Spinal neural tube formation and tail development in human embryos
Figures
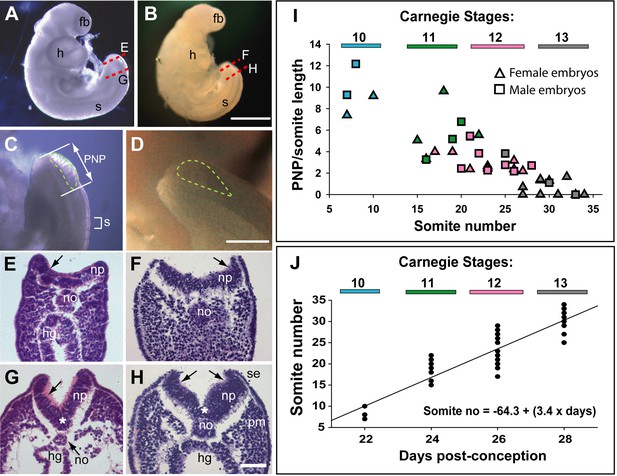
Morphology and timing of human posterior neuropore (PNP) closure.
(A,B) Two CS12 embryos viewed from right side, each with 22–23 somites (s), and a looped heart (h). Neural tube closure is complete along most of the body axis, including the forebrain (fb), whereas the PNP remains open caudally. (C,D) Magnified oblique views from upper right side of the caudal region; the open PNP is outlined with dashed lines. (E–H) Haematoxylin and eosin (H&E)-stained transverse sections, through the PNP, with section planes as indicated by dashed lines in A,B. The most caudally located sections (E,F) show a relatively flat neural plate (np), although incipient dorsolateral hinge points (DLHPs; arrows) are visible. Note the midline notochord (no) underlying the neural plate, and hindgut (hg) beneath the notochord (in E only). More rostral sections (G,H) show elevated neural folds with DLHPs clearly visible (arrows: unilateral in G, bilateral in H), located where basal contact of the neural plate changes from surface ectoderm (se), to paraxial mesoderm (pm). A median hinge point (MHP; asterisks in G,H) overlies the notochord. (I) PNP length (double headed arrow in C), normalised to somite (s) length (bracketed in C), determined from photographic images of 40 human embryos (24 females; 16 males) at CS10 (n=4), CS11 (n=8), CS12 (n=16), and CS13 (n=12). Symbol colours indicate the Carnegie stages assigned at the time of collection. The PNP shows gradual closure, with completion around the 30 somite stage. (J) Somite number of the 40 embryos in I, plotted against days post-conception, as reported for each Carnegie stage by O’Rahilly and Muller, 1987. The linear regression equation is shown, with R2=0.82 and p<0.001. Scale bars: 1 mm in A,B; 0.4 mm in C,D; 0.1 mm in E–H.
-
Figure 1—source data 1
Measurements from photographic images of individual human embryos, as described in Materials and methods.
- https://cdn.elifesciences.org/articles/88584/elife-88584-fig1-data1-v1.xlsx
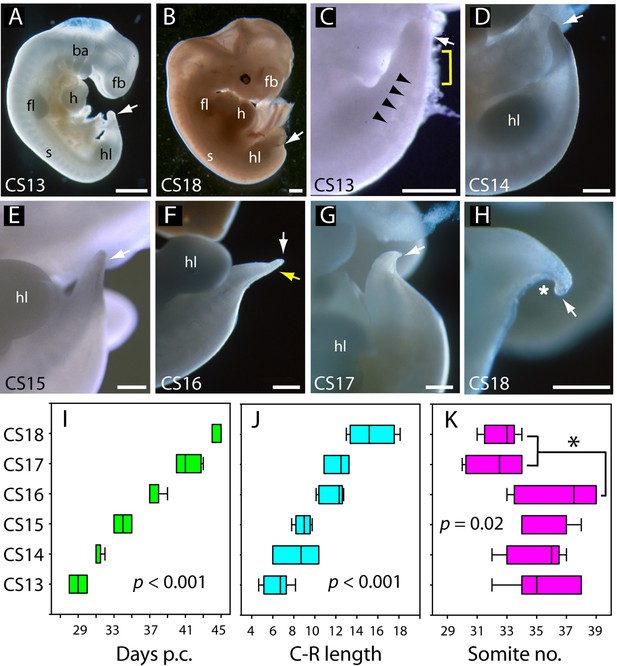
Development of the tail in human embryos.
