NAD+ prevents septic shock-induced death by non-canonical inflammasome blockade and IL-10 cytokine production in macrophages
Figures
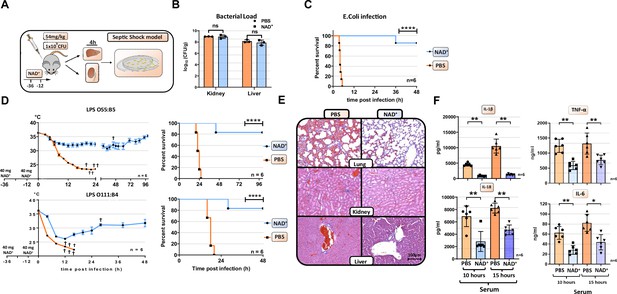
NAD+ protects mice from lethal bacterial infection and endotoxic shock by dampening systemic inflammation.
(A) Mice were treated with PBS or NAD+ prior to administration of a lethal dose of either pathogenic E. coli or lipopolysaccharide (LPS) by intraperitoneal injection. (B) After the death of each animal, lungs, kidney and livers were removed and bacterial load was determined by counting colony-forming unit (CFU). Column plots display mean with standard deviation (n=3). (C) Survival was monitored over 48 hr after bacterial infection and (D) LPS injection of both serotypes (n=6, 3 independent survival experiments). In addition, body temperature was monitored in the kinetics of up to 100 hr. (E) Lungs, kidneys, and livers were removed and IHC was performed for hematoxylin and eosin (H&E) staining. (F) Systemic levels (serum) of IL-6, TNFα, IL-1β, and IL-18 were assessed by ELISA. Column plots display mean with standard deviation (n=5). Statistical significance was determined by using Student’s t-test or one-way ANOVA while survival data were compared using log-rank Mantel-Cox test. Asterisks indicate p-values *=p<0.05, **=p<0.01, and ***=p<0.001, only significant values are shown. All data depicted in this figure are provided as source data.
-
Figure 1—source data 1
Raw data for Figure 1B: Bacterial load.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-data1-v1.xlsx
-
Figure 1—source data 2
Raw data for Figure 1C: E. coli infection.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-data2-v1.xlsx
-
Figure 1—source data 3
Raw data for Figure 1D: Lipopolysaccharide (LPS) infection.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-data3-v1.xlsx
-
Figure 1—source data 4
Raw data for Figure 1E: Histology.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-data4-v1.zip
-
Figure 1—source data 5
Raw data for Figure 1F: ELISA.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-data5-v1.xlsx
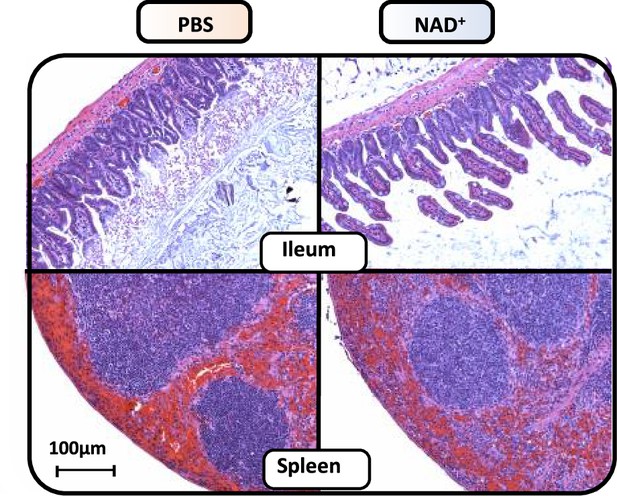
NAD+ preserves ileal villi structure and reduces splenic hemorrhage during lipopolysaccharide (LPS)-induced septic shock.
C57BL/6 mice were treated with PBS or NAD+ for 2 days prior to administration of a lethal dose of LPS (O55:B5/O111:B4) by intraperitoneal injection. Ileum and spleen were removed after 15 hr and subsequently IHC for hematoxylin and eosin (H&E) staining was performed. All data depicted in this figure are provided as source data.
