Hierarchical morphogenesis of swallowtail butterfly wing scale nanostructures
Figures

Hierarchical nanostructure of wing scales in Parides eurimedes (Papilionidae).
(A) Adult male with structurally colored green patches on their dorsal forewings. Credit: iDigBio YPM Ent 433579. (B) Scanning electron microscopy (SEM) top view and (C) Focused ion beam (FIB)-SEM cross-sectional image of an adult green scale showing ridges and honeycomb lattice ending in trabeculae on top of a perforated multilayer lattice. Scale bars: (A) 1 cm, (B, C) 1 µm.
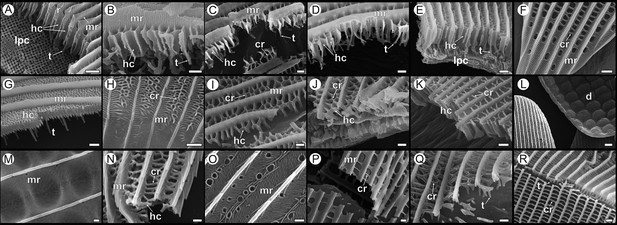
Diversity of adult wing scale nanostructure in Papilionidae shown in comparison to (R) a dorsal forewing (DFW) black scale of Hypolimnas bolina (Nymphalidae) with regular rectilinear crossribs.
P. arcas: (A) male DFW green cover, (B) male DFW black, (C) female DFW yellow cover, (D) female dorsal hindwing (DHW) red cover; P. eurimedes: (E) male DFW green cover, (F) proximal part of male DFW black; P. nireus: (G, H) male DFW blue cover; P. memnon: (I) DFW navy, (J) ventral hindwing (VHW) black, (K) VHW red; P. palinurus: (L) DHW green cover, (M) DFW green cover, (N) ventral forewing (VFW) dark gray, (O) VHW blue; Graphium agamemnon: (P) light pink, (Q) dark pink; (R) DFW black; Hypolimnas bolina. All scale bars 1 µm except for (L) 5 µm. r, ridges; mr, microribs; cr, crossribs; hc, honeycombs; t, trabeculae; lpc, lumen photonic crystal; d, dimples.
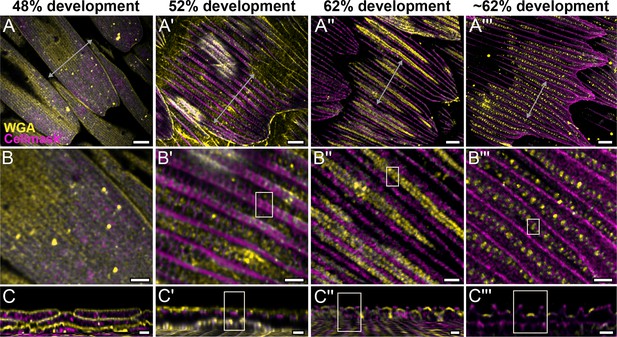
Morphogenetic time series of the development of cuticular disks in pupal P. eurimedes dorsal forewing scales, acquired with a 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and CellMask (magenta) stains plasma membrane. By 52% development, hollow vein-like crossribs appear on plasma membrane in between ridges that serve as a scaffold for cuticle accretion. As scales mature, more cuticle is deposited into these rows of disks bounded by plasma membrane. See also Figure 2—figure supplements 3–5. (B–B''') Closeup views of (A–A'''). (C–C''') xz cross-sections of the scale at locations marked with gray lines in (A–A''') reveal the planar aspect of the cuticular disks. Yellow regions of interest (ROIs) in (C'–C''') correspond to those in (B'–B'''). Scale bars (A–A''') 5 µm, (B–B''' and C–C''') 2 µm.
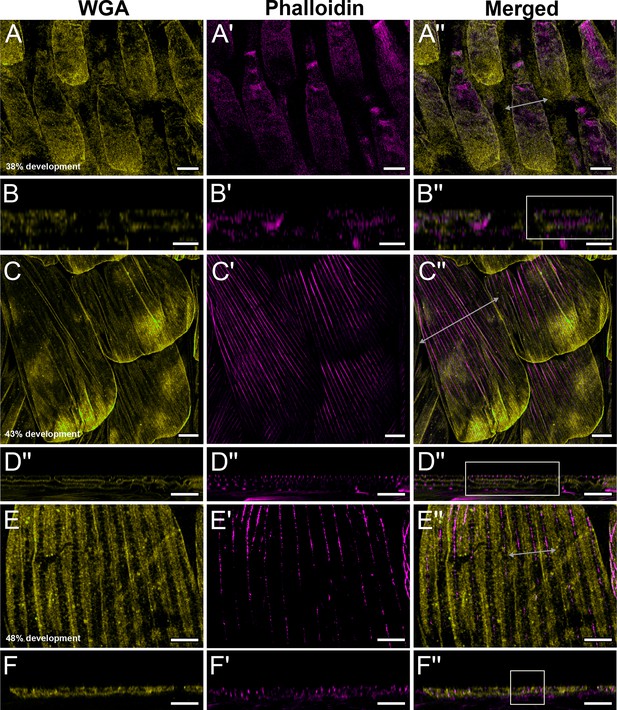
Early development of dorsal forewing green cover scales in pupal male P. eurimedes acquired with 60× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains plasma membrane and AF-647 phalloidin (magenta) stains F-actin. (A–A'') 38% development: young scales extend out from the wing epithelium. F-actin filaments are beginning to organize into bundles. (C–C'') 43% development: F- actin bundles span the scale length along the proximal–distal axis. Plasma membrane of scale cells begins to pleat. (E–E'') 48% development: ridge-like projections are seen in between adjacent F-actin bundles with WGA faintly staining crossribs. (B–B'', D–D'', F–F'') xz cross-sections of the scales shown in (A–A'', C–C'', E–E''). All scale bars 5 μm.