(A,B) Whole embryos at CS13 (A) and CS18 (B), showing the range of stages studied (4–6.5 weeks post-conception). The tailbud (arrow) is well formed at CS13 following completion of posterior neuropore (PNP) closure at CS12, whereas, by CS18, tail development and regression are largely complete and only a small tail remnant remains (arrow). (C–H) Higher magnification views of the caudal region at CS13–18. At CS13, the tailbud is relatively massive, tapering gradually and with a rounded end (arrow in C). Somites are visible rostral to the tailbud (arrowheads) with an intervening region of presomitic mesoderm (yellow bracket). At CS14 and CS15 the tail narrows progressively, with distal tapering (arrows in D–E). By CS16, this has yielded a slender structure with a narrow pointed end (white arrow in F) in which somites extend almost to the tail tip (yellow arrow in F). Thereafter, the tail shortens progressively (arrows in G,H), develops a marked flexion (asterisk in H), and becomes increasingly translucent (G, H). (I–K) Analysis of embryos in the range CS13–16 (Table 2), plotting CS against: (I) days post-conception (p.c., see Materials and methods), (J) crown-rump (C–R) length in mm, and (K) somite no. One-way analysis of variance (ANOVA) on ranks shows all three parameters vary significantly with CS (p-values on graphs). Somite no. reduces significantly between CS16 and CS17/18 (*p<0.05). Abbreviations: ba, branchial arches; fb, forebrain; fl, forelimb; h, heart; hl, hindlimb; s, somites. Scale bars: 1 mm in A,B; 0.5 mm in C–H.
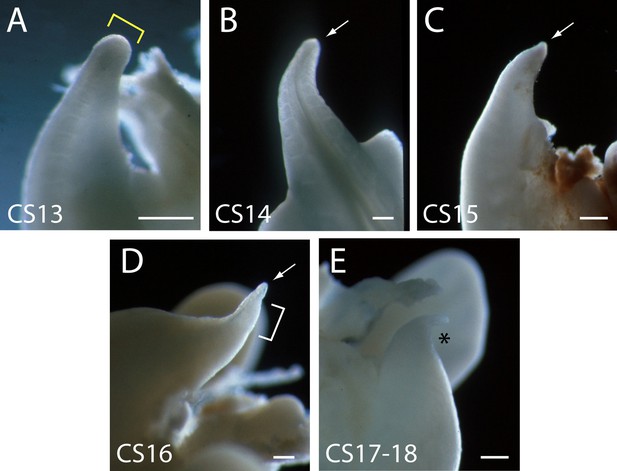
Human embryonic tails (additional to those in Figure 2) to show the reproducibility of tail morphology changes between CS13 and CS18.
Note the broad tailbud at CS13 with rounded tip (yellow bracket in A), which gradually narrows and becomes increasingly pointed, through CS14, CS15, and CS16 (arrows in B,C,D). At CS16, the tail tip has a markedly translucent appearance (bracket in D). By CS17–18, the tail has shortened and is dorsally curved at its tip (asterisk in E). Scale bars: 0.5 mm.
