Spontaneous human CD8 T cell and autoimmune encephalomyelitis-induced CD4/CD8 T cell lesions in the brain and spinal cord of HLA-DRB1*15-positive multiple sclerosis humanized immune system mice
Figures
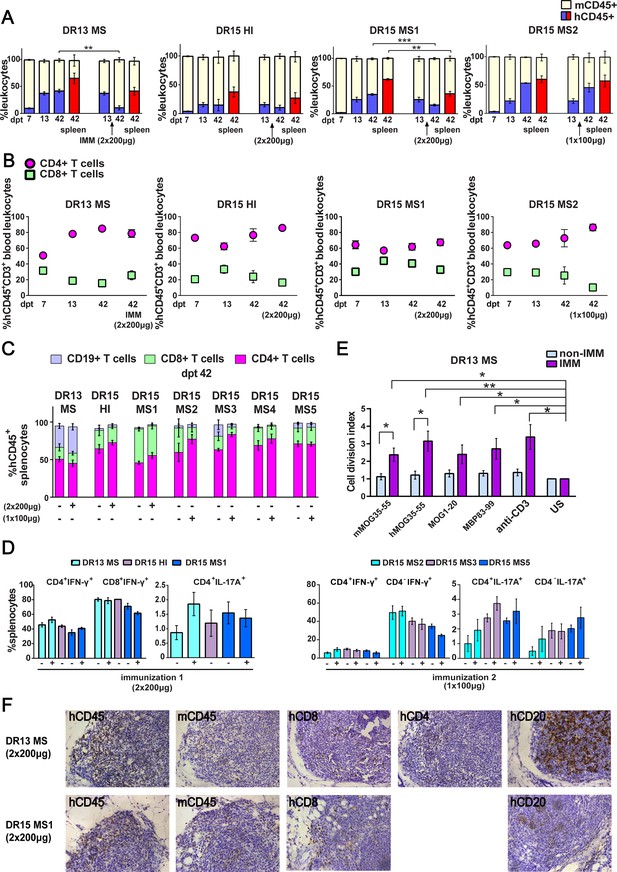
Reconstitution of a human adaptive immune system in B2m-NOG mice engrafted with peripheral blood mononuclear cell (PBMC) from multiple sclerosis (MS) patient and healthy donors.
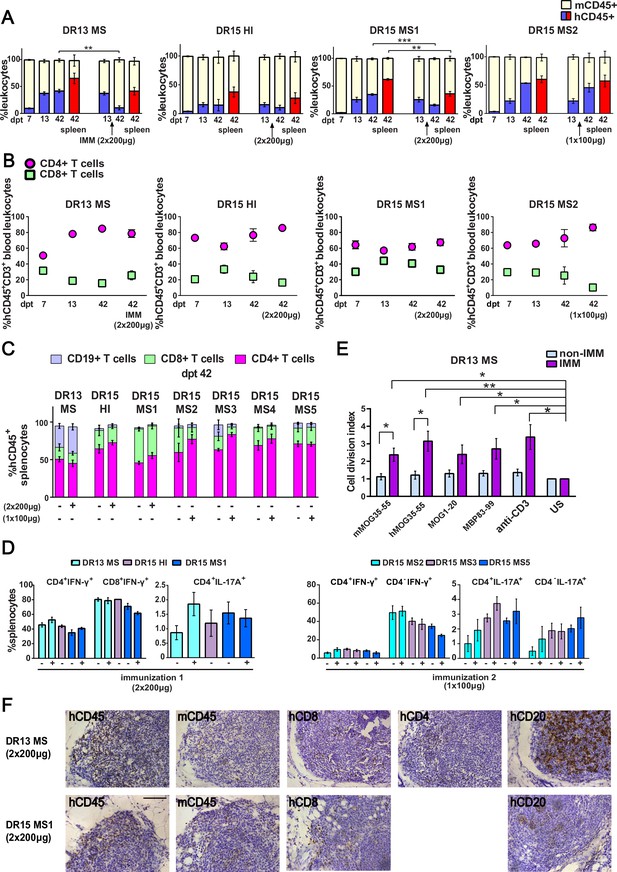
(A) Progressive engraftment of human (h) CD45+ leukocytes from selected donors in groups of B2m-NOG mice, non-immunized (left bars) and immunized for experimental autoimmune encephalomyelitis (EAE) (right bars) using a myelin peptide cocktail in repeat immunizations with 200 μg/each myelin peptide (EAE experiment 1), or single immunization with 100 μg/each myelin peptide (EAE experiment 2), measured in peripheral blood samples taken at different time points , and in spleen recovered at sacrifice 42 days’ post-transplantation (dpt 42) by fluorescence-activated cell sorting (FACS) (non-immunized mice, dpt 7 & 13, n=7-8 mice/group; dpt 42, n=3 mice/group; immunized mice, dpt 42, n=4-5 mice/group). (B) Proportions of hCD4+ and hCD8+ T cells in blood hCD45+CD3+ T cells at different time points by FACS (non-immunized mice, dpt 7 & 13, n=7-8 mice/group; dpt 42, n=3 mice/group; immunized mice, dpt 42, n=4-5 mice/group). (C) Proportions of human immune cell subpopulations in spleens of immunized and non-immunized mice at dpt 42 by FACS (non-immunized mice n=3 mice/group; immunized mice n=4-5 mice/group). (D) Proportions of interferon-γ- and IL-17A-producing CD4+ and CD8+ (or CD4−) T cells in splenocytes recovered from mice in the different groups at dpt 42 by FACS (non-immunized mice n=2-3 mice/group; immunized mice n=4-5 mice/group). (E) Antigen-specific T cell proliferation responses to the immunizing antigens (mMOG35-55, hMOG35-55, MOG1-20, and MBP83-99), anti-hCD3 (positive