The PMA phorbol ester tumor promoter increases canonical Wnt signaling via macropinocytosis
Figures
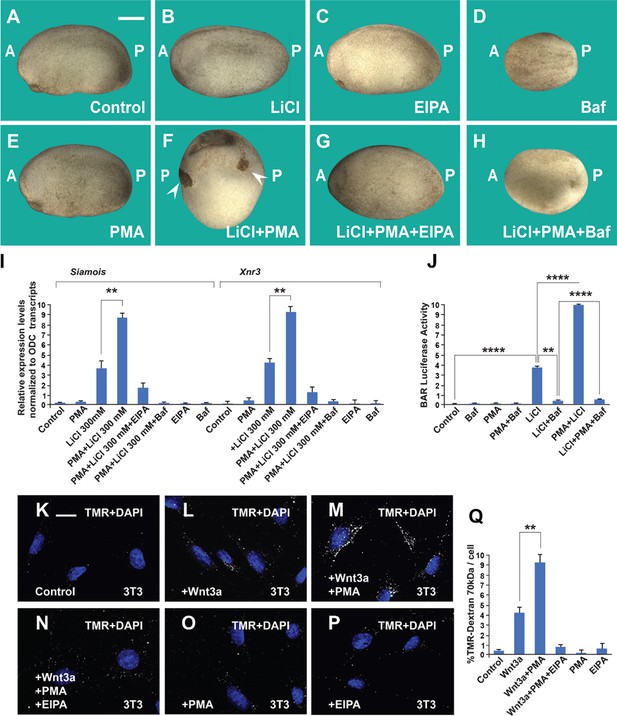
The phorbol ester phorbol 12-myristate 13-acetate (PMA) synergizes with GSK3 inhibition and Wnt3a and this cooperation requires macropinocytosis and lysosome acidification.
(A) Uninjected control embryo at stage 24. (B) Lithium Chloride (LiCl) (4 nl 300 mM, 1x ventral) dorsalized the embryo only slightly. (C) 5-(N-Ethyl-N-isopropyl) Amiloride (EIPA) (1 mM, 1x ventral) alone did not produce a distinctive phenotype at this concentration. (D) The vacuolar ATPase (V-ATPase) inhibitor Bafilomycin A1 (Baf) (incubation with 5 μM for 7 min at 32 cells) ventralized embryos. (E) A single injection of PMA (500 nM) did not show any phenotypic effect. (F) PMA and LiCl strongly synergized, resulting in embryos with radial heads. (G) Co-injection of the EIPA macropinocytosis inhibitor blocked dorsalization caused by LiCl plus PMA. (H) Incubation with Baf lysosomal acidification inhibitor blocked dorsalization caused by LiCl plus PMA. Number of embryos was as follows: A = 70, 100%; B = 80; 98%; C = 75, 94%; D = 82, 92%; E = 76, 100%; F = 112, 98%; G = 75, 93%; H = 85, 98%. Scale bar, 500 μm. (I) PMA increases the effects of LiCl in Wnt signaling in early embryos. qRT-PCR analysis at blastula stage 9.5 of the Wnt target genes Siamois and Xnr3 normalized for Ornithine decarboxylase (ODC) transcripts. Embryos received a single injection of 4 nl of LiCl 300 mM with or without PMA (500 nM). Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01). (J) Luciferase assay in HEK293BR cells showing cooperation between PMA and LiCl, and its inhibition by Baf. (K–P) PMA and Wnt3a cooperated in stimulating macropinocytosis, which was sensitive to inhibition by EIPA, in 3T3 cells. TMR-dextran 70 kDa was added for 1 hr. (Q) Quantification of the Wnt3a + PMA results. Experiments with cultured cells were biological triplicates. Error bars denote standard deviation (****p < 0.0001 and **p < 0.01).
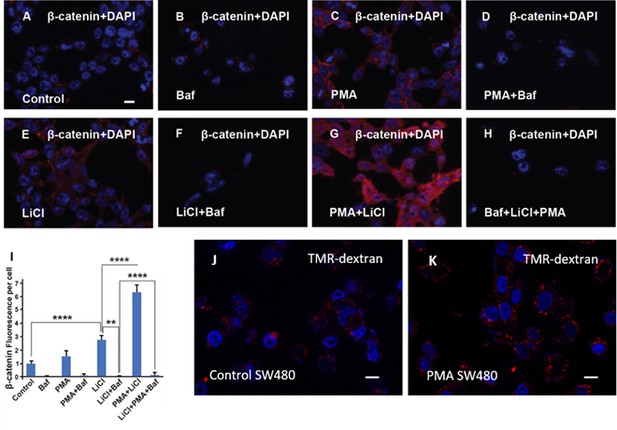
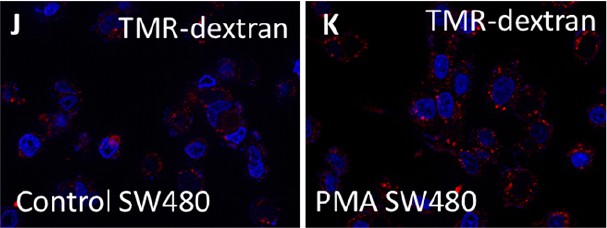
In cultured cells, the phorbol ester phorbol 12-myristate 13-acetate (PMA) cooperates with GSK3 inhibition in β-catenin signaling and increases dextran macropinocytosis.