-
Figure 1—figure supplement 1—source data 1
Raw data for Figure 1—figure supplement 1: Histology.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-figsupp1-data1-v1.zip
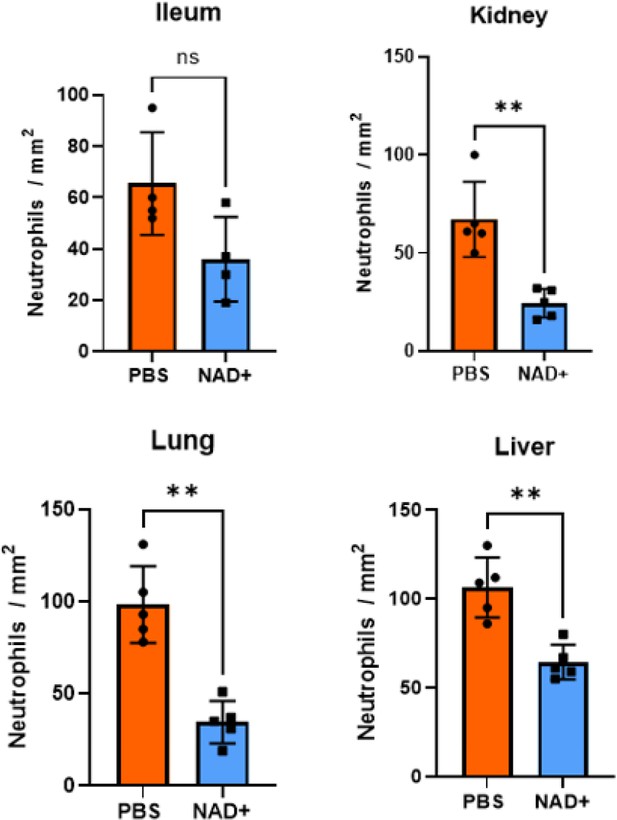
Neutrophils per mm2 infiltrating mice: ileum, kidney, lung, and liver in the IHC stains.
C57BL/6 mice were treated with PBS or NAD+ for 2 days prior to administration of a lethal dose of lipopolysaccharide (LPS) (O55:B5/O111:B4) by intraperitoneal injection. After a 15 hr interval, the ileums, lungs, kidneys, and livers were extracted and subjected to immunohistochemical staining with hematoxylin and eosin (H&E). Neutrophil quantification was performed subsequent to the immunohistochemical staining in various tissue samples. Notably, in the kidney, lung, and liver tissues, the number of neutrophils was observed to be significantly higher in the PBS-treated mice group as compared to the NAD+-treated mice group. Column plots display mean with standard deviation (n=4–5). Statistical significance was determined by using Student’s t-test or one-way ANOVA. Asterisks indicate p-values *=p<0.05, **=p<0.01. All data depicted in this figure are provided as source data.
-
Figure 1—figure supplement 2—source data 1
Raw data for Figure 1—figure supplement 2: Neutrophil count.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig1-figsupp2-data1-v1.xlsx
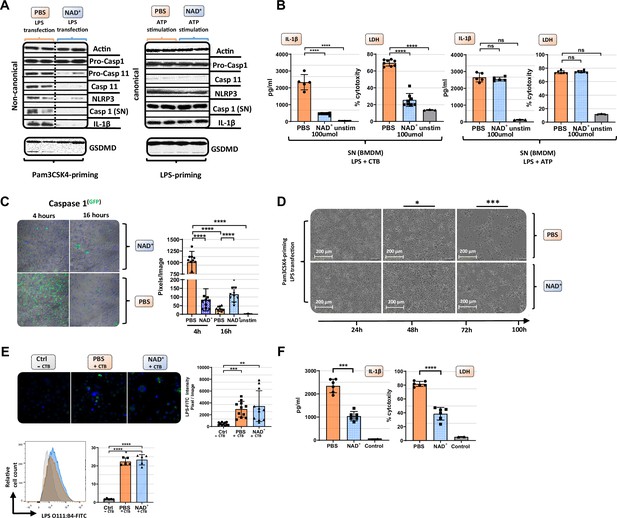
NAD+ specifically inhibits the non-canonical inflammasome by targeting caspase-11.
Bone marrow was isolated from mice and bone marrow-derived macrophages (BMDMs) were differentiated in vitro. Subsequently, BMDMs were cultured in the presence of NAD+ or PBS. BMDMs were then primed with either Pam3CSK4 or lipopolysaccharide (LPS) O111:B4. Next primed BMDMs were stimulated with ATP or LPS and cholera toxin B (CTB). (A) Pro-casp-1, pro-casp-11, casp-11, NLRP3, casp-1, IL1β, and gasdermin D (GSDMD) expression were determined using western blot and (B) IL-1β secretion and LDH release were assessed in the supernatant. Column plots display mean with standard deviation (n=5-8). (C) Time-dependent caspase-1 expression was determined via active staining and assessed using a confocal microscope. Column plots display mean with standard deviation (n=5) (D) Cell viability and apoptosis were monitored using the IncuCyte live microscopy system. (E) LPS transfection with CTB was visualized by using FITC-coupled LPS and DAPI staining and quantified by confocal microscopy and flow cytometry. Column plots display mean with standard deiation (n=6) (F) For human experiments macrophages were differentiated from PBMC, primed with Pam3CSK4 and subsequently transfected with LPS and 0.25% Fugene HD Plus. Column plots display mean with standard deviation (n=6). Statistical significance was determined by using Student’s t-test or one-way ANOVA. Asterisks indicate p-values *=p<0.05, **=p<0.01, and ***=p<0.001, only significant values are shown. All data depicted in this figure are provided as source data.