control) and medium (unstimulated; US), in splenocytes recovered from non-immunized and immunized DR13 MS PBMC humanized mice at dpt 42 by FACS (non-immunized mice n=3 mice/group; immunized mice n=3-4 mice/group). Results are expressed as a cell division index. (F) Draining lymphoid structures in inguinal fat of mice engrafted with DR13 MS and DR15 MS PBMC and immunized with myelin peptides, recovered at dpt 42. Immunohistochemistry revealed the presence of hCD45- and mCD45-positive leukocytes, hCD4- and hCD8-positive T cells, and hCD20-positive B cells (latter only DR13 MS mice). Scale bar 200 μm; ×20 objective (F). All results are depicted as mean ± standard error of the mean (SEM). Statistical significance is shown after pairwise comparisons between groups using Student’s t test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
-
Figure 1—source data 1
Proportions of human and mouse immune cell subpopulations in peripheral blood and spleen of humanized B2m-NOG mice at different time points measured by FACS.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-data1-v1.docx
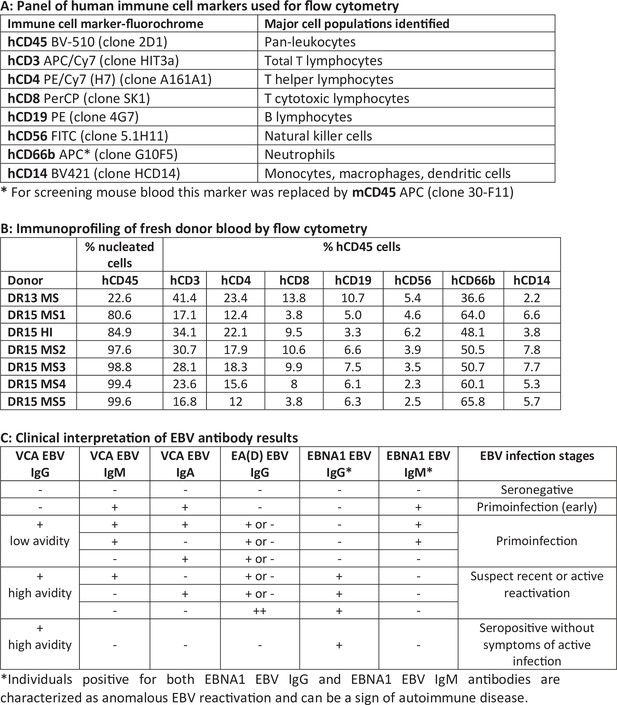
Analyses of peripheral blood samples from multiple sclerosis (MS) and healthy peripheral blood mononuclear cell (PBMC) donors.
(A) Panel of human immune cell markers used for fluorescence-activated cell sorting (FACS). (B) Immunoprofiling of fresh peripheral blood from donors by FACS using antibody panel in A. (C) Clinical interpretation of enzyme-linked immunosorbent assay (ELISA) results for detection of Epstein–Barr virus (EBV) antibodies in plasma from donors.
-
Figure 1—figure supplement 1—source data 1
C: Levels of EBV infection markers measured in the plasma of MS and healthy individual blood donors by ELISA.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-figsupp1-data1-v1.xlsx
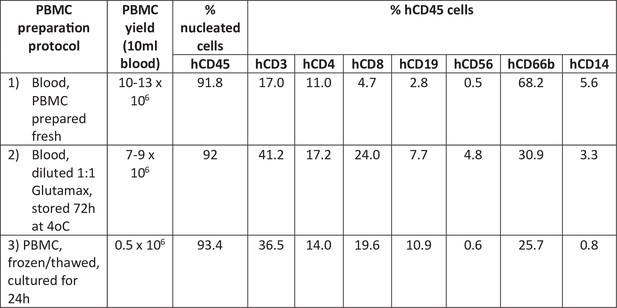
Optimization of human peripheral blood mononuclear cell (PBMC) isolation protocol.