(A) HEK293BR (β-catenin activity reporter [BAR]/Renilla) cells stained with total β-catenin antibody. (B) HEK293BR cells treated with Baf (20 μM) overnight show no changes in β-catenin levels. (C) Treatment with PMA (1.5 μM) moderately increased β-catenin levels. (D) HEK293BR cells treated with PMA and Baf had low β-catenin levels. (E) Cells treated with Lithium Chloride (LiCl) (40 mM) showed an increase in β-catenin. (F) Baf treatment blocked the increase in β-catenin caused by LiCl. (G) PMA strongly cooperated with LiCl in the induction of β-catenin levels. (H) Baf treatment blocked the cooperative effect of LiCl and PMA, indicating a requirement for lysosome acidification. (I) Quantification of β-catenin fluorescence per cell with the different treatments indicated above. (J) SW480 colorectal cancer (CRC) cells showing basal levels of TMR-dextran 70 kDa. (K) Treatment with PMA for 2 hr increased dextran uptake by macropinocytosis in SW480 cells. All experiments with cultured cells were biological triplicates. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01),.**p < 0.001, (**** p < 0.0001). Scale bars, 10 μm.
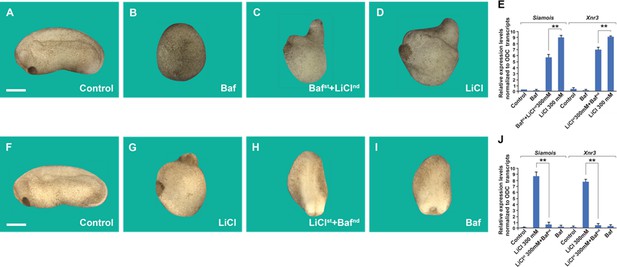
Order-of-addition experiment showing that lysosomal acidification at 32-cell stage is required for early Wnt/β-catenin activation.
(A) Untreated embryo. (B) Baf treatment (5 μM for 7 min) inhibited endogenous axis formation resulting in ventralized embryos lacking heads at early tailbud. (C) Embryos treated with Baf, washed, and then immersed in 300 mM Lithium Chloride (LiCl) an additional 7 min formed heads, resulting in dorsalized embryos with enlarged heads and short trunks. Note that all treatments were done at 32-cell stage, which is critical for the early Wnt signal. (D) LiCl treatment alone dorsalized embryos. (E) Quantitative RT-PCR (qPCR) for Wnt target genes Siamois and Xnr3 at blastula, confirming that the phenotypic effects are due to early activation of the Wnt pathway. (F) Untreated embryo. (G) Embryos incubated with LiCl at 32-cell were dorsalized. (H) Embryos first treated with LiCl and then subsequently with Baf resulted in ventralized embryos lacking heads. (I) Baf treatment caused ventralization and small heads. (J) Quantitative RT-PCR for Wnt target genes Siamois and Xnr3 showing that inhibiting V-ATPAse at the 32-cell stage blocks the effect of earlier LiCl treatment. The numbers of embryos analyzed were as follows: A = 11 5, 100%; B = 124, 98% with phenotype; C = 132, 97%; D = 99%; F = 132, 100%; G = 135, 99%; H = 126, 97%; I = 129, 98% (scale bars, 500 μm.). Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01).
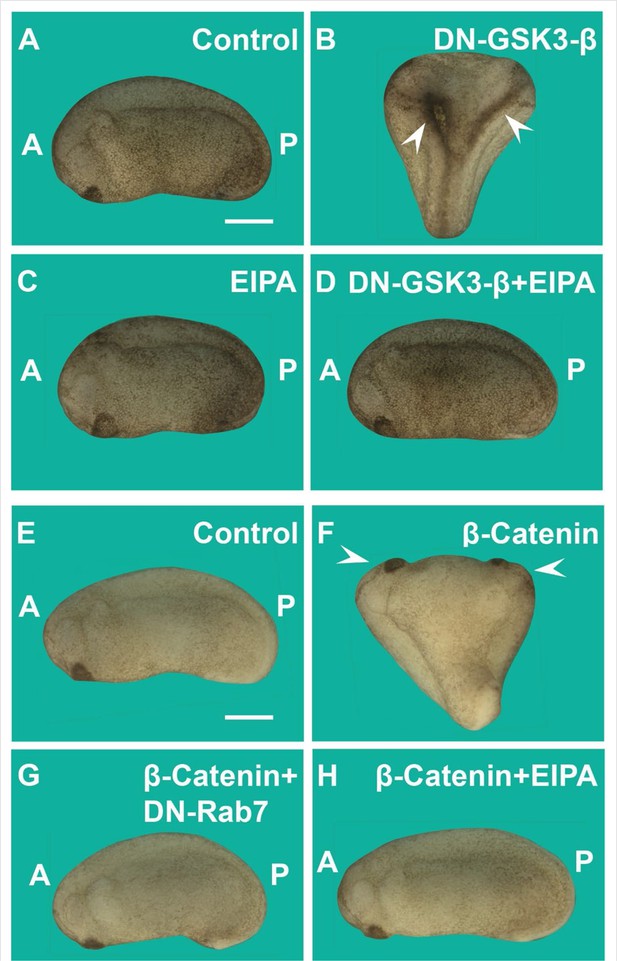
Induction of secondary axes by the GSK3/β-catenin pathway requires membrane trafficking.