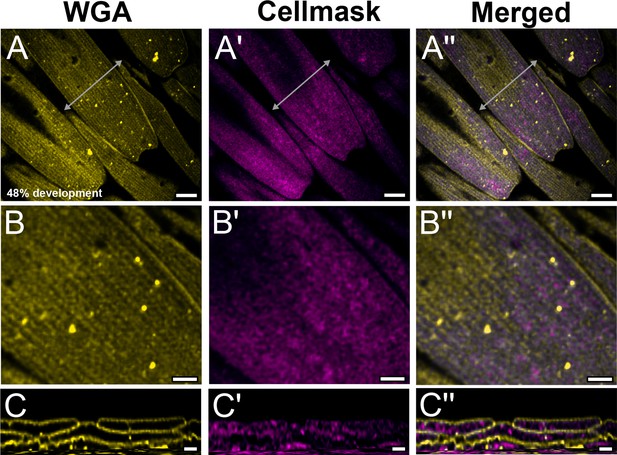
Yellow dorsal forewing cover scales of pupal female P. eurimedes at 48% development acquired with 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and CellMask (magenta) stains plasma membrane. Scales are relatively young with a long and narrow appearance. (B–B'') Closeup views of (A–A''). Ridges seen on the surface of scale cells are indistinct while the membrane has a mottled appearance. (C–C'') xz cross-sections of the scale at location marked with gray line in (A'') showing chitin is deposited on the cell membrane, as expected. Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
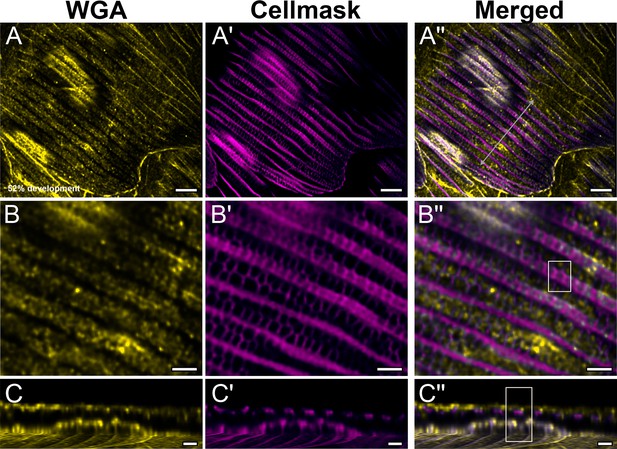
Yellow dorsal forewing cover scales of pupal female P. eurimedes at 52% development acquired with 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and CellMask (magenta) stains plasma membrane. CellMask staining reveals hollow anastomozing vein-like crossribs on the plasma membrane in between ridges that will serve as a scaffold for cuticle accretion. (B–B'') Closeup views of (A–A'') showing chitin is deposited within the hollow membranous outlines. (C–C'') xz cross-sections of the scale at location marked with gray line in (A''). Most of the chitin deposition is on the scale surface. Yellow region of interest (ROI) in (C'') corresponds to that in (B''). Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
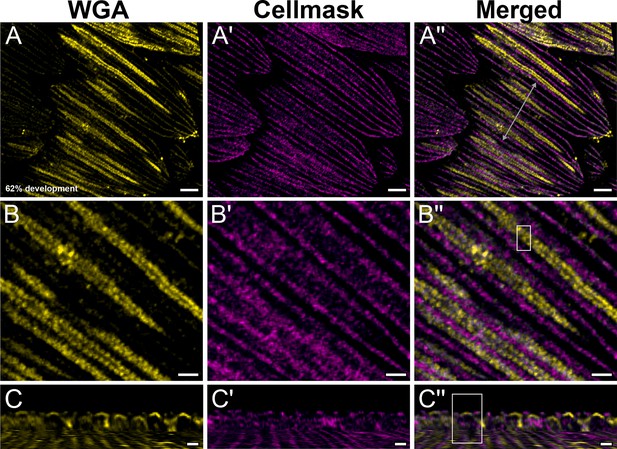
Yellow dorsal forewing cover scales of pupal female P. eurimedes at 62% development acquired with 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and CellMask (magenta) stains plasma membrane. As scales mature, more cuticle is deposited on rows of disks bounded by the membraneous crossribs compared to the rest of the scale cell surface. (B–B'') Closeup views of (A–A''). (C–C'') xz cross-sections of the scale at location marked with gray line in (A'') reveal the planar aspect of the cuticular disks, for the honeycomb lattice has not formed yet. Yellow region of interest (ROI) in (C'') corresponds to that in (B''). Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
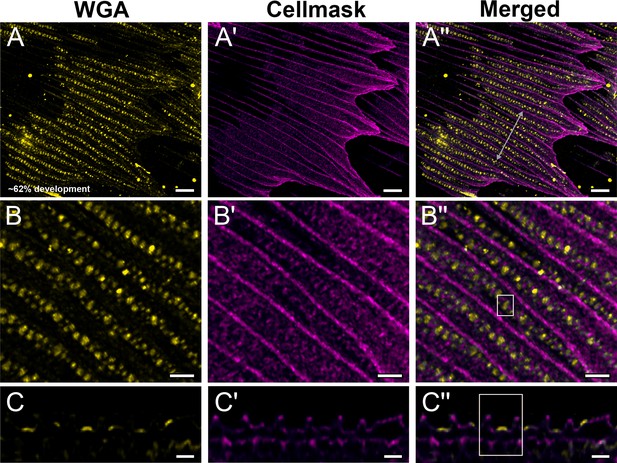