-
Figure 2—source data 1
Raw data for Figure 2A: Original western blots.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data1-v1.zip
-
Figure 2—source data 2
Raw data for Figure 2A: Western blots with highlighted bands and sample labels.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data2-v1.zip
-
Figure 2—source data 3
Raw data for Figure 2B: ELISA mouse bone marrow-derived macrophages (BMDMs).
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data3-v1.xlsx
-
Figure 2—source data 4
Raw data for Figure 2C: Caspase-1 staining.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data4-v1.zip
-
Figure 2—source data 5
Raw data for Figure 2D: IncuCyte live microscopy.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data5-v1.zip
-
Figure 2—source data 6
Raw data for Figure 2E: Lipopolysaccharide (LPS) transfection staining.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data6-v1.zip
-
Figure 2—source data 7
Raw data for Figure 2F: ELISA human macrophages.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-data7-v1.xlsx
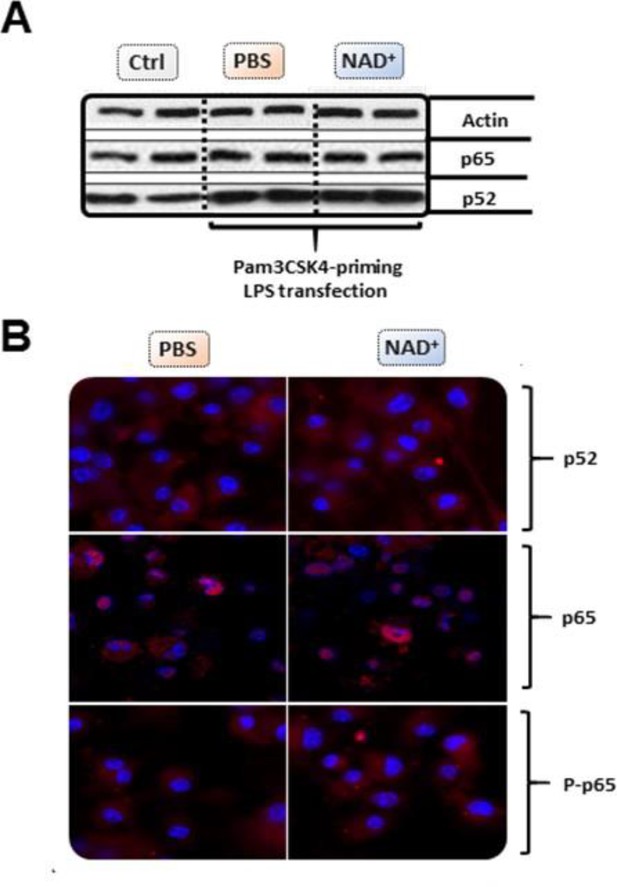
NAD+ does not alter bone marrow-derived macrophage (BMDM)-derived NF-κB expression or phosphorylation.
Differentiated BMDMs were cultured in the presence of 100 µmol NAD+ or PBS for 2 following days. BMDMs were then primed with 1 µg/ml Pam3CSK4 and subsequently stimulated with 2 µg/ml lipopolysaccharide (LPS) O111:B4 and 20 µg/ml cholera toxin B (CTB). Unstimulated BMDMs served as controls. (A) P52 and p65 expression was determined using western blot. (B) Stimulated BMDMs were stained with p52, p65, and phospho-p65 and expression levels assessed using confocal microscopy. All data depicted in this figure are provided as source data.
-
Figure 2—figure supplement 1—source data 1
Raw data for Figure 2—figure supplement 1A: Western blot.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Raw data for Figure 2—figure supplement 1A: Western blots bands with highlighted and sample labels.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-figsupp1-data2-v1.zip
-
Figure 2—figure supplement 1—source data 3
Raw data for Figure 2—figure supplement 1B: Immunofluorescence.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-figsupp1-data3-v1.zip
Unstimulated bone marrow-derived macrophage (BMDM) cell viability and apoptosis.