(A) Control embryo at early tailbud. (B) Embryo injected with DN-GSK3 (dominant-negative GSK3-β, 150 pg 1x ventral) mRNA, showing double axes (arrowheads). (C) Injection of 5-(N-Ethyl-N-isopropyl) Amiloride (EIPA) 1x ventral alone showed no phenotypic effect (4 nl,1 mM). (D) Co-injection of DN-GSK3 and EIPA blocked double axis formation. (E) Uninjected control embryo. (F) Activation of Wnt signaling via injection of β-catenin mRNA (80 pg) induced complete twinned axes (arrowheads). (G) Embryo co-injected with β-catenin mRNA and DN-Rab7 (500 pg) showing that membrane trafficking is required for secondary axis formation. (H) Co-injection of β-catenin mRNA and the macropinocytosis inhibitor EIPA blocks axial duplication. The numbers of embryos analyzed were as follows: A = 62, 100%; B = 75, 94% with double axes; C = 76, 100%; D = 70, 98%; E = 70, 100%; F = 73, 98%; G = 69, 75%; H = 82, 97%; four independent experiments (scale bars, 500 μm).
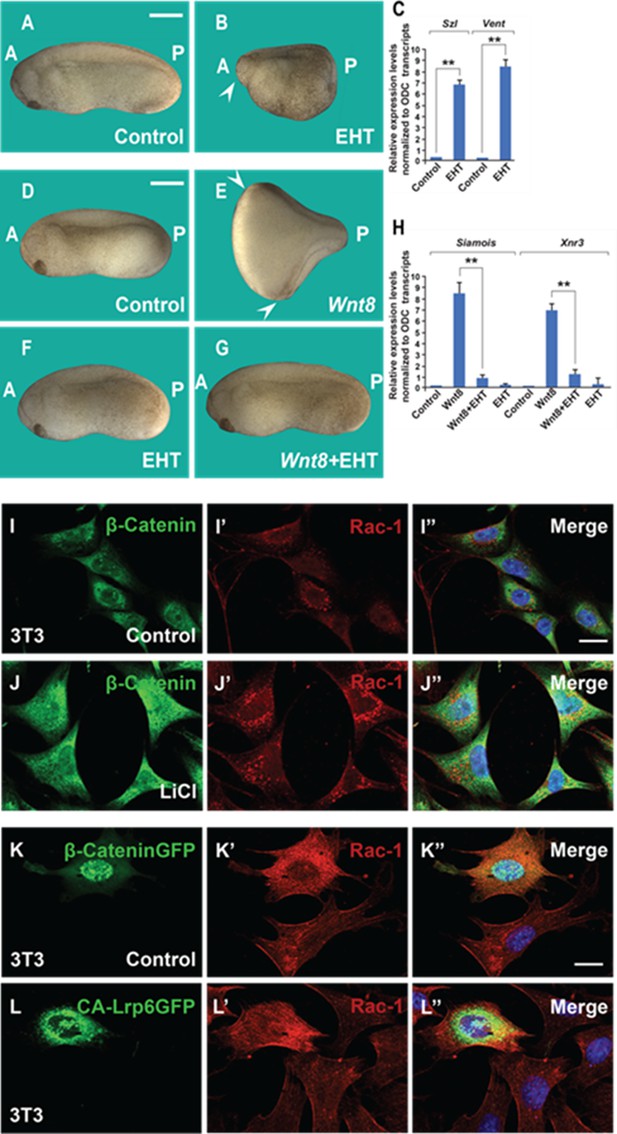
The Rac1 inhibitor EHT1864 blocks Wnt signaling in Xenopus embryos, and Rac1 levels are stabilized by treatments that increase Wnt/β-catenin signaling.
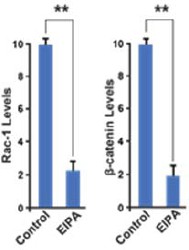
(A) Uninjected control embryo. (B) Incubation of the Xenopus embryos with the Rac1 inhibitor EHT at 32-cell stage (7 min, 10 mM) resulted in a ventralized phenotype with a small head in the anterior (A, arrowhead) and expanded ventral structures in the posterior (P). Rac1 activity is required for macropinocytosis, see Figure 4—figure supplement 1. (C) qRT-PCR of gastrula stage embryos showing increased ventral markers Szl and Vent1 after Rac1 inhibition. (D) Control embryo. (E) Injection of Wnt8. mRNA (2 pg) induces complete duplicated axes (arrows). (F) Injected embryos with EHT (1 mM, 4 nl 1x ventral) alone showed no phenotypic effect at this concentration. (G) EHT co-injected with Wnt8 mRNA blocked double axis formation. (H) qRT-PCR of Wnt target genes Siamois and Xnr3 at blastula confirming that Rac1 is required for early Wnt signaling. (I–J’’) Treatment of 3T3 cells with 40 mM Lithium Chloride (LiCl) increases β-catenin and Rac1 levels. (K–L’’) Transfection of 3T3 cells with stabilized constitutively active forms of β-cateninGFP or Lrp6GFP increased levels of Rac1 protein in transfected cells compared to untransfected ones. The numbers of embryos analyzed were as follows: A = 52, 100%; B = 47, 95% with ventralized small head phenotype; D = 58, 100%; E = 67; 97%; F = 64, 96%; G = 62, 97%, four independent experiments. Scale bars for embryos 500 μm; scale bars for immunofluorescence, 10 μm. qRT-PCR experiments represent biological replicates. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01).