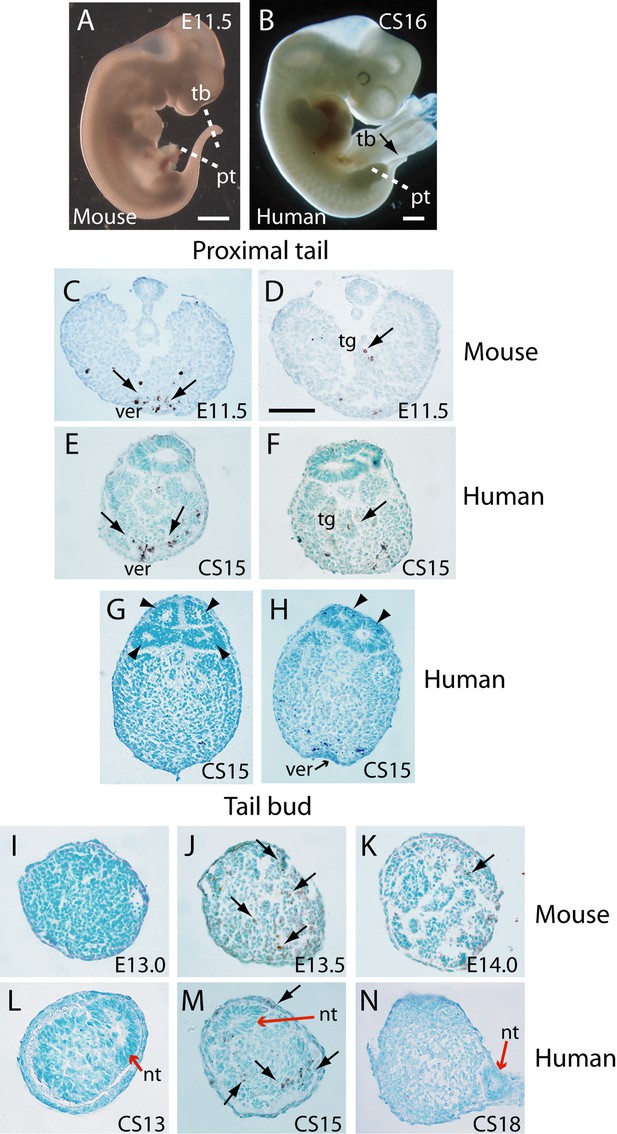
Tail morphology and apoptosis in mouse and human embryos.
(A,B) Mouse E11.5 (A) and human CS16 (B) embryos to illustrate the level of transverse sections through the proximal tail (pt) and tailbud (tb) regions of mouse (C,D,I–K) and human (E–H,L–N) embryos at the stages indicated on the panels. Immunohistochemistry was performed on paraffin wax sections for activated caspase 3 (brown stain), with counterstaining by methyl green. (C–F) In the proximal tail region, intense programmed cell death is observed in the ventral midline mesoderm overlying the ventral ectodermal ridge (ver) of both mouse (arrows in C) and human (arrows in E) embryos. Cell death can also be detected in the tailgut (tg) of both mouse (arrow in D) and human embryos (arrow in F). (C,D) are sections from a single E11.5 mouse embryo; (E,F) are sections from a single CS15 human embryo. (G,H) Multiple neural tube profiles in two human embryonic tails at CS15: four lumens are visible in one embryo (arrowheads in G) and two lumens in a second (arrowheads in H). (I–K) In mouse, the tailbud displays a stage-dependent burst of apoptotic cell death at E13.5 (arrows in J), with absence of caspase 3-positive cells 12 hr earlier, at E13.0 (I), and only occasional dying cells 12 hr later, at E14.0 (arrow in K). Note the absence of a neural tube at the mouse tailbud tip, and the sparse nature of the tailbud mesenchyme at E14.0. (L–N) Human embryonic tailbuds show a similar developmental sequence to the mouse, with absence of cell death at CS13 (L), abundant dying cells at CS15 (arrows in M) and cessation of cell death by CS18 (N). Unlike the mouse, the secondary neural tube extends to the tailbud tip (red arrows in L–N), and this terminal neural tube portion has a single lumen in all three embryos. Scale bars in A,B, 1 mm; bar in C represents: 70 µm (C,D), 50 µm (E–K,N), and 30 µm (L,M).
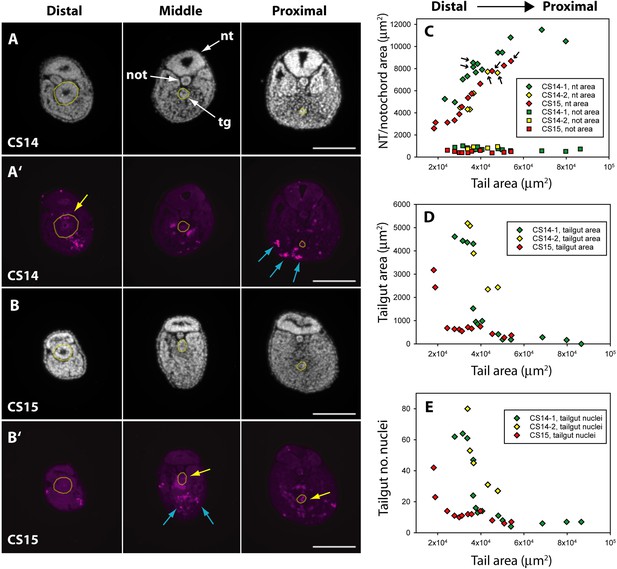
Programmed cell death and tissue size along the developing human tail.