Differentiated BMDMs were cultured in the presence of 100 µmol NAD+ or PBS for 2 following days. BMDMs were then primed with 1 µg/ml Pam3CSK4, subsequently stimulated with 2 µg/ml lipopolysaccharide (LPS) O111:B4 and 20 µg/ml cholera toxin B (CTB), and cell viability and apoptosis were monitored for 100 hr using the IncuCyte live microscopy system. All data depicted in this figure are provided as source data.
-
Figure 2—figure supplement 2—source data 1
Raw data for Figure 2—figure supplement 2: IncuCyte live microscopy.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig2-figsupp2-data1-v1.zip
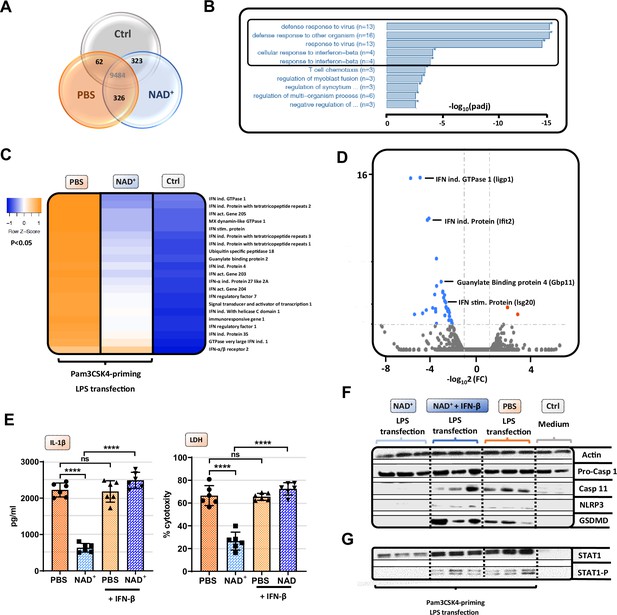
NAD+-mediated inhibition of the non-canonical inflammasome is based on an impaired response to IFN-β.
Differentiated bone marrow-derived macrophages (BMDMs) were cultured in the presence of NAD+ or PBS. BMDMs were then primed with Pam3CSK4, subsequently stimulated with lipopolysaccharide (LPS) and cholera toxin B (CTB) and RNA-sequencing was performed. Unstimulated BMDMs served as controls. (A) Venn diagram plotting common gene expression between all three groups. (B) Gene ontology enrichment analysis displaying the highest significant pathways differing when comparing NAD+ and PBS-treated BMDMs. (C) Expression cluster analysis of genes involved in IFN-β signaling through cluster analysis depicted in a heat map. (D) Volcano plot displaying the most significant genes up- or downregulated comparing NAD+ and PBS-treated BMDMs. (E) Stimulated BMDMs were additionally treated with recombinant INF-β, and IL-1β and LDH release were measured. Column plots display mean with standard deviation (n=6) (F) Moreover, pro-casp-1, casp-11, NLRP3, gasdermin D (GSDMD), (G) signal transducer activator of transcription-1 (STAT-1), and phospho-STAT-1 expression were assessed by western blot. Statistical significance was determined by using Student’s t-test or one-way ANOVA. Asterisks indicate p-values *=p<0.05, **=p<0.01, and ***=p<0.001, only significant values are shown. All data depicted in this figure are provided as source data.
-
Figure 3—source data 1
Raw data for Figure 3E: ELISA bone marrow-derived macrophage (BMDM).
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Raw data for Figure 3F: Original western blots.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig3-data2-v1.zip
-
Figure 3—source data 3
Raw data for Figure 3F: Western blots with highlighted bands and sample labels.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig3-data3-v1.zip
-
Figure 3—source data 4
Raw data for Figure 3G: Original western blots.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig3-data4-v1.zip
-
Figure 3—source data 5
Raw data for Figure 3G: Western blots with highlighted bands and sample labels.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig3-data5-v1.zip
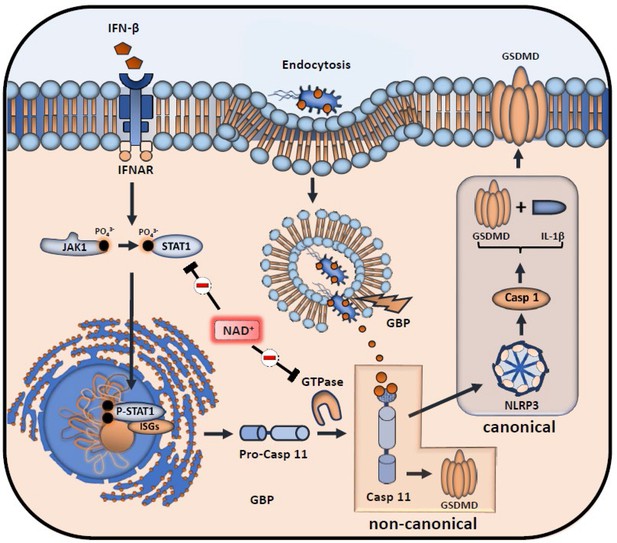
Inhibitory effects of NAD+ on IFN-β downstream signaling and inflammasome activation.