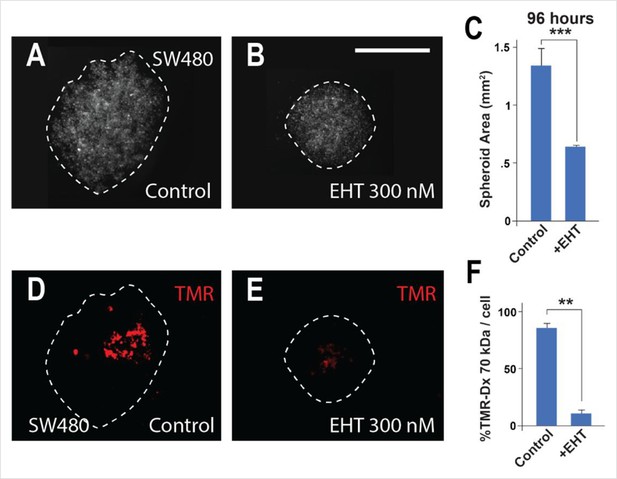
Macropinocytosis is inhibited in SW480 colorectal cancer (CRC) spheroids by treatment with the Rac1 inhibitor EHT.
(A) Control SW480 spheroids at 96 hr of inverted drop culture. (B) Treatment with 300 nM Rac1 inhibitor (EHT1864) reduced the diameter of SW480 spheroids at 96 hr. (C) Quantification of spheroid area after EHT treatment. (D) The same spheroids incubated with the macropinocytosis marker TMR-dextran 70 kDa for 1 hr. (E) EHT treatment strongly inhibited macropinocytosis. (F) Quantification of macropinocytosis inhibition. Six spheroids were plated per condition, in triplicate. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01) (***p < 0.001) (scale bars, 500 μm).
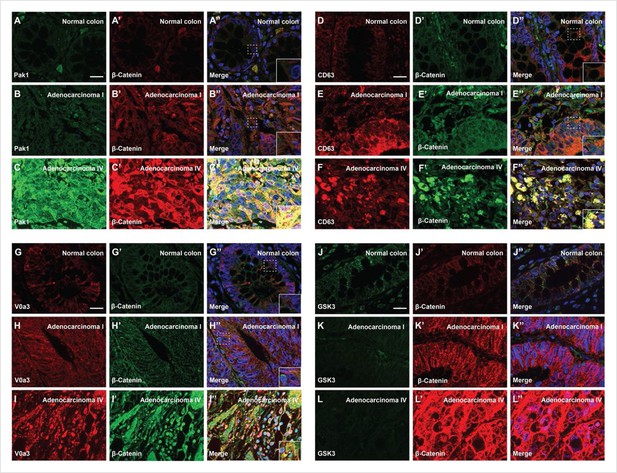
Degree of human colorectal cancer (CRC) malignancy is associated with increased macropinocytosis/multivesicular body (MVB)/lysosome markers and decreased GSK3 levels.
(A–A’) Normal human colon paraffin section stained with the macropinocytosis marker Pak1 and β-catenin, respectively.(A’’) Merged image with DAPI (4’,6-diamidino-2-phenylindole), a few cells colocalize both markers. (B–B’’) Pak1 and β-catenin levels are moderately increased at an early stage I CRC adenocarcinoma. (C’–C’’) Strong colocalization between Pak1 and β-catenin was observed in advanced stage IV CRC (inset). (D–D’’) Normal human colon section stained with the MVB marker CD63 and β-catenin; colocalization was not observed. (E–E’’) CD63 and β-catenin were stabilized in adenocarcinoma I, and moderate colocalization was found between CD63 and β-catenin. (F–F’) CD63 and β-catenin were strongly stabilized and colocalized in adenocarcinoma stage IV CRC. (F’’) Merge; note the striking colocalization between the MVB marker CD63 and β-catenin in advanced stages of cancer (inset). (G–G’’) Normal colon stained for V0a3 (a subunit of V-ATPase that marks lysosomes) and β-catenin. (H–H’’) Stage I adenocarcinoma with moderately increased levels of lysosomes and β-catenin. (I–I’’) Strong co-localization of lysosomes and β-catenin in stage IV CRC (see inset). (J–J’’) Human colon array stained with the GSK3 and β-catenin. (K–K’’) GSK3 decreases, and β-catenin increases, at early stages of carcinogenesis, (L–L’’) GSK3 levels are very low in advanced CRC compared to normal human colon, while β-catenin levels are very high. Scale bars, 10 μm. Also see Figure 5—figure supplement 1 for quantifications and mouse xenografts of CRC cell.