Black dorsal forewing scales of pupal male P. eurimedes at approximately 62% development acquired with 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and CellMask (magenta) stains plasma membrane. (B–B'') Closeup views of (A–A''). Cuticular disks are clearly seen in between ridges and within the hollow outlines (crossribs) defined by the plasma membrane. (C–C'') xz cross-sections of the scale at location marked with gray line in (A'') again shows the cuticular disks are flat and bulk of the cuticle deposition is in the cuticular disks. Yellow region of interest (ROI) in (C'') corresponds to that in (B''). Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
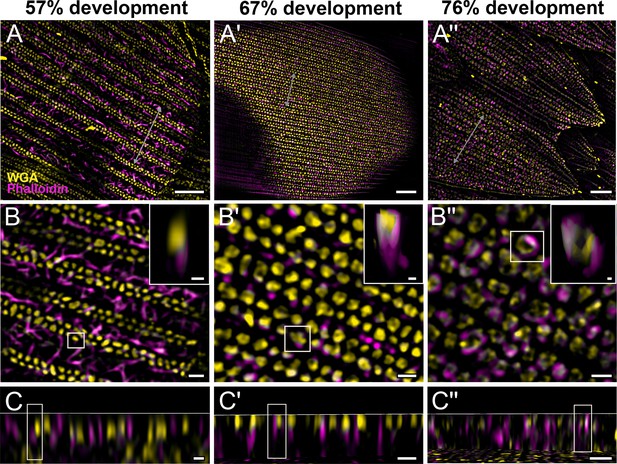
Morphogenetic time series of the development of columnar honeycomb lattice in pupal P. eurimedes dorsal forewing scale cells acquired with super-resolution lattice structured illumination microscopy (SIM).
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta) stains F-actin. AF-555 WGA shows a gradual evolution of the cuticular disks from filled-in planar to hollow tubular outgrowths. The disintegrating F-actin bundles show evidence of reorganization from linear to reticulated features with ring-like cross-sections. See also Figure 3—figure supplements 1–3. (B–B'') Closeup views of (A–A''). Insets correspond to the regions of interest (ROI) marked in yellow shown with a 3D aspect. (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Scale bars (A–A'') 5 µm, (B–B'', C–C'', and insets) 1 µm.
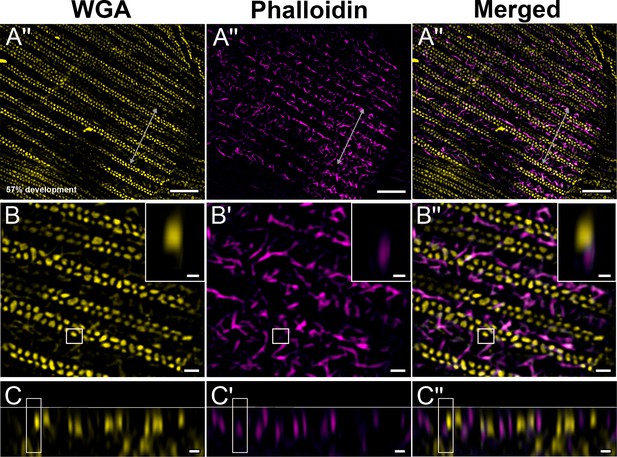
Green dorsal forewing cover scales of pupal male P. eurimedes at 57% development acquired with super-resolution lattice structured illumination microscopy (SIM).
(A–A'') AF-555 wheat germ agglutinin (WGA) (yellow) stains double rows of cuticular disks along the upper lamina. F-actin bundles as stained by AF-647 phalloidin are seen disintegrating into shorter fibrils that are distributed around the cuticular disks. The scale is still soft (ridges are not fully sclerotized) at this point, given the irregular, dimpled appearance. (B–B'') Closeup views of (A–A''). Insets correspond to the regions of interest (ROI) marked in yellow shown with a 3D aspect. (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Yellow ROIs correspond to those in (B–B''). Scale bars (A–A'') 5 µm, (B–B'', C–C'', and insets) 1 µm.
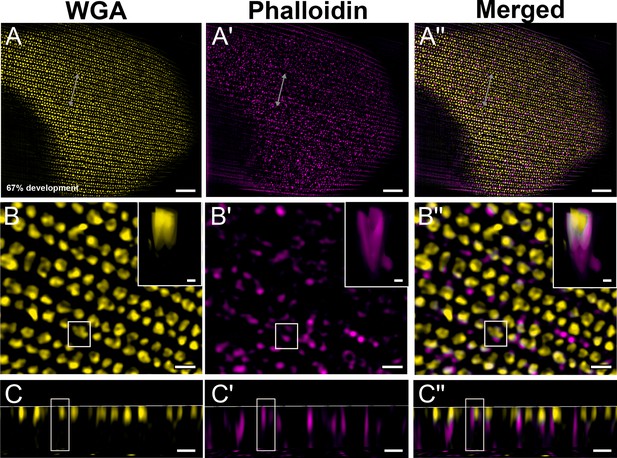
Green dorsal forewing cover scales of pupal male P. eurimedes at 67% development acquired with super-resolution lattice structured illumination microscopy (SIM).