Three different PBMC isolation protocols were compared for the preparation of cells for transplantation into B2m-NOG mice. Specifically, PBMC were isolated from peripheral blood samples from a healthy individual, fresh on the day of transplantation (protocol 1), after dilution of fresh blood with culture medium and 3 days’ storage at 4°C (protocol 2), freshly isolated, frozen, thawed, and cultured for 24 hr (protocol 3).
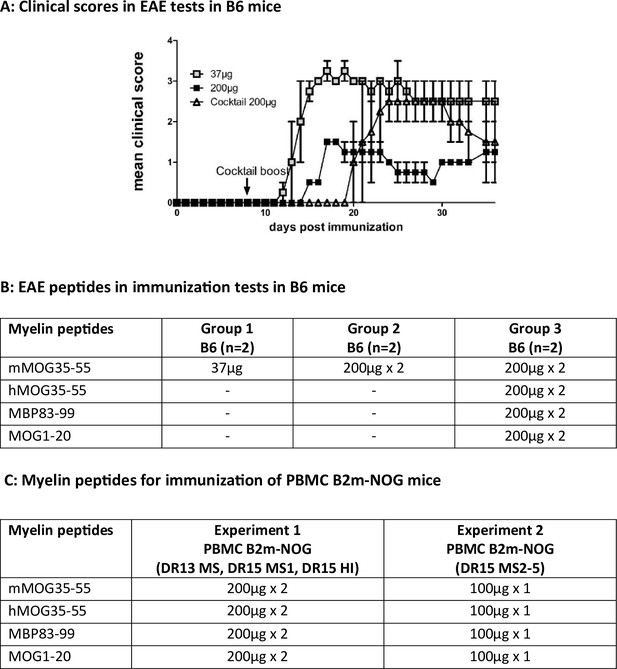
Experimental autoimmune encephalomyelitis (EAE) protocols used in C57BL/6 and humanized B2m-NOG mice.
Previously, we found that humanized HLA-DR2b transgenic mice lacking all mouse MHCII genes required greater amounts of peptide antigen to induce clinical EAE than wild-type C57BL/6 (B6) mice (Dagkonaki et al., 2020). (A, B) To test for possible toxicity of EAE induced by high amounts of myelin peptide antigens, we compared increasing dosages of peptide in three groups of 6- to 8-week-old female B6 mice (n=2 mice/ group): Group 1 was immunized using our standard EAE protocol in B6 mice; Group 2 was immunized using an EAE protocol for HLA-DR2b transgenic mice; Group 3 was immunized using a myelin peptide cocktail. (C) EAE immunization protocols used for immunization of humanized B2m-NOG mice. In experiment 1, humanized B2m-NOG mice were immunized with the myelin peptide cocktail at 200 μg/peptide, followed by a repeat boost immunization 7 days later. In experiment 2, humaized B2m-NOG mice were immunized once with the myelin peptide cocktail at 100 μg/peptide. (A) Results are depicted as mean ± standard error of the mean (SEM).
-
Figure 1—figure supplement 3—source data 1
A: Clinical scores of individual C57BL/6 mice immunized for EAE with the different peptide protocols shown in B.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-figsupp3-data1-v1.docx
Reconstitution of a human adaptive immune system in B2m-NOG mice engrafted with peripheral blood mononuclear cell (PBMC) from multiple sclerosis (MS) patiients.
(A) Progressive engraftment of human (h) CD45+ leukocytes from donors in groups of B2m-NOG mice, non-immunized (left) and immunized (right) for experimental autoimmune encephalomyelitis (EAE) using the single immunization protocol with 100 μg/each myelin peptide (EAE experiment 2), measured in peripheral blood samples taken at different time points, and in spleen recovered at sacrifice 42 days’ post-transplantation (dpt 42) by fluorescence-activated cell sorting (FACS) (non-immunized mice, dpt 7 & 13, n=7 mice/group; dpt 42, n=2-3 mice/group; immunized mice, dpt 42, n=4 mice/group). (B) Proportions of hCD4+ and hCD8+ T cells in blood hCD45+CD3+ T cells at different time points by FACS. (C) Proportions of CFSElow (proliferating) splenocytes recovered from non-immunized (−) and EAE-immunized (+) PBMC B2m-NOG mice at sacrifice on dpt 42 and cultured for 120 hr in the presence of individual myelin peptides, anti-CD3 and phytohemaglutinin (PHA), or unstimulated (US) by FACS analysis (non-immunized mice, dpt 7 & 13, n=7 mice/group; dpt 42, n=3 mice/group; immunized mice, dpt 42, n=4 mice/group). All results are depicted as mean ± standard error of the mean (SEM). Statistical analysis was performed by pairwise comparisons between different groups of mice using Student’s t test.