(A,A’,B,B’) Transverse sections at distal (left), middle (centre), and proximal (right) levels of the tail. Panels show DAPI (4′,6-diamidino-2-phenylindole) (A,B) and anti-cleaved caspase 3 immunostaining (A’,B’) of the same sections at CS14 (A,A’) and CS15 (B,B’). Yellow dotted lines outline the tailgut. Apoptotic cell death occurs mainly in tailgut (tg, yellow arrows) and ventral mesoderm (blue arrows). Note the diminishing diameter of the tailgut from distal to proximal. (C) Change in transverse sectional areas of neural tube (nt, diamonds) and notochord (not, squares) along the body axis in CS14 (x2; green and yellow symbols) and CS15 (red symbols) embryos. Embryos CS14-1 and CS15 are shown in (A,B). Tissue-specific areas (y-axis) are plotted against total tail area (x-axis), which increases from left (distal sections) to right (proximal sections). In all embryos, neural tube area increases in a proximal direction, whereas notochord area is relatively constant along the axis. Arrows: sections in which neural tube shows multiple lumens (see Figure 6). (D,E) Similar analysis for tailgut area (D) and tailgut nuclear number (E). Both show a dramatic reduction in a distal-to-proximal direction, in contrast to neural tube and notochord. Scale bars: 50 μm.
-
Figure 4—source data 1
Quantification of total tail, neural tube (NT), notochord, and tailgut areas (in square microns), and tailgut nuclear counts in three human embryos at CS14 (x2) and CS15.
- https://cdn.elifesciences.org/articles/88584/elife-88584-fig4-data1-v1.xlsx
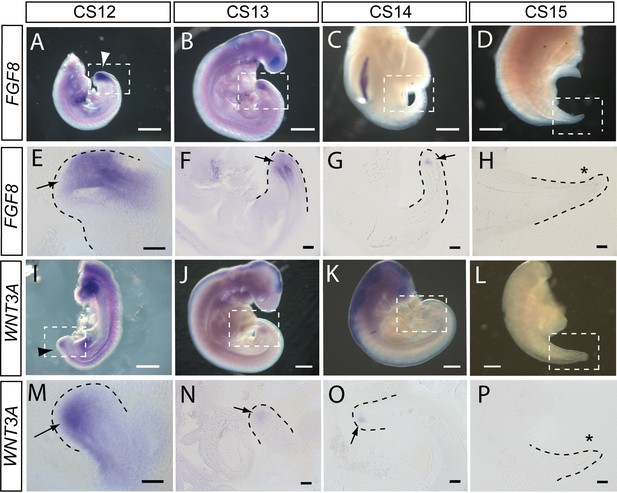
FGF8 and WNT3A expression in the elongating caudal region of human embryos.
Whole-mount in situ hybridisation (A–D,I–L) and sagittal vibratome sections through the caudal region (E–H,M–P) for FGF8 (A–H) and WNT3A (I–P) in embryos at CS12 (A,E,I,M), CS13 (B,F,J,N), CS14 (C,G,K,O), and CS15 (D,H,L,P). Both genes show prominent expression domains in the tailbud at CS12 (arrows in E,M) when axial elongation is underway and the posterior neuropore (PNP) is closing (arrowheads in A,I). At CS13, following PNP closure, expression of FGF8 and WNT3A remains prominent although less intense and more localised to the terminal tailbud than at CS12 (arrows in F,N). By CS14, both genes exhibit much smaller, highly localised expression domains that each appears as a ‘dot’ within the tailbud region (arrows in G,O). By CS15, axial elongation has ceased, the tail tip has narrowed and is increasingly transparent. At this stage, expression of neither gene can be detected (asterisks in H,P). Whole embryos shown in B,J,K; isolated trunk/caudal regions shown in A,C,D,I,L. No. embryos analysed: FGF8, n=2 for each stage; WNT3A, n=2 for each stage except n=3 for CS13. Scale bars: A–D, I–L, 1 mm; E–H, M–P, 100 μm.