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta) stains F-actin. (A–A'') The cuticular disks are more regularly arranged, as the scale cells expand and flatten. (B–B'') Closeup views of (A–A'') show the disintegrating F-actin bundles have reorganized into a mesh-like network and associate with the cuticular disks to a greater degree. The cuticular disks now appear elongated and slightly tubular in cross-sections. Insets correspond to the region of interest (ROI) marked in yellow shown with a 3D aspect. (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Yellow ROIs correspond to those in (B–B''). The cuticular disks are located along the upper lamina surface, whereas F-actin structures extend further into the lumen. Scale bars (A–A'') 5 µm, (B–B'', C–C'', and insets) 1 µm.
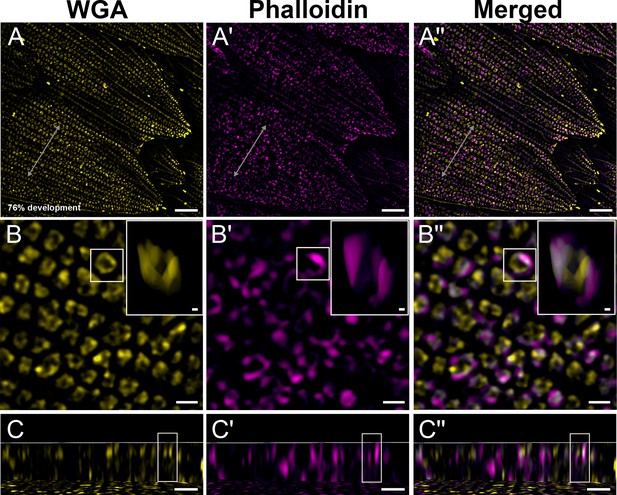
Black dorsal forewing scales of pupal male P. eurimedes at 76% development acquired with super-resolution lattice structured illumination microscopy (SIM).
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta) stains F-actin. (A–A'') Scale cells take on the overall appearance of adult black scale cells with finger-like projections on the distal tip. (B–B'') Closeup views of (A–A'') show F-actin has completely disintegrated. The reorganized F-actin enmeshes the cuticular disks, which have extruded into the lumen to become hollow tubes at this stage. Insets correspond to the region of interest (ROI) marked in yellow shown with a 3D aspect. (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Yellow ROIs correspond to those in (B–B'') and show higher degree of association compared to earlier stages. Scale bars (A–A'') 5 µm, (B–B'', C–C'', and insets) 1 µm.
Planar sections through the same dataset (57% development) as depicted in Figure 3—figure supplement 1.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta), F-actin. The xy planes move through increasing z depth, showing cuticular disks and F-actin mesh-like structures at different z positions of the scale cell.
Planar sections through the same dataset (67% development) as depicted in Figure 3—figure supplement 2.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta), F-actin. Scale cells are relatively flatter at this stage.
Planar sections through the same dataset (76% development) as depicted in Figure 3—figure supplement 3.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta), F-actin. Cuticular disks have become extruded into hollow tubes and are enmeshed by tulip bulb-like F-actin structures.
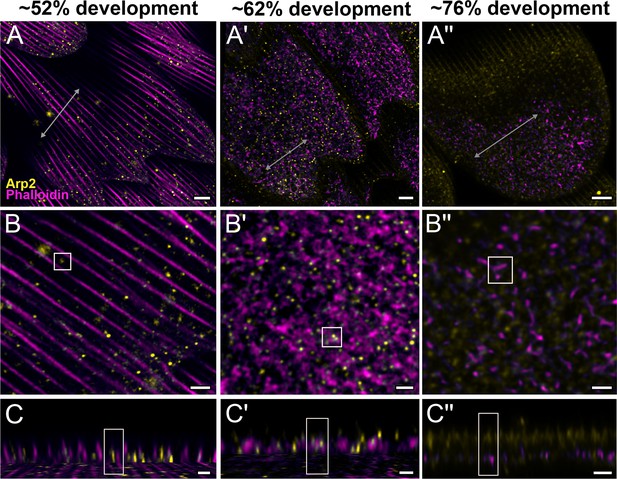
Arp2/3 complex is involved in F-actin reorganization in pupal P. eurimedes wing scales.
Dorsal forewing scales stained with AF-594 anti-Arp2 (yellow) and AF-647 phalloidin (magenta) acquired with 100× confocal microscope. Initially (~52% development), Arp2/3 complex appear as sparse punctate dots while the F-actin bundles are still intact. As F-actin bundles disintegrate and reorganize, a relatively higher density of punctate Arp2/3 signal is seen in close association with the reticulate F-actin network. Our oldest timepoint in P. eurimedes (~76% development) has a large amount of cuticle autofluorescence overlapping with the AF594 signal, but the punctate pattern can be still discerned. See also Figure 4—figure supplements 1–3. (B–B'') Closeup views of (A–A''). (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Yellow regions of interest (ROIs) correspond to those in (B–B''). Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
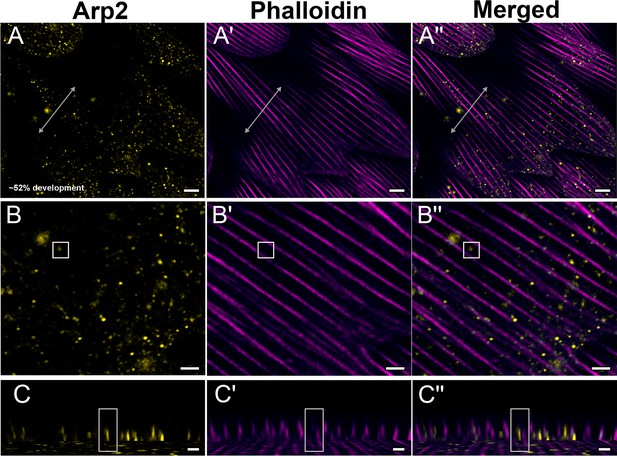
Yellow dorsal forewing cover scales of pupal female P. eurimedes at approximately 52% development acquired with 100× confocal microscope.