-
Figure 1—figure supplement 4—source data 1
Proportions of CFSE-low (highly proliferating) splenocytes isolated from the different B2m-NOG humanized mice at the end of the experiment (dpt 42), stimulated with myelin peptides or polyclonal stimuli ex vivo, and measured by FACS.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-figsupp4-data1-v1.docx
Human immune cell engraftment in peripheral blood mononuclear cell (PBMC) humanized B2m-NOG mouse splenocytes.
Analysis of splenocytes recovered from non- and experimental autoimmune encephalomyelitis (EAE)-immunized (+) PBMC B2m-NOG mice at sacrifice on day post-transplantation 42 (dpt 42) by fluorescence-activated cell sorting (FACS), showing engraftment by human CD45+ leukocyte populations.
-
Figure 1—figure supplement 5—source data 1
Proportions of human immune cell subpopulations in splenocytes isolated from humanized B2m-NOG mice at the end of the experiment (dpt 42) and measured by FACS.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-figsupp5-data1-v1.docx
Comparative fluorescence-activated cell sorting (FACS) analysis of mouse CD11b+ myeloid cell subpopulations in the peripheral blood of non-peripheral blood mononuclear cell (PBMC)-engrafted mouse strains.
Analysis of blood mCD11b+ myeloid cell subpopulations in non-immunized (naive) B2m-NOG (n = 4), NOD-scid (n = 2), and C57BL/6 (B6) (n = 5), as well as in groups of experimental autoimmune encephalomyelitis (EAE) immunized NOD-scid (n = 2) and B6 (n = 5) mice at dpi 8. EAE immunization was performed by subcutaneous (s.c.) tail-base injection of CFA emulsion supplemented with H37Ra and without myelin peptides, followed by two injections of Bordetella pertussis toxin, as described in Materials and methods.
-
Figure 1—figure supplement 6—source data 1
Proportions of mouse myeloid cell subpopulations in peripheral blood of non-PBMC engrafted B2m-NOG, NOD-scid and C57BL/6 mice, either non-immunized (naive) or immunized with CFA and measured by FACS.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig1-figsupp6-data1-v1.docx
Human T cells accumulate at brain borders in non-immunized DR15 MS and DR15 HI mice, and form spontaneous parenchymal lesions in brain of DR15 MS mice.
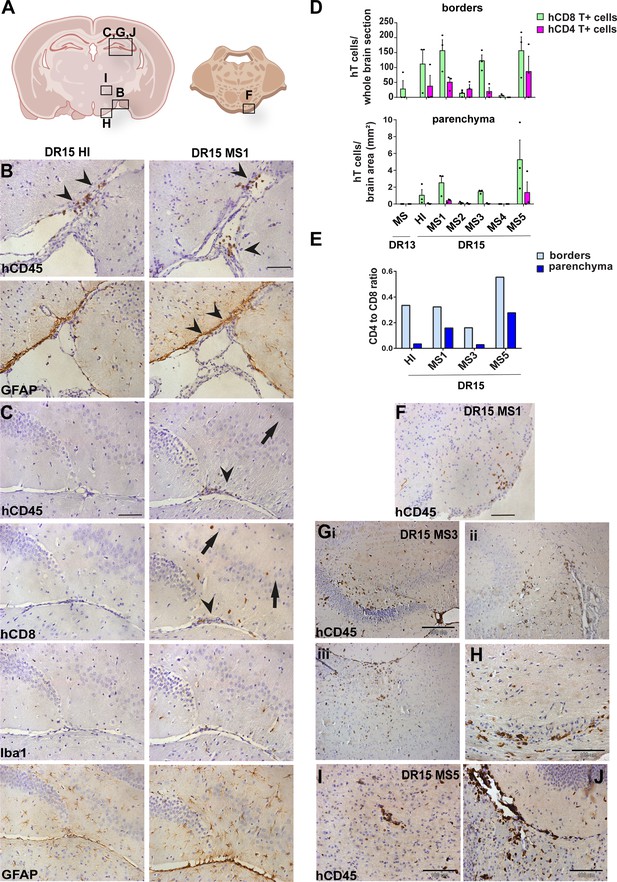
Immunohistochemical analysis of the brain from non-immunized peripheral blood mononuclear cell (PBMC) B2m-NOG mice showing infiltration by human (h) and mouse (m) CD45-positive leukocytes, and hCD8-positive T cells, in brain border and parenchymal regions (denoted in diagrams of brain and brainstem A). (B, C) Accumulation of hCD45- and mCD45-positive leukocytes and hCD8-positive T cells, together with local activation of Iba1-positive microglia and glial fibrillary acid protein (GFAP)-positive astrocytes, at border regions in the brains of DR15 HI (left panels) and DR15 MS (right panels) mice, specifically at meninges close to the optic tract (B, arrowheads) and in the connective tissue of the interventricular foramen joining the lateral and third ventricles (C, arrowheads). Scattered hCD45-positive leukocytes and hCD8 T cells in brain parenchyma of DR15 MS mice (C, arrows). (D) Counting of border-associated and parenchymal hCD8- and hCD4-positive T cells in whole coronal sections of brain of humanized mice, represented as total cells and cells/mm2 (n=3 mice/group) (E) Ratios of hCD4/hCD8 T cells at borders and parenchyma of selected humanized mice. (F) Small hCD45-positive immune cell lesion in brainstem of DR15 MS1 mice (see also diagram A). (G) hCD45-positive immune cell lesions in gray matter of hippocampus (i, ii) and sub-hippocampus/thalamus (iii), and white matter of optic chiasm (H) of DR15 MS3 mice. (I, J) hCD45-positive immune cell lesions in gray matter of thalamus and hippocampus, respectively, of DR15 MS5 mice. Scale bars 100 μm; ×20 objective (B, C, F), 200 μm; ×10 objective (G), 100 μm; ×40 objective (H–J). All results are depicted as mean ± standard error of the mean (SEM). Statistical analysis was performed by pairwise comparisons between different groups of mice using Student’s t test.