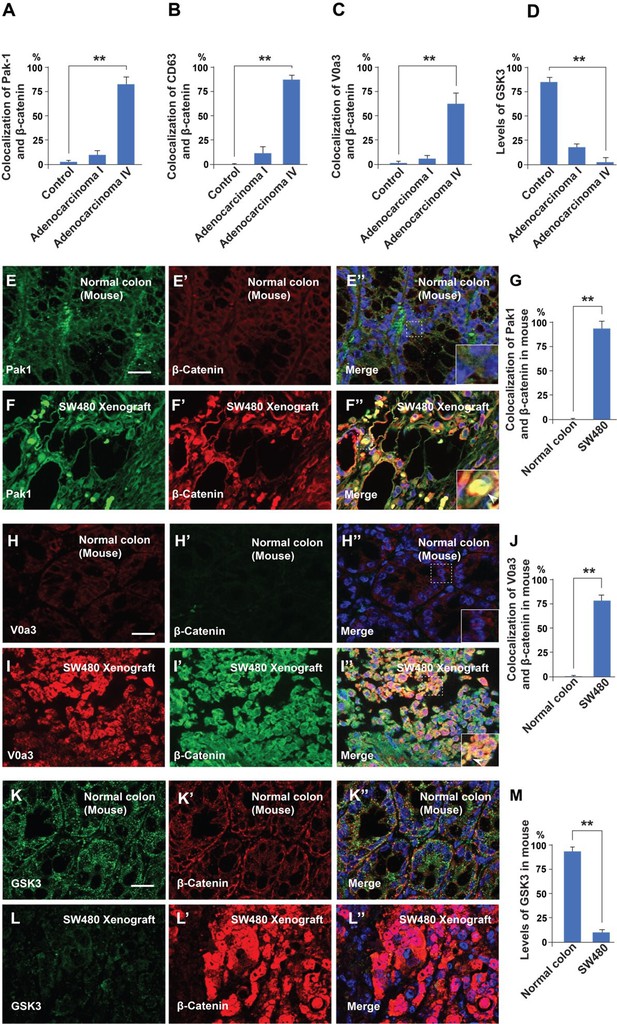
Malignancy of colorectal cancer positively correlates with macropinocytosis, multivesicular body (MVB), and lysosome markers, and inversely correlates with GSK3 levels.
(A–D) Quantification of the colocalization between Pak1, CD63, V0a3, and GSK3 with β-catenin in normal colon and at different stages of cancer from the histological sections shown in Figure 5. (E–E’ ) Immunohistochemistry of normal colon tissue from the CD1 NU/NU nude mice stained for the macropinocytosis marker Pak1 and β-catenin. (E’’) Merge with DAPI. (F–F’’) SW480 xenograft showing correlation between high levels of Pak1 and β-catenin colocalization (see inset). (G) Quantification of colocalization of Pak1 and β-catenin. (H–H’’) Control colon immunostained for V0a3 and β-catenin. (I–I’’) SW480 tumor showing a strong increase in the lysosomal marker V0a3 and β-catenin. (K–K’) Normal colon sample from mouse stained for GSK3 and β-catenin staining. Note that normal colon has GSK3 staining. (L–L’’) SW480 xenograft tumor showing reduced levels of GSK3, and high levels of β-catenin. (M) Quantification of the levels of GSK3 in normal conditions and in tumor cells; note that the decrease in GSK3 levels supports the GSK3 sequestration model of Taelman et al., 2010. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01). (Scale bars, 10 μm). Human tissue arrays were quantified in triplicate fields.
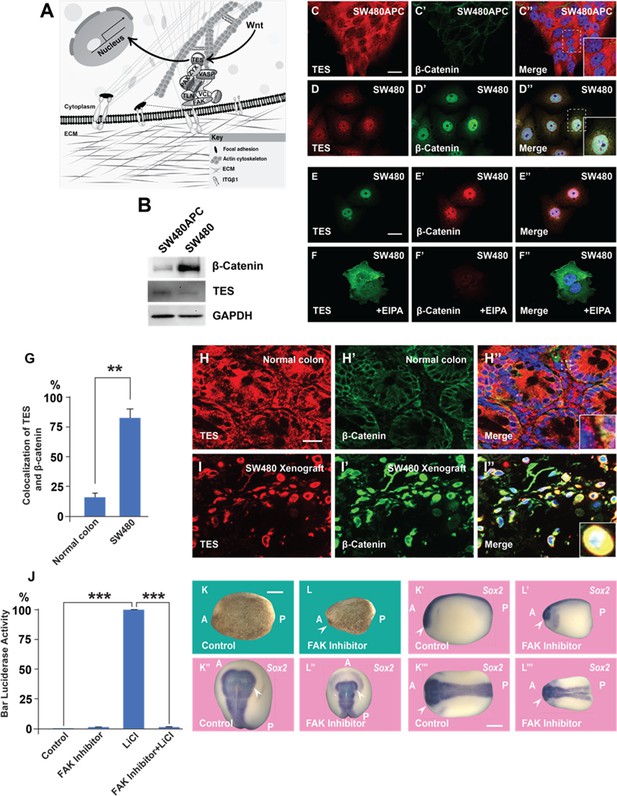
A focal adhesion protein changes its nucleocytoplasmic distribution after Wnt signaling activation, and focal adhesion kinase inhibition affects Wnt/β signaling.
(A) Diagram showing how focal adhesions connect the actin cytoskeleton to the extracellular matrix via integrins; Wnt signaling changes the distribution of the focal adhesion protein Tes from focal adhesion sites to the nucleus. (B) Western blot of SW480 cells with and without stable expression of Adenomatous Polyposis Coli (APC); note the reduction in Tes expression and increase in β-catenin levels in SW480 cells lacking full-length APC. GADPH was used as loading control. (C–C’) Colon cancer cell line SW480 stably restored with full-length APC stained with Tes and β-catenin antibodies. Note that in SW480APC cells TES is absent from the nucleus (inset). (D–D’’) In SW480 colorectal cancer (CRC) cells both Tes and β-catenin are located the nucleus (inset). (E–E’’) Colon cancer cell line SW480 stained with Tes (in green) and β-catenin (in red) antibodies as a control. (F–F’’) Inhibiting macropinocytosis with 5-(N-Ethyl-N-isopropyl) Amiloride (EIPA) (40 μM) treatment restored the cytoplasmic localization of the focal adhesion protein Tes in SW480 cells and strongly inhibited β-catenin levels. (G) Quantification of colocalization between Tes and β-catenin in normal mouse colon and SW480 xenografts. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01) (H–H’) Immunohistochemistry of the CD1 NU/NU normal mouse colon showing Tes and β-catenin distribution. (H’’) Merge showing modest co-localization (inset). (I–I’’) Immunohistochemistry images of the CD1 NU/NU mouse xenograft model showing nuclear TES, which is strongly colocalized with β-catenin levels in transplanted human SW480 cancer cells (inset). Scale bars, 10 μm. (J) Reporter assay in HEK293T cells stably expressing β-catenin activity reporter (BAR) and Renilla, showing strong inhibition of the β-catenin transcriptional activity induced by Lithium Chloride (LiCl) (40 mM) by focal adhesion kinase (FAK) inhibitor PF-00562271 (20 μM) after overnight treatment. Error bars denote standard error of the mean (SEM) (n ≥ 3) (***p < 0.001). (K–L’’’) Immersion of Xenopus embryos in FAK inhibitor (7 min at 32-cell stage) inhibits head and dorsal development. Bright field and several angles of in situ hybridization with pan-neural marker Sox2 are shown to demonstrate reduction in brain structures indicated by the arrowhead; scale bar 500 μm.