AF-594 anti-Arp2 (yellow) stains Arp2/3 complex and AF-647 phalloidin (magenta) stains F-actin. (A–A'') Arp2/3 complex shows a punctate, sparse distribution, while F-actin bundles are intact. (B–B'') Closeup views of (A–A''). (C–C'') xz cross-sections of the scale at locations marked with gray lines in (A–A''). Yellow regions of interest (ROIs) correspond to those in (B–B''). Scale bars (A–A'') 5 µm, (B–B'' and C–C'') 2 µm.
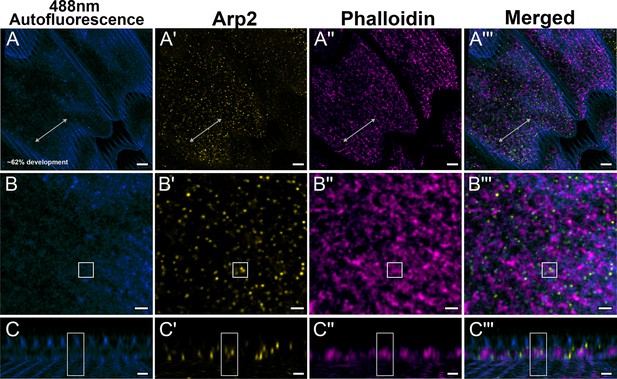
Yellow dorsal forewing cover scales of pupal female P. eurimedes at approximately 62% development acquired with 100× confocal microscope.
488 nm channel shows cuticular autofluorescence (blue) from scales. AF-594 anti-Arp2 (yellow) stains Arp2/3 complex and AF-647 phalloidin (magenta) stains F-actin. (A–A''') F-actin bundles have disintegrated at this stage and reorganized into a reticulate network seen closely associating with a relatively higher concentration of punctate Arp2/3 complex. (B–B''') Closeup views of (A–A'''). (C–C''') xz cross-sections of the scale at locations marked with gray lines in (A–A'''). Yellow regions of interest (ROIs) correspond to those in (B–B'''). Ridges are seen in the autofluorescence channel. Scale bars (A–A''') 5 µm, (B–B''' and C–C''') 2 µm.
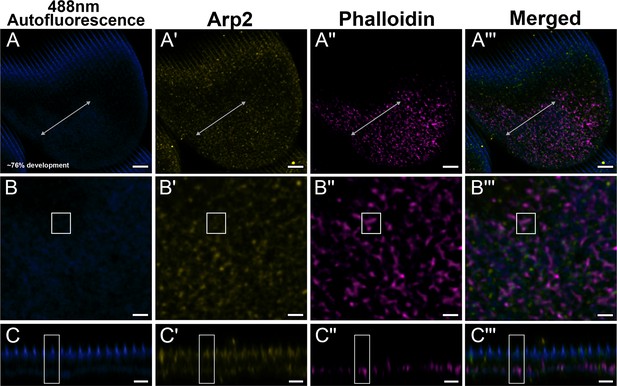
Green dorsal forewing cover scales of pupal male P. eurimedes at approximately 76% development acquired with 100× confocal microscope.
488 nm channel shows cuticular autofluorescence (blue) from scales. AF-594 anti-Arp2 (yellow) stains Arp2/3 complex and AF-647 phalloidin (magenta) stains F-actin. (B–B''') Closeup views of (A–A'''). (C–C''') xz cross-sections of the scale at locations marked with gray lines in (A–A'''). Yellow regions of interest (ROIs) correspond to those in (B–B'''). F-actin network shows a greater degree of reorganization. A large amount of the Arp2 antibody signal overlaps with cuticle autofluorescence, but the punctate pattern can still be discerned. Scale bars (A–A''') 5 µm, (B–B'' and C–C''') 2 µm.
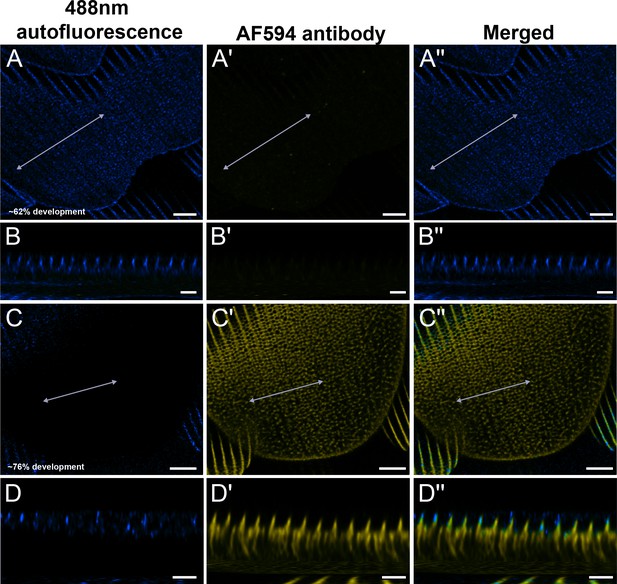
Negative controls for Arp2 antibody staining.