-
Figure 2—source data 1
Numbers of human CD4 and CD8 T cells counted at borders (total cells in comparable whole coronal brain sections) and in parenchyma (cells/mm2) (D), as well as the human CD4/CD8 T cell ratios derived from these measurements (E).
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig2-data1-v1.docx
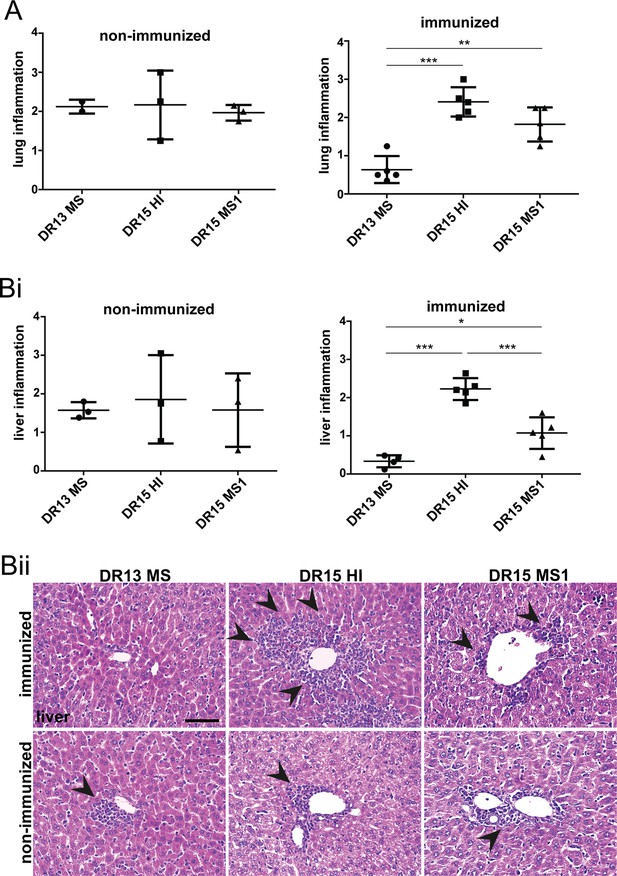
Inflammation in peripheral graft versus host disease (GVHD) target tissues in humanized B2m-NOG mice.
Histochemical analysis of peripheral tissues recovered from non- and experimental autoimmune encephalomyelitis (EAE)-immunized humanized B2m-NOG mice at sacrifice on day post-transplantation 42 (dpt 42). Inflammation was evaluated in lung (A) and liver (Bi) by hematoxylin and eosin (H&E) staining of paraffin section prepared from non-immunized (n=3 mice/group) and immunized mice (n=5 mice/group). Representative photomicrographs of liver from the different mouse groups are shown (Bii). All results are depicted as mean ± standard error of the mean (SEM). Statistical comparisons between lung and liver inflammation in three different non-immunized and immunized mouse groups at the same time points using one-way analysis of variance (ANOVA) (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
-
Figure 2—figure supplement 1—source data 1
Inflammation scores in the liver and lung of non-immunized and immunized humanized B2m-NOG mice, as measured by semi-quantitative analysis of H&E stained sections isolated at the end of the experiment.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig2-figsupp1-data1-v1.docx
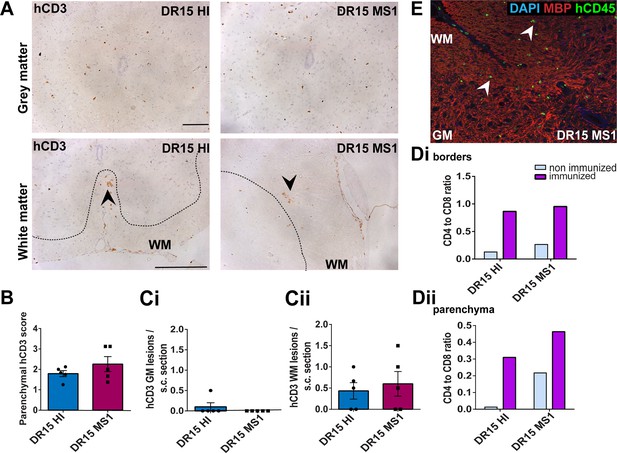
Human T cells infiltrate spinal cord white matter in non-immunized DR15 MS and DR15 HI mice, and form gray matter lesions in DR15 MS mice.