-
Figure 6—source data 1
Original files with the uncropped western blots used in Figure 6B.
- https://cdn.elifesciences.org/articles/89141/elife-89141-fig6-data1-v1.zip
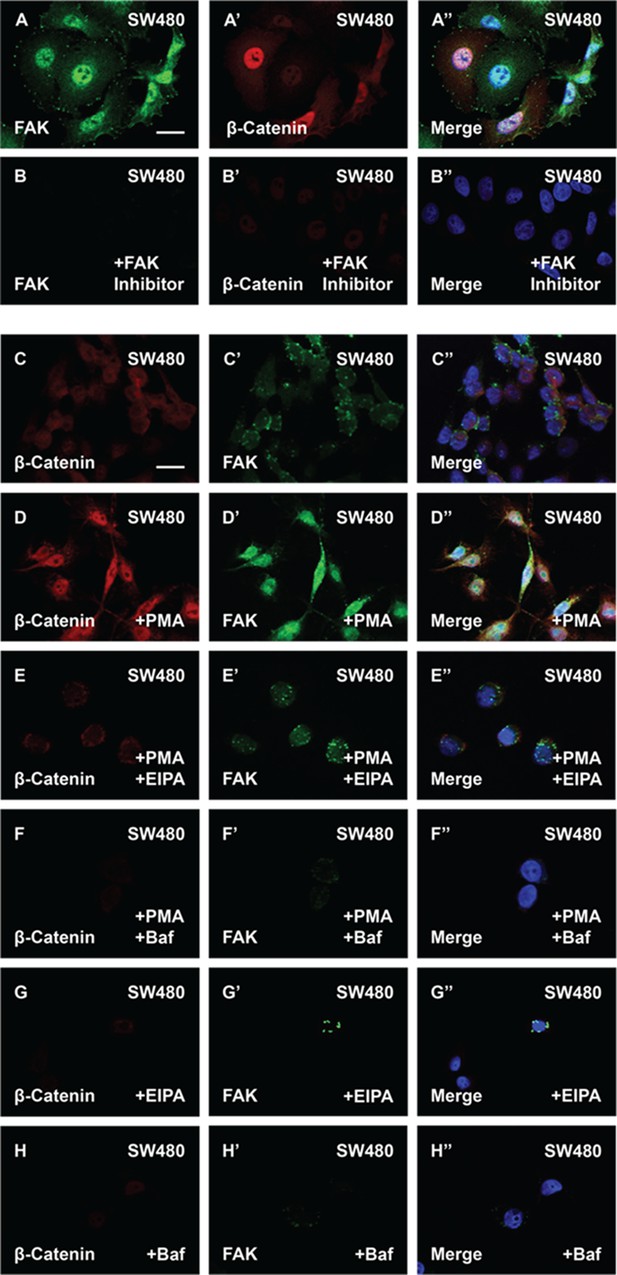
The phorbol ester phorbol 12-myristate 13-acetate (PMA) stabilizes focal adhesion kinase (FAK) and enhances β-catenin levels in cells with constitutive Wnt signaling.
(A–B’’) FAK inhibitor PF-00562271 (100 nM) reduces FAK and β-catenin immunostaining levels. (C–D’’) SW480 cells treated with PMA showed increased in β-catenin and FAK levels. (E–E’’) 5-(N-Ethyl-N-isopropyl) Amiloride (EIPA) (40 μM) overnight treatment blocked the effect of PMA in SW480 cells. (F–F’’) Baf (500 nM) treatment also blocked the effect of PMA in colorectal cancer (CRC) cells. (G–G’’) EIPA treatment alone was sufficient to reduce β-catenin and FAK levels in SW480 cells. (H–H’’) Baf treatment also reduced β-catenin and FAK protein levels in SW480 cells. Experiments in cultured cells represent biological triplicates. Error bars denote standard error of the mean (SEM) (n ≥ 3) (**p < 0.01). Scale bars, 10 μm.