Red dorsal hindwing cover scales from the same pupal male P. eurimedes from Figure 4—figure supplement 2 (A–A'' at approximately 62% development), and Figure 4—figure supplement 3 (C–C'' at approximately 76% development) acquired with 100× confocal microscope. 488 nm channel shows cuticular autofluorescence (blue) from scales while AF-594 antibody (yellow) serves as a negative control for unspecific binding from the secondary antibody. Cuticle shows autofluorescence at longer wavelengths as scale matures. (B–B'', D–D'') xz cross-sections of the scale at locations marked with gray lines in (A–A'') and (C–C''), respectively. Scale bars (A–A'', C–C'') 5 µm, (B–B'', D–D'') 2 µm.
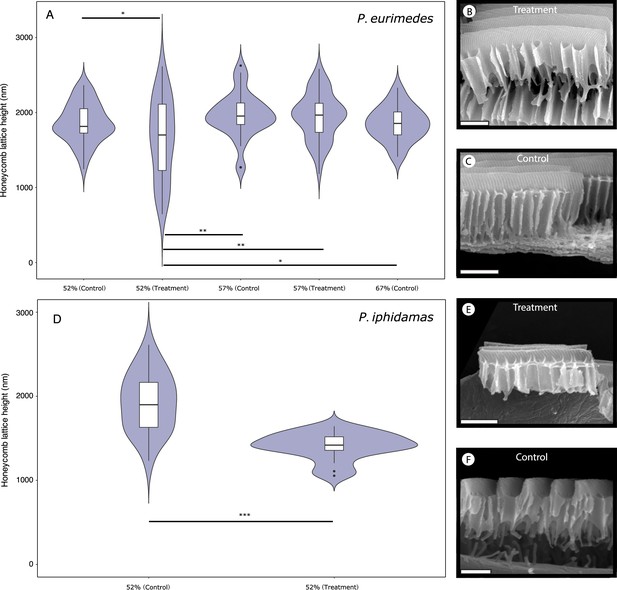
Pharmacological disruption of Arp2/3 with CK-666 in Parides pupae.
Violin plots depict the distribution of honeycomb lattice heights measured from multiple scales of a single emerged male individual either treated with CK-666 or DMSO (negative control) as pupae, along with representative scanning electron microscopy (SEM) images of scale cross-sections used in measurements for P. eurimedes (A–C) and P. iphidamas (D–F). Developmental stage of the pupae at the time of injection are as marked. Results of pairwise t-tests (after corrections for multiple comparisons) are indicated as follows: *p<0.05, **p<0.01, ***p<<0.01.
-
Figure 4—figure supplement 5—source data 1
FIJI/ImageJ (version 2.1.0/1.53c) measurements of scanning electron microscopy (SEM) images of developing Parides eurimedes and P. iphidamas scales respectively used to generate the violin plots in Figure 4—figure supplement 5A and D, along with a summary of the pairwise t-tests.
- https://cdn.elifesciences.org/articles/89082/elife-89082-fig4-figsupp5-data1-v1.xlsx
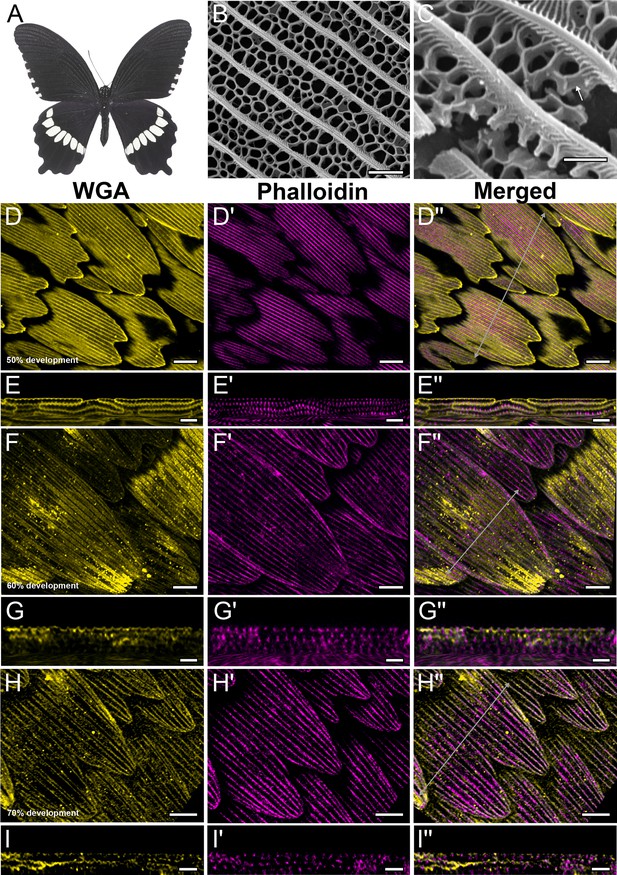
Development of dorsal forewing black cover scales in pupal P. polytes acquired with 100× confocal microscope.
AF-555 wheat germ agglutinin (WGA) (yellow) stains chitin and AF-647 phalloidin (magenta) stains F-actin. (A) Adult P. polytes. Credit: iDigBio YPM ENT 815256 (reproduced as CC0 1.0). (B) Scanning electron microscopy (SEM) top view and (C) cross-sectional image of an adult black scale showing irregular crossribs (white arrow). (D–D'') 50% development: chitinous ridges developed in between adjacent F-actin bundles. (E–E'') xz cross-sections of the scale at location marked with gray line in (A’’) shows F-actin bundles along the periphery of scale cells, with thicker bundles at the lower surface of the cell. (F–F'') 60% development: WGA signal shows irregular crossrib pattern that resemble the final morphology in adult scales. (H–H'') 70% development: WGA signal is fuzzier, possibly due to the increased amount of lipids and waxes deposited in the cuticle. Irregular crossribs are still visible in between the ridges. (G–G'' and I–I'') xz cross-sections of the scale at location marked with gray lines in (F'') and (H''), respectively. Scale bars (B) 2 µm, (D–D'', F–F'', and H–H'') 10 µm, (E–E'', G–G'', and I–I'') 5 µm.