NAD+ inhibits signal transducer activator of transcription-1 (STAT-1) expression and phosphorylation, thus compromising the intracellular response to IFN-β. Subsequently, stimulation of the IFNAR receptor by IFN-β leads to a decreased transcription of pro-caspase-11 as well as IFN-stimulated genes (ISGs) (IFN-inducible GTPases and GBPs). Due to diminished caspase-11 levels, non-canonical inflammasome activation through intracellular, gram-negative bacteria opsonization by GBPs is significantly inhibited.
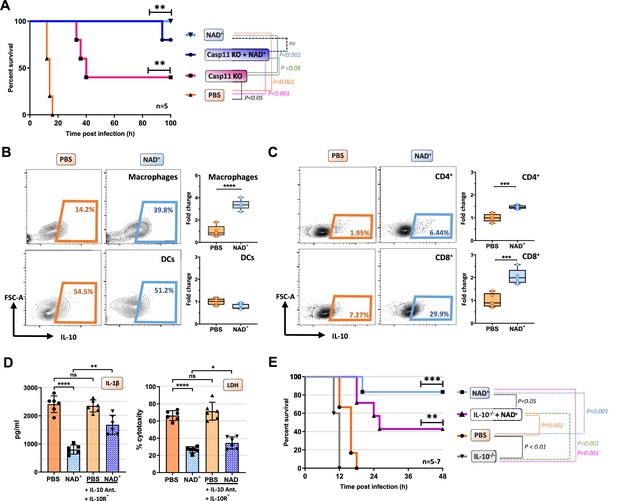
IL-10 constitutes an additional mechanism mediating the protective capacities of NAD+ in the context of septic shock.
(A) Caspase-11 KO (knockout) mice were treated with NAD+ or PBS. Subsequently mice were subjected to poly(I:C) prior to lipopolysaccharide (LPS) injection and survival was monitored (n=5, 2 independent survival experiments). Mice treated with either NAD+ or PBS were injected with LPS and after 10 hr, splenic frequencies of IL-10 producing (B) macrophges and dendritic cells (C) and CD4+ and CD8+ T cells were assessed by flow cytometry. Box plots display fold change of leukocyte proportions as mean with standard deviation (n=5) (D) Bone marrow-derived macrophages (BMDMs) treated with NAD+ or PBS were stimulated with LPS and cholera toxin B (CTB) in the presence of IL-10 neutralizing antibodies and IL-10 receptor antagonists. Subsequently IL-1β and LDH release were assessed. Column plots display mean with standard deviation (n=6) (E) IL-10-/- mice treated with NAD+ or PBS were challenged with LPS and survival was monitored (n=5–7, 2 independent survival experiments). Statistical significance was determined by using Student’s t-test or one-way ANOVA while survival data were compared using log-rank Mantel-Cox test. Asterisks indicate p-values *=p<0.05, **=p<0.01, and ***=p<0.001, only significant values are shown. All data depicted in this figure are provided as source data.
-
Figure 5—source data 1
Raw data for Figure 5A: Casp11 knockout (KO) survival.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Raw data for Figure 5B: FACS macrophages and DCs.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig5-data2-v1.xlsx
-
Figure 5—source data 3
Raw data for Figure 5C: FACS CD4+ and CD8+ T cells.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig5-data3-v1.zip
-
Figure 5—source data 4
Raw data for Figure 5D: ELISA bone marrow-derived macrophage (BMDM).
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig5-data4-v1.xlsx
-
Figure 5—source data 5
Raw data for Figure 5E: IL-10-/- survival.
- https://cdn.elifesciences.org/articles/88686/elife-88686-fig5-data5-v1.xlsx