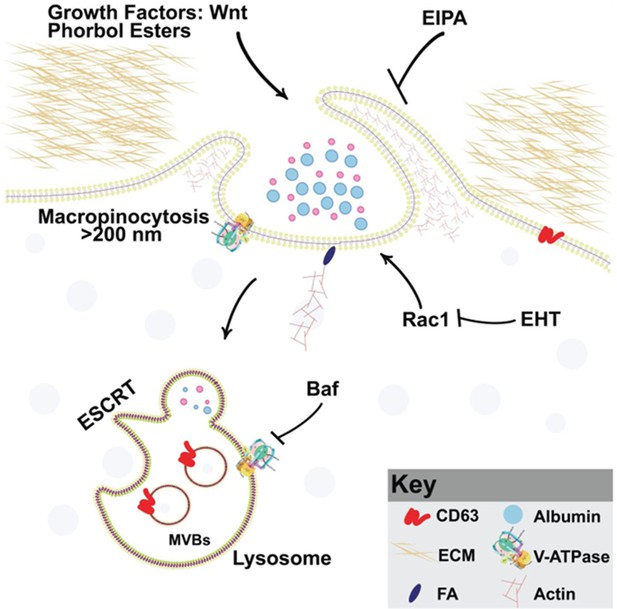
Model of phorbol 12-myristate 13-acetate (PMA) synergy with Wnt signaling through macropinocytosis and membrane trafficking Wnt and PMA are activators of macropinocytosis, which is a cell drinking process driven by an actin meshwork that requires the small GTPase Rac1.
Membrane trafficking into multivesicular bodies (MVBs)/lysosomes (marked by CD63) requires acidification by V-ATPase, which is inhibited by Bafilomycin A (Baf). Phorbol esters such as PMA stimulate macropinocytosis and synergize with Wnt signaling. Inhibiting macropinocytosis with the membrane trafficking inhibitors 5-(N-Ethyl-N-isopropyl) Amiloride (EIPA), Baf, or the Rac1 inhibitor EHT1864 block Wnt signaling and its cooperation with the tumor promoter PMA.
Videos
An SW480 colorectal cancer (CRC) cell before and after treatment with phorbol 12-myristate 13-acetate (PMA) (0.3 µM); the plasma membrane was marked by transfection of myristoylated-GFP (mGFP) and filmed for 15 min.
Note that PMA greatly enhanced plasma membrane vesicular activity typical of macropinocytosis. Control cell was treated with DMSO alone and shows low but detectable macropinocytosis cup formation. The arrowheads indicate two prominent macropinocytosis vesicles.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HeLa (human cervical adenocarcinoma) | ATCC | RRID: CVCL_0030 | |
| Cell line (Homo sapiens) | SW480 (human colorectal) | ATCC | RRID:CVCL_0546 | |
| Biological sample (Xenopus laevis male) | Sperm | Xenopus I | 5215 Albino Xenopus laevis Sexually Mature Male/-/-/M | Used for fertilization |
| Biological sample (Xenopus laevis female) | Egg | Xenopus I | 4280 Sexually Mature Female 10.5–11 cm/-/-/F | Used for fertilization |
| Biological sample (CD1 NU/NU nude mouse female) | Charles River Laboratories | Xenograft | ||
| Antibody | Pak1 antibody rabbit polyclonal | Abcam | Cat# ab131522, RRID:AB_11156726 | IF (1:100) |
| Antibody | FAK antibody rabbit polyclonal | Cell Signaling | Cat# 3285, RRID:AB_ 2269034 | IF (1:100) |
| Antibody | Rac-1 antibody rabbit polyclonal | Thermo Fisher | Cat# PA1-091 | IF (1:100) |
| Antibody | CD63 antibody mouse monoclonal | Abcam | Cat# ab59479 RRID:AB_ 940915 | IF (1:100) |
| Antibody | TES antibody rabbit polyclonal | ATLAS | Cat# HPA018123 RRID:AB_1857900 | IF (1:100) |
| Antibody | GSK3 antibody mouse monoclonal | Abcam | Cat# ab93926, RRID:AB_10563643 | IF (1:100) |
| Antibody | Transferrin receptor (TfR) antibody mouse monoclonal | Thermo Fisher | Cat# 13-6800 | IF (1:100) |
| Antibody | ATP6V0a3 antibody rabbit polyclonal | Novus | Cat# nbp1-89333 RRID:AB_11016312 | IF (1:100) |
| Antibody | GAPDH antibody rabbit monoclonal | Cell Signaling | mAb #2118 | WB (1:1000) |
| Antibody | β-Catenin antibody rabbit polyclonal | Thermo Fisher Scientific | Cat# 71-2700; RRID:AB_ 2533982 | IF (1:100) |
| Antibody | β-Catenin antibody mouse monoclonal | Thermo Fisher Scientific | Cat# MA1-2001; RRID:AB_ 326078 | IF (1:100) |
| Antibody | IgG, HRP-linked antibody (horse anti-mouse polyclonal) | Cell Signaling | Cat# 7076; RRID:AB_330924 | WB (1:2500) |
| Antibody | IgG, HRP-linked antibody (goat anti-mouse polyclonal) | Cell Signaling | Cat# 7074; RRID:AB_2099233 | WB (1:2500) |
| Antibody | IgG H&L (Alexa Fluor 594) preadsorbed (goat anti-mouse polyclonal) | Abcam | Cat# ab150120; RRID:AB_2631447 | IF (1:200) |
| Antibody | IgG H&L (Alexa Fluor 594) preadsorbed (goat anti-rabbit polyclonal) | Abcam | Cat# ab150084; RRID:AB_ 2734147 | IF (1:200) |
| Antibody | IgG H&L (Alexa Fluor 488) preadsorbed (goat anti-mouse polyclonal) | Abcam | Cat# ab150117; RRID:AB_ 2734147 | IF (1:200) |
| Antibody | IgG H&L (Alexa Fluor 488) preadsorbed (ab150081) (goat anti-rabbit polyclonal) | Abcam | Cat# ab150081; RRID:AB_2734747 | IF (1:200) |
| Antibody | IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (goat anti-mouse polyclonal) | Invitrogen | Cat# A-11001; RRID:AB_2534069 | IF (1:200) |