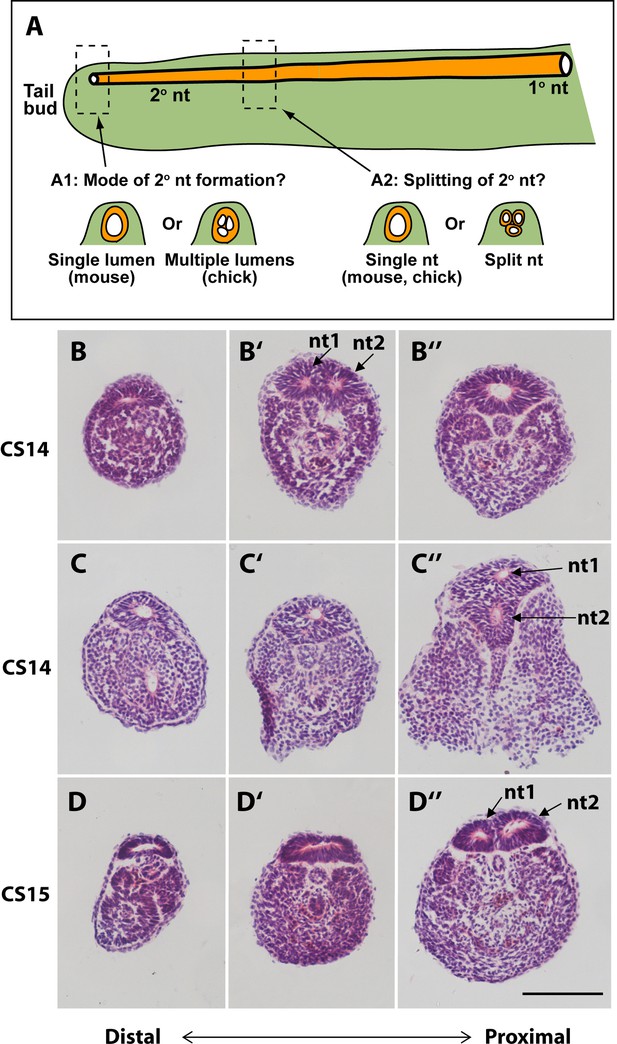
Mode of formation and proximal splitting of the human secondary neural tube.
(A) Hypotheses on mode of formation of the human secondary neural tube (2° nt). A single lumen may be formed in the tailbud as in mouse or, by analogy to chick, multiple lumens may form initially, which then coalesce to form the secondary neural tube (A1). Alternatively, the finding of multiple neural tube lumens in sections of some human tails may reflect splitting of the secondary neural tube at more rostral levels (A2). (B–D’’) Representative serial transverse sections (haematoxylin and eosin) through three human embryonic tails at CS14 (x2; B,C) and CS15 (D). Sections close to the tailbud tip (left side: B,C,D) show a broad, dorsoventrally flattened neural tube with a single lumen. There is no evidence of multiple lumens coalescing in the tailbud of any embryos. Further rostrally (middle and right side panels: B’,B’’,C’,C’’,D’,D’’), some sections show a neural tube with single lumen (C’,D’), whereas others show evidence of secondary neural tube splitting, with two lumens (nt1, nt2 in B’,C’’,D’’). One CS14 embryo shows re-establishment of a single lumen in more proximal sections (B’’), after splitting more distally (B’). These findings support a mouse-like formation of the human secondary neural tube with, additionally, splitting at various rostro-caudal levels along the tail. Scale bar: 50 µm.
Tables
Number of human embryos in the study, with breakdown by analysis type, sex, and method of pregnancy termination*.
| Analysis type | Figures in paper | Total no. | No. females | No. males | No. sex unknown | No. medical | No. surgical |
|---|---|---|---|---|---|---|---|
| PNP morphology | 1A–H | 2** | 0 | 2 | 0 | 2 | 0 |
| PNP closure timing | 1I, J | 40 | 24 | 16 | 0 | 40 | 0 |
| Tail morphology, histology, cell death | 2, 3 | 37** | 14 | 21 | 2 | 36 | 1 |
| Serial section analysis, cell death | 4, 6 | 11 | 5 | 6 | 0 | 10 | 1 |
| FGF8, WNT3A expression | 5 | 18 | 9 | 9 | 0 | 18 | 0 |
| Totals | 108 | 52 | 54 | 2 | 106 | 2 |
-
*Medical: mifepristone- and misoprostol-induced delivery; Surgical: ultrasound-guided vacuum aspiration.