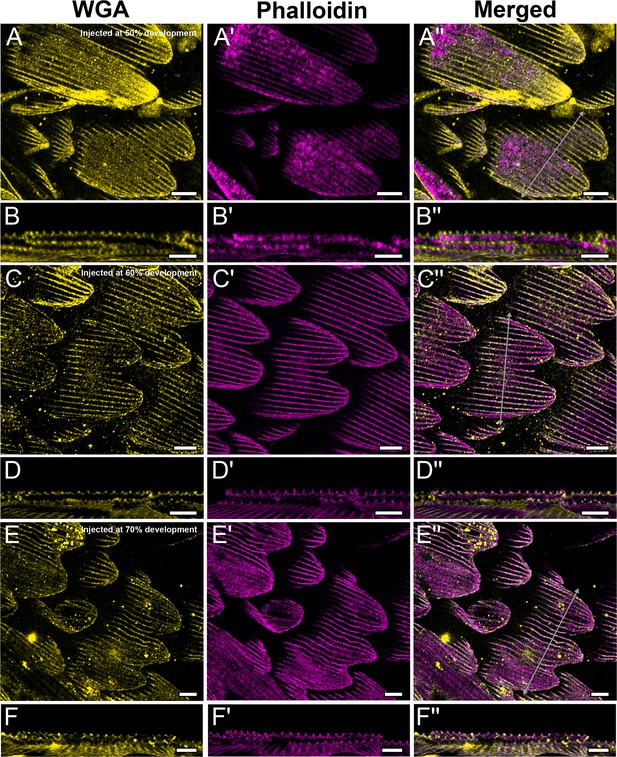
CK-666 inhibition does not affect F-actin structure in P. polytes.
P. polytes pupae were injected with 100 µM CK-666 at 50, 60, and 70% development, and dissected at 80% development. Dorsal forewing cover scales are stained with AF-555 wheat germ agglutinin (WGA) (yellow) and AF-647 phalloidin (magenta) and acquired with 100× confocal microscope. Ridges and irregular crossribs developed across all treatment days. (B–B'', D–D'', and F–F'') xz cross-sections of the scale at locations marked with gray lines in (A''), (C''), and (E''), respectively. All scale bars 10 µm.
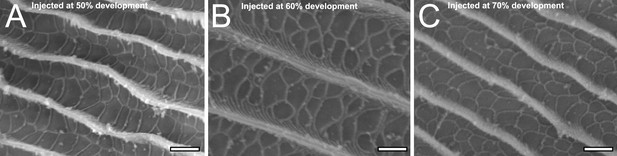
Scanning electron microscopy (SEM) micrographs of P. polytes pupal scales injected with CK-666 at 50 (A), 60 (B), and 70% (C) development.
Wing scales are from the same pupa corresponding to Figure 4—figure supplement 7, but are different black scales. Irregular crossrib formation is not disrupted after CK-666 treatment. All scale bars 1 µm.
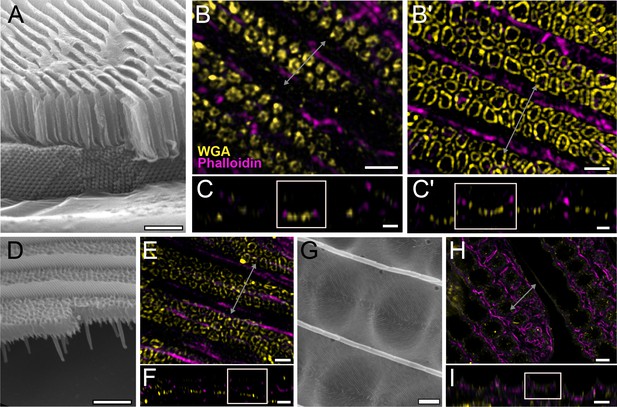
Conservation of honeycomb lattice development in Papilionidae.
Scanning electron microscopy (SEM) cross-sectional view of (A) adult green scales in P. arcas, (D) blue scales in P. nireus, and (G) green scales in P. palinurus. All pupal dorsal forewing scales are stained either with AF-555 wheat germ agglutinin (WGA) or FITC-WGA (yellow) showing chitin, and AF-647 phalloidin or TRITC-phalloidin (magenta) showing F-actin. (B, C) Maximum projected 3D structured illumination microscopy (3D-SIM) micrograph of green cover scales in pupal male P. arcas featuring planar cuticular disks similar in shape and arrangement to P. eurimedes. (B'–C') Maximum projected 3D-SIM micrograph of green cover scales of a different male P. arcas pupa showing irregular crossribs patterns. (E, F) Maximum projected 3D-SIM micrograph of blue cover scales of pupal P. nireus, similarly with crossribs and intact linear actin bundles. (H, I) Maximum projected 60× confocal micrographs of green cover scales of pupal P. palinurus. A concave network of F-actin underlies the cuticular dimples. At lower z, the actin rings (in cross-section) are smaller in size and show a foam-like appearance. (C–C', F, and I) xz cross-sections of the scales with region of interest (ROI) at locations marked with gray lines in (B–B'), (E), and (H), respectively. Scale bars (A, D, and G) 5 µm, (B–B', C–C', E, and F) 1 µm, (H, I) 5 µm.