Immunohistochemical analysis of spinal cord from non-immunized peripheral blood mononuclear cell (PBMC) B2m-NOG mice showing infiltration by human (h) CD3-positive T cells in the gray and white matter regions. (A) Infiltrating hCD3-positive T cells were scattered individually throughout spinal cord gray and white matter and formed small lesions in the white matter (WM) of both DR15 MS and DR15 HI mice (lower panels, arrowheads), and gray matter lesions only in DR15 MS mice (upper panels, arrowheads). The dotted lines mark the boundary between gray matter and white matter. (B) Semi-quantitative estimation of hCD3-positive T cells in comparable whole spinal cord sections from DR15 HI and DR15 MS mice (n=3 mice/group). (C) Counting of parenchymal hCD3-positive T cell lesions (≥3 adjacent cells) in gray matter (GM) (i) and white matter (WM) (ii) in whole spinal cord sections from DR15 HI and DR15 MS mice. (D) Ratios of hCD4/hCD8 T cells at borders and parenchyma of DR15 HI and DR15 MS mice. (E) Double immunofluorescence staining for hCD45-positive leukocytes (green, arrowheads) and MBP-positive myelin (red), with 4′,6-diamidino-2-phenylindole (DAPI) counterstained nuclei (blue), in spinal cord WM of DR15 MS mice. Scale bars 100 μm; ×20 objective (A, top panels); ×40 objective (A, bottom panels). All results are depicted as mean ± standard error of the mean (SEM). Statistical analysis was performed by pairwise comparisons between different groups of mice using Student’s t test (**p ≤ 0.01).
-
Figure 3—source data 1
Semi-quantitative analysis of human CD3-positive T cells (B) and numbers of human CD3-positive lesions (defined as 3 or more adjacent cells) in grey matter (GM) and white matter (WM) regions (C) in comparable whole spinal cord sections from DR15 HI and DR15 MS mice, as well as human CD4/CD8 T cell ratios at borders and in parenchyma of these sections.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig3-data1-v1.docx
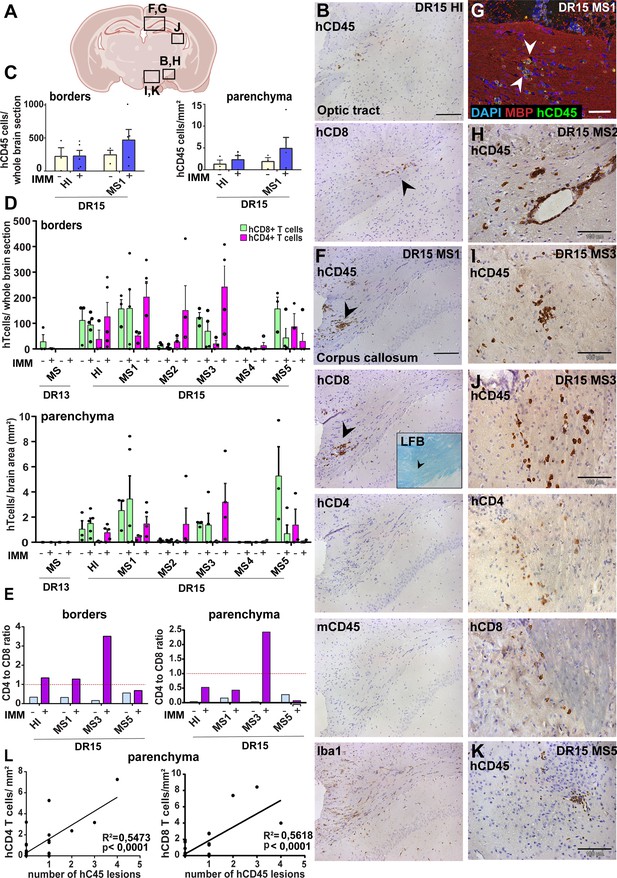
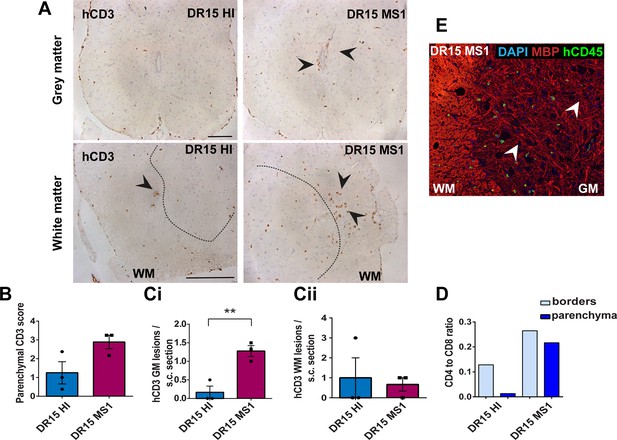
Immunization with myelin peptides increases hCD4 T cell infiltration of brain parenchyma resulting in mixed hCD4/hCD8 T cell lesions in brain of DR15 MS and DR15 HI mice.