-
**Embryos that are included in Table 2.
Measurements of human embryos, CS12–18*.
| Carnegie stage (CS) | Age range (days post-fertilisation) | Number of embryos | Somite number † | Crown-rump length ‡ | Tail length (total) ‡ | Tail length distal to somites ‡ |
|---|---|---|---|---|---|---|
| 12 | 25–27 | 2 | 22, 22 | 3.0, 3.0 | N/A | N/A |
| 13 | 28–30 | 7 | 35.4±2.3 | 6.4±1.3 | 1.06±0.44 | 0.49±0.16 |
| 14 | 31–32 | 5 | 35.0±2.0 | 8.4±2.2 | 0.99±0.21 | 0.56±0.06 |
| 15 | 33–35 | 7 | 35.9±1.8 | 8.9±0.8 | 1.18±0.55 | 0.67±0.15 |
| 16 | 37–39 | 8 | 36.6±2.6 | 11.7±1.2 | 1.29±0.48 | 0.46±0.13 |
| 17 | 40–43 | 4 | 32.3±2.1 | 12.2±1.2 | 1.18±0.20 | 0.34±0.01 |
| 18 | 44–45 | 6 | 32.6±1.1 | 15.4±2.2 | 1.14±0.48 | N/D |
-
N/A: not applicable; N/D: not determined.
-
*
Somite numbers: mean ± SD (except CS12, where actual somite numbers are shown). Somite number was available for all embryos except n=5 at CS18.
-
†
Summary of embryos that underpin Figure 1A–H (CS12) and Figures 2 and 3 (CS13–18). For full data set, see Supplementary file 2.
-
‡
Lengths (mm): mean ± SD (except CS12, where actual lengths are shown). Length measuements were available only for a subset of embryos. See Supplementary file 2 for full details.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (mouse) | CD1 | Charles River UK | Strain Code 022 | https://emodels.criver.com/en/page/species |
| Biological sample (human embryos) | Human embryos | MRC/Wellcome Human Developmental Biology Resource | NA | https://www.hdbr.org/ |
| Antibody | Rabbit polyclonal anti-cleaved caspase-3 (Asp 175) antibody | Cell Signalling | Cat. No. 9661 | Used at 1/1000 (wax sections) and 1/250 (cryosections) https://www.cellsignal.com/browse?categories=Primary%20Antibodies |
| Antibody | Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat. No. A-31573 | Used at 1/250 https://www.thermofisher.com/antibody/product/Donkey-anti-Rabbit-IgG-H-L-Highly-Cross-Adsorbed-Secondary-Antibody-Polyclonal/A-31573 |
| Sequence-based reagent | Human FGF8 DNA sequence | NIH National Library of Medicine | NM_033165.5 | https://www.ncbi.nlm.nih.gov/nuccore/NM_033165 |
| Sequence-based reagent | Human WNT3A DNA sequence | NIH National Library of Medicine | NM_033131.4 | https://www.ncbi.nlm.nih.gov/nuccore/NM_033131.4 |
| Commercial assay or kit | ApopTag Peroxidase In Situ Apoptosis Detection Kit | Sigma-Aldrich | Cat. No. S7100 | https://www.merckmillipore.com/GB/en/product/ApopTag-Peroxidase-In-Situ-Apoptosis-Detection-Kit,MM_NF-S7100 |
| Software, algorithm | Fiji software | ImageJ | Free downloads | https://imagej.net/software/fiji/downloads |
| Software, algorithm | AxioVision v4.8.2 software | Carl Zeiss | 410130-0600-000 | https://www.fishersci.pt/shop/products/axiovision-rel-4-8-2-software/11875113 |
Additional files
-
Supplementary file 1
Table of publications from a review of the literature on human secondary neural tube and body formation.
Rows show individual publications; columns show the topics covered in publications.
- https://cdn.elifesciences.org/articles/88584/elife-88584-supp1-v1.docx
-
Supplementary file 2
Source data on human embryos as summarised in Figure 2I–K and Table 2.
Each line in the table corresponds to a different human embryo (n=37). Measurements of crown-rump length, tail length, and tail length distal to somites were not available for all embryos. Data in Figure 2I–K and Table 2 are based on the available data.
- https://cdn.elifesciences.org/articles/88584/elife-88584-supp2-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/88584/elife-88584-mdarchecklist1-v1.docx