Planar sections through the same dataset as depicted in Figure 5H and I, for P. palinurus.
FITC wheat germ agglutinin (WGA) (yellow) stains chitin and TRITC phalloidin (magenta), F-actin. A concave whorl-like network of F-actin closely follows and underlies the cuticular dimples in between ridges.
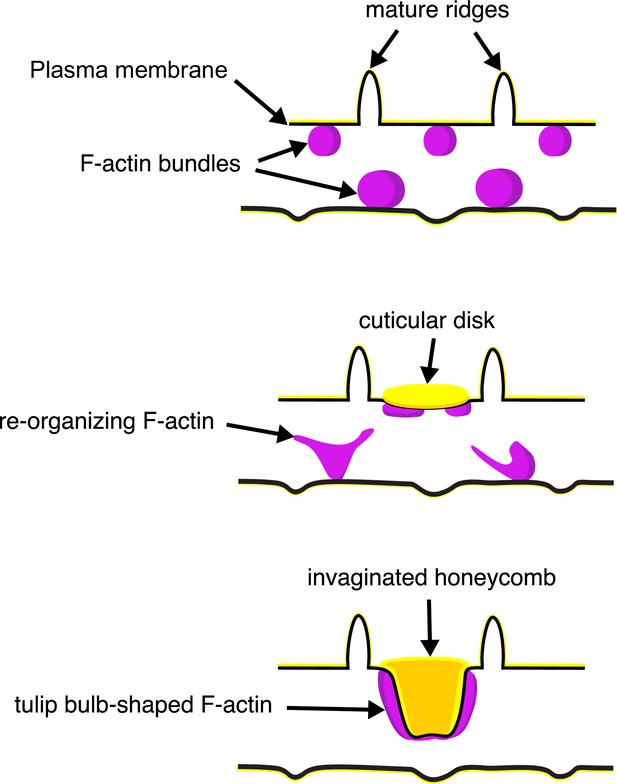
A schematic illustrating the proposed morphogenesis of honeycomb lattices in papilionid wing scales.
During early stages, F-actin bundles prefigure the loci where ridges form in between adjacent actin bundles. Next the plasma membrane (black lines) forms a scaffold where cuticle (green lines and structures) accumulates into the spaces and forms irregular disk-like structures. Reorganization of F-actin around the disks subsequently extrudes the plasma membrane along with the deposited cuticle, forming the honeycomb lattice walls.
Tables
| Reagent type(species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Parides eurimedes) | P. eurimedes | Stratford-upon-Avon Butterfly Farm (UK); Mariposario del Bosque Nuevo (Costa Rica) | developing pupae | |
| Strain, strain background (Parides arcas) | P. arcas | Stratford-upon-Avon Butterfly Farm (UK); Mariposario del Bosque Nuevo (Costa Rica) | developing pupae | |
| Strain, strain background (Parides iphidamas) | P. iphidamas | Mariposario del Bosque Nuevo (Costa Rica) | developing pupae | |
| Strain, strain background (Papilio polytes) | P. polytes | Marl Insect and Butterfly Culture (Philippines) | developing pupae | |
| Strain, strain background (Papilio nireus) | P. nireus | Stratford-upon-Avon Butterfly Farm (UK); Marl Insect and Butterfly Culture (Philippines) | developing pupae | |
| Strain, strain background (Papilio palinurus) | P. palinurus | Marl Insect and Butterfly Culture (Philippines) | developing pupae | |
| Antibody | Anti-Arp2 (rabbit) | Abcam | ab47654 | 1:500 |
| Antibody | Anti-rabbit Alexa Fluor 594 (goat) | Abcam | ab150088 | 1:300 |
| Antibody | Alexa Fluor 555-conjugated wheat germ agglutinin | Invitrogen | W32464 | 1:200 |
| Antibody | Alexa Fluor 647-conjugated phalloidin | Invitrogen | A22287 | 1:40 |
| Antibody | FITC-conjugated wheat germ agglutinin | EY-Labs | F-2101-5 | 1:100 |
| Antibody | TRITC-conjugated phalloidin | Sigma-Aldrich | P1951 | 1:100 |
| Chemical compound, drug | CellMask Deep Red Plasma Membrane Stain | Invitrogen | C10046 | 1:300 |
| Chemical compound, drug | CK-666 | Sigma-Aldrich | SML0006 | 100µM |
| Chemical compound, drug | ProLong Gold Antifade Mountant | Life Technologies | P36930 | |
| Software, algorithm | Imaris Viewer v9.5.1 | Bitplane AG | RRID:SCR_007370 | |
| Software, algorithm | Imaris v9.1 | Bitplane AG | RRID:SCR_007370 | |
| Software, algorithm | Shotcut v19.07.15 | Meltytech, LLC | ||
| Software, algorithm | Packages for R: ggplot2, base | R statistical environment (v4.1.2) | RRID:SCR_001905 | http://www.r-project.org |
| Software, algorithm | Huygens Professional v20.04 | Scientific Volume Imaging B.V. | ||
| Software, algorithm | FIJI/ImageJ (v2.1.0/1.53c) | 10.1038/nmeth.2019 | RRID:SCR_002285 | https://fiji.sc |
Additional files
-
Supplementary file 1
Details of CK-666 inhibition experiments performed in different batches of purchased pupae and their eventual fates.
- https://cdn.elifesciences.org/articles/89082/elife-89082-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/89082/elife-89082-mdarchecklist1-v1.docx