Immunohistochemical analysis of the brain from peripheral blood mononuclear cell (PBMC) B2m-NOG mice immunized for experimental autoimmune encephalomyelitis (EAE) showing infiltration by human (h) and mouse (m) CD45-positive leukocytes, hCD8- and hCD4-positive T cells, and local activation of Iba1-positive microglia and GFAP-positive astrocytes, in brain regions denoted in the diagram (A). (B) Individual hCD45-positive leukocytes and hCD8-positive T cells form small lesions in the optic tract (arrowhead) in immunized DR15 HI mice. (C) Counting of hCD45-positive immune cells at borders (total cells in section) and parenchyma (cells/mm2) in whole coronal sections of brain from non-immunized (n=3/group) and immunized (n=5 mice/group) DR15 HI and DR15 MS mice. (D) Counting of hCD4- and hCD8-positive T cells at borders (total cells in section) and parenchyma (cells/mm2) in whole coronal sections of brain from non-immunized (n=3 mice/group) and immunized DR13 MS, DR15 HI and DR15 MS1 (all n=5 mice/group), DR15 MS2-5 (n=4 mice/group). (E) Ratios of hCD4/hCD8 T cells at borders and parenchyma of non-immunized and immunized DR15 HI and DR15 MS mice. (F) Prominent lesions in the corpus callosum white matter of two of five DR15 MS1 mice (arrowheads), containing hCD45- and mCD45-positive leukocytes, hCD4- and hCD8-positive T cells, and locally activated Iba1-positive microglia. Inset shows a serial section stained by Luxol fast blue showing absence of demyelination. (G) Double immunofluorescence staining for hCD45-positive leukocytes (green, arrowheads) and MBP-positive myelin (red), with DAPI counterstained nuclei (blue), in corpus callosum in immunized DR15 MS1 mice, showing inflammatory lesion without demyelination. (H) Small white matter lesion in DR15 MS2 mouse. (I) Prominent white and (J) gray matter lesions containing both hCD4 and hCD8 T cells in DR15 MS3 mice. (K) Small lesion containing human hCD45-positive immune cells in sub-thalamic area of DR15 MS5 mice. (L) Correlation analysis between numbers of hCD4 or hCD8 with number of hCD45 lesions in brain parenchyma of all combined immunized DR15 HI and DR15 MS1-5 mice. Scale bars 100 μm; ×20 objective (B, F); ×40 objective (G, K). All results are depicted as mean ± standard error of the mean (SEM). Statistical analysis was performed by pairwise comparisons between different groups of mice using Student’s t test.
-
Figure 4—source data 1
Numbers of human CD45-positive immune cells counted at brain borders (total cells in comparable whole coronal brain sections) and in parenchyma (cells/mm2) (C), as well as of human CD8 and human CD4 T cell-positive cells counted at brain borders (total cells in comparable whole coronal brain sections) and in parenchyma (cells/mm2) (D) of non-immunized and immunized humanized B2m-NOG mice, as well as the human CD4/CD8 T cell ratios derived from these measurements (E).
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig4-data1-v1.docx
Immunization with myelin peptides increases hCD4 T cell infiltration of spinal cord white matter in both DR15 MS and DR15 HI mice.
Immunohistochemical analysis of spinal cord from peripheral blood mononuclear cell (PBMC) B2m-NOG mice immunized for experimental autoimmune encephalomyelitis (EAE) showing infiltration by human (h) CD3-positive T cells in the gray and white matter regions. (A) Infiltrating hCD3-positive T cells scattered throughout spinal cord gray and white matter and forming small lesions in the white matter (WM) of both DR15 MS1 and DR15 HI mice (bottom panels, arrowheads) The dotted lines mark the boundary between gray and white matter. (B) Semi-quantitative estimation of hCD3-positive T cells in comparable whole spinal cord sections from DR15 HI and DR15 MS mice (n=5 mice/group). (C) Counting of hCD3-positive T cell lesions (≥3 adjacent cells) in gray matter (GM) (i) and white matter (WM) (ii) in whole spinal cord sections from DR15 HI and DR15 MS1 mice (n=5 mice/group). (D) Ratios of hCD4/hCD8 T cells at borders (i) and in parenchyma (ii) of spinal cord in non-immunized and immunized DR15 HI and DR15 MS mice. (E) Double immunofluorescence staining for hCD45-positive leukocytes (green, arrowheads) and MBP-positive myelin (red), with DAPI counterstained nuclei (blue), in white matter (WM) of immunized DR15 MS spinal cord. Scale bars 100 μm; ×40 objective (A). All results are depicted as mean ± standard error of the mean (SEM). Statistical analysis was performed by pairwise comparisons between different groups of mice using Student’s t test.