| Antibody | IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 (goat anti-rabbit polyclonal) | Invitrogen | Cat# A-11011; RRID:AB_143157 | IF (1:200) |
| Recombinant DNA reagent | Dominant-negative (DN)-GSK3-GFP (Xenopus laevis) (plasmid) | Addgene | RRID:Addgene_29681 | K85R and K86R |
| Recombinant DNA reagent | DN-Rab7 (Homo sapiens) (plasmid) | Addgene | RRID:Addgene_12660 | Dominant-negative plasmid |
| Recombinant DNA reagent | pCS2-mGFP (plasmid) | Addgene | RRID:Addgene_14757 | Membrane bound form of EGFP |
| Recombinant DNA reagent | CD63-RFP (Homo sapiens) (plasmid) | Addgene | RRID:Addgene_62964 | RFP tag |
| Recombinant DNA reagent | xWnt8myc (wnt8a.L Frog) (plasmid) | Addgene | RRID:Addgene_16863 | |
| Recombinant DNA reagent | β-Catenin-activated reporter (BAR) (plasmid) | Addgene | RRID:Addgene_12456 | Beta-catenin reporter. TCF/LEF sites upstream of a luciferase reporter |
| Recombinant DNA reagent | Renilla reporter (plasmid) | Addgene | RRID:Addgene_62186 | MuLE (Multiple Lentiviral Expression) Entry vector containing a CMV promoter and renilla luciferase module |
| Recombinant DNA reagent | Lipofectamine 3000 | Thermo Fisher | Cat# L3000001 | |
| Peptide, recombinant protein | Wnt3a | Peprotech | Cat# 315-20 | |
| Commercial assay or kit | mMESSAGE mMACHINE SP6 Transcription Kit | Thermo Fisher | Cat# AM1340 | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | Cat# E1500 | |
| Chemical compound, drug | Fibronectin | Thermo Fisher | Cat# 33016015 | |
| Chemical compound, drug | Dextran Tetramethylrhodamine (TMR-Dx) 70,000 | Thermo Fisher | Cat# D1818 | |
| Chemical compound, drug | 5-(N-Ethyl-N-isopropyl) amiloride (EIPA) | Sigma | Cat# A3085 | |
| Chemical compound, drug | Bafilomycin A1 | Selleckchem | Cat# S1413 | |
| Chemical compound, drug | Digitonin | Sigma | Cat# 300410 | |
| Chemical compound, drug | Phalloidin | Abcam | Cat# ab176759 | |
| Chemical compound, drug | Lithium chloride (LiCl) | Sigma | Cat# L4408 | |
| Chemical compound, drug | PF-00562271 (FAK inhibitor) | Selleckchem | Cat# S2672 | |
| Chemical compound, drug | Phorbol 12-myristate 13-acetate (PMA) | TOCRIS | Cat# 1201 | |
| Chemical compound, drug | 5-(N-Ethyl-N-isopropyl) amiloride (EIPA) | Sigma | Cat# A3085 | |
| Chemical compound, drug | EHT 1864 | Selleckchem | Catalog# S7482 | |
| Chemical compound, drug | Triton X-100 | Thermo Fisher | Cat# HFH10 | |
| Chemical compound, drug | Paraformaldehyde | Sigma | Cat# P6148 | |
| Chemical compound, drug | Fibronectin | Sigma | Cat# F4759 | |
| Chemical compound, drug | Protease inhibitors | Roche | Cat# 04693132001 | |
| Chemical compound, drug | Phosphatase inhibitors | Calbiochem | Cat# 524629 | |
| Software, algorithm | ImageJ | NIH | http://imagej.nih.gov/ij/ | |
| Software, algorithm | AxioVision 4.8 | Zeiss | http://Zeiss.com | |
| Software, algorithm | Zen 2.3 imaging software | Zeiss | http://Zeiss.com | |
| Software, algorithm | R | R Core Team | https://cran.r-project.org | |
| Other | 10 cm dish | Thermo Fisher | Cat# 174903 | |
| Other | 8-Well glass-bottom chamber slides | ibidi | Cat# 80827 | |
| Other | Circular coverslips | ibidi | Cat# 10815 | |
| Other | 12-Well dish | Thermo Fisher | Cat# 150628 | |
| Other | DMEM (culture medium) | Thermo Fisher | Cat# 11965092 | |
| Other | L-15 (culture medium) | Thermo Fisher | Cat# 11415064 | |
| Other | Glutamine | Thermo Fisher | Cat# 25030081 | |
| Other | Fetal bovine serum (FBS) | Thermo Fisher | Cat# 16000044 | |
| Other | Bovine serum albumin (BSA) | Thermo Fisher | Cat# 9048468 | |
| Other | Pen-Strep antibiotics | Thermo Fisher | Cat# 15140122 | |
| Other | PBS | Gibco | Cat# 10-010-023 | |
| Other | PBS | Fisher Scientific | Cat# BP3994 | |
| Other | Fluoroshield Mounting Medium with DAPI | Abcam | Cat# ab104139 | |
| Other | IM 300 microinjection pump | Narishige International USA, Inc | N/A | |
| Other | Axio Observer Z1 Inverted Microscope with Apotome | Zeiss | N/A |