +36 hr and L1 larvae at mid-L4 +48 hr.
-
Figure 15—source data 1
Excel file containing source data for Figure 15.
- https://cdn.elifesciences.org/articles/88253/elife-88253-fig15-data1-v1.xlsx
Tables
QTL detected by GWA mapping for mean and coefficient of variation (CV) of egg number in utero (N=316 strains).
| Trait | Chromosome | Interval (bp) | Peak | Log10(p) | Variance explained (%) |
|---|---|---|---|---|---|
| Mean | III | 8,532,784–10,019,713 | 9,312,552 | 6.01 | 24.14 |
| CV | II | 13,477,760–14,077,923 | 13,790,719 | 4.45 | 6.74 |
| CV | IV | 15,491,767–16,170,655 | 15,885,539 | 4.81 | 6.74 |
Potential candidate genes (with known roles in C. elegans egg laying) and variants in the QTL interval on chromosome III (GWA mapping for egg number in utero).
Potential high-impact variants are predicted to disrupt gene function, for example, through nonsense or frameshift mutations; low impact variants are predicted to have little or no impact on gene function, such as synonymous mutations (Cook et al., 2017).
| Gene | Chromosome | Interval (bp) | Number of variants(predicted high impact) | Number of variants(predicted low impact) |
|---|---|---|---|---|
| pat-2 | III | 8,818,898–8,825,266 | 1 | 2 |
| lin-12 | III | 9,060,220–9,071,472 | 0 | 2 |
| ina-1 | III | 9,168,072–9,172,802 | 1 | 2 |
| lin-52 | III | 9,824,082–9,824,750 | 0 | 1 |
| cbp-2 | III | 9,923,173–9,924,800 | 20 | 11 |
Natural variation in egg laying in response to a gradient of low exogenous serotonin concentrations in wild type and mod-5(n822) animals (Figure 8C, Figure 8—figure supplement 1B).
Results for statistical analyses testing for the effects of and interactions between genetic background, presence of mod-5(lf) and Treatment (concentration of serotonin) on egg laying (ANOVA).
| Source | DF | Sum of Squares | F Ratio | p |
|---|---|---|---|---|
| mod-5 | 1 | 67.45 | 186.42 | <.0001* |
| Background | 7 | 149.21 | 58.91 | <.0001* |
| Treatment | 3 | 120.21 | 110.73 | <.0001* |
| mod-5 x Background | 7 | 8.94 | 3.53 | 0.0010* |
| mod-5 x Treatment | 3 | 15.77 | 14.53 | <.0001* |
| Background x Treatment | 21 | 95.77 | 12.60 | <.0001* |
| mod-5 x Background x Treatment | 21 | 13.31 | 1.75 | 0.021* |
| Error | 510 | 184.55 |
Additional files
-
Supplementary file 1
A table of strains used in this study.
- https://cdn.elifesciences.org/articles/88253/elife-88253-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/88253/elife-88253-mdarchecklist1-v1.docx