-
Figure 5—source data 1
Semi-quantitative analysis of human CD3-positive T cells (B), and numbers of human CD3-positive lesions (defined as 3 or more adjacent cells) in grey matter (GM) and white matter (WM) regions (C), in comparable whole spinal cord sections from DR15 HI and DR15 MS mice, as well as human CD4/CD8 T cell ratios at borders and in parenchyma of these sections in non-immunized and immunized humanized B2m-NOG mice.
- https://cdn.elifesciences.org/articles/88826/elife-88826-fig5-data1-v1.docx
Tables
Clinical and demographic data of MS patient and healthy control blood donors.
| HLA genotypeDiagnosis(donor type) | G | Age | MS duration (year) | Therapies | Relapse(12/24 months) | MRIBrain/Gd | MRISpine/Gd | EDSS | Viral infections(titre) | EBV clinical interpretation (Figure 1—figure supplement 1C) | T cell responses [Dagkonaki et al., 2020] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DR13 1302/1303 RRMS (donor for DR13 MS mice) | F | 42 | 27 | Azathioprine Interferon beta-1a Interferon beta-1b Fingolimod Natalizumab (5 months) Cortisone (for 1 month prior to sampling) | 1/1 Last relapse 05/23 | (+) stable | (+) GD (+) | 2.5 | JC+ (1.24) EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA+ EBV EA IgG+ EBV EBNA1 IgG+ EBV EBNA1 IgM− | Suspect recent or ongoing reactivation | MBP83-99 hMOG35-55 [Patient 17 Dagkonaki et al., 2020] |
| DR15 0402/1501 RRMS (donor for DR15 MS1 mice) | F | 38 | 15 | Interferon beta-1a Fingolimod Natalizumab (24 months) To Cladribine (after sampling) | 1/1 No relapse during the last year | (+) stable | (+) stable | 2.5 | JC+ (1.47) EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA− EBV EA IgG− EBV EBNA1 IgG+ EBV EBNA1 IgM+ | Anomalous reactivation | MBP13-32 MBP83-99 hMOG35-55 [Patient 11 Dagkonaki et al., 2020] |
| DR15 04/15 healthy (donor for DR15 HI mice) | F | 28 | NA | None | NA | NA | NA | NA | JC ND EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA− EBV EA IgG+ EBV EBNA1 IgG+ EBV EBNA1 IgM+ | Anomalous reactivation | None [Normal 1 Dagkonaki et al., 2020] |
| DR15 RRMS (donor for DR15 MS2 mice) | F | 47 | 10 | Dimethyl Fumarate Natalizumab (1 year and ongoing) | 0/0 No relapses during the last year | (+) stable | (+) stable | 1.5 | JC+ (0,96) EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA− EBV EA IgG− EBV EBNA1 IgG+ EBV EBNA1 IgM- | Seropositive without symptoms of active infection | ND |
| DR15 RRMS (donor for DR15 MS3 mice) | F | 39 | 15 | Interferon beta-1b Natalizumab (8 years and ongoing) | 0/0 No relapse during the last year | (+) stable | (+) stable | 2.0 | JC (−) EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA+ EBV EA IgG+ EBV EBNA1 IgG+ EBV EBNA1 IgM− | Suspect recent or ongoing reactivation | ND |
| DR15 RRMS (donor for DR15 MS4 mice) | F | 53 | 23 | Interferon beta-1a Interferon beta-1b Glatiramer Acetate Natalizumab (13 years and ongoing) | 0/0 No relapse during the last year | (+) stable | (+) stable | 3.5 | JC (−) EBV VCA IgG+ EBV VCA IgM− EBV VCA IgA− EBV EA IgG+ EBV EBNA1 IgG+ EBV EBNA1 IgM+ | Anomalous reactivation | ND |
| DR15 RRMS (donor for DR15 MS5 mice) | F | 30 | 13 | Interferon beta-1a Interferon beta-1b Fingolimod Natalizumab (8 years and ongoing) | 0/0 No relapse during the last year | (+) stable | (+) stable | 1.5 | JC (−) EBV VCA IgG+ EBV VCA IgM+ EBV VCA IgA+ EBV EA IgG− EBV EBNA1 IgG+ EBV EBNA1 IgM− | Suspect recent or ongoing reactivation | ND |
-
KEY: EBV, Epstein-Barr virus; EDSS, expanded disability status scale; G, gender; Gd, gadolinium-enhancing lesions; JC; John Cunningham virus; NA, not applicable; ND, not done; RRMS, relapse-remitting MS.





