The reciprocal regulation between mitochondrial-associated membranes and Notch signaling in skeletal muscle atrophy
Figures
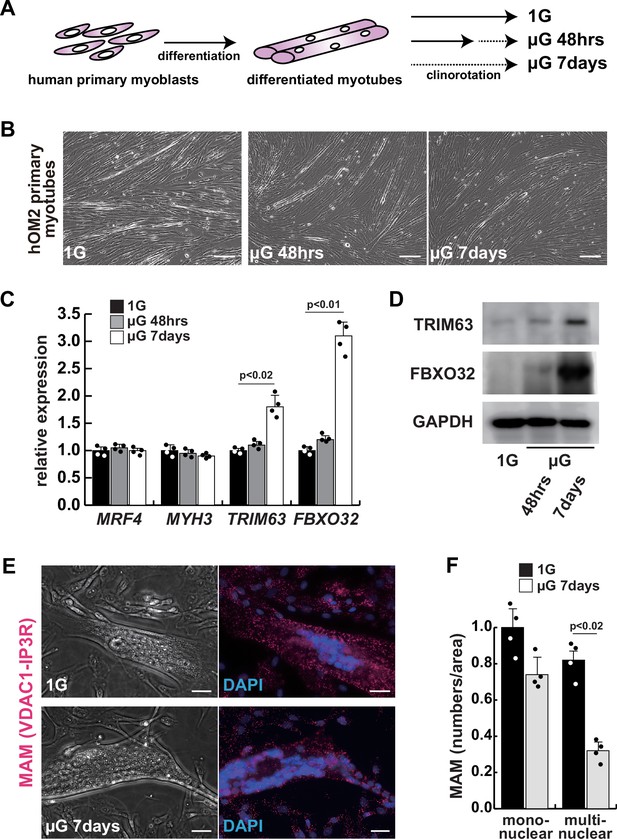
The integrity of the mitochondrial-associated endoplasmic reticulum (ER) membrane is compromised by microgravity in differentiated primary human myotubes.
(A) A diagrammatic representation of differentiated human myotubes under normal conditions or exposed to clinorotation. µG, microgravity. (B) Phase contrast images of differentiated primary human myotubes under 1G (upper panel) and µG for 48 hr (left lower panel), for 7 days (right lower panel). Scale bars: 50 µm. (C) Transcription levels of MRF4 (myogenic determination), MYH3 (early differentiation), TRIM63, and FBXO32 (muscle atrophy) in differentiated myotubes with or without microgravity, measured by RT-qPCR and presented relative to transcripts of ribosomal protein RPL13a. (D) Western blotting analyses of lysates from differentiated myotubes with or without microgravity. Nuclear lysates were analyzed with antibodies against TRIM63 (40 kDa) and FBXO32 (42 kDa). GAPDH was used as a loading control (36 kDa). (E) Phase contrast images and detectable adjacent mitochondria-associated ER membranes (MAM) by proximal ligation assay in differentiated myotube. Scale bars: 20 µm. (F) The number of MAM in mononuclear myoblasts (mononuclear) or multinucleated fused myotubes (multinuclear) per fixed area. All error bars indicate ± SEM (n=4). p-Values are determined by non-parametric Wilcoxon tests or one-way ANOVA and Tukey’s test for comparisons.
-
Figure 1—source data 1
Original western blotting images of Figure 1D with anti-GAPDH, anti-TRIM63, and anti-FBXO32 antibodies.
- https://cdn.elifesciences.org/articles/89381/elife-89381-fig1-data1-v1.zip
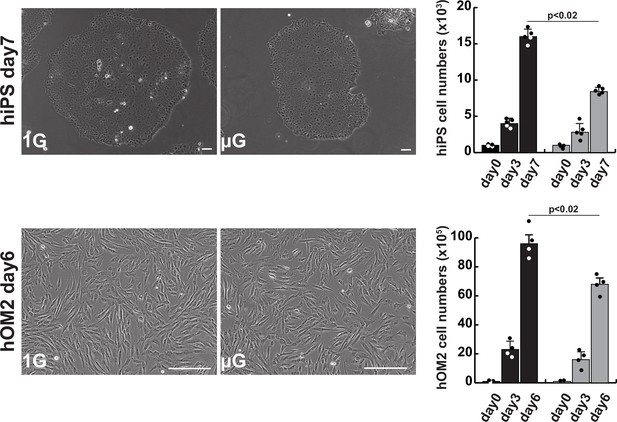
The effects of microgravity on cell proliferation in human induced pluripotent stem (iPS) cells and hOM2 primary myogenic cells.
Phase contrast images showing cell morphology diagram after approximately 1 week of cell culture in normal (1G) or microgravity environment (µG) (left panels), and histograms showing the number of proliferating cells in cell culture (right). Scale bars: 100 µm. All error bars indicate ± SEM (n=5). p-Values are determined by one-way ANOVA and Tukey’s test for comparisons.
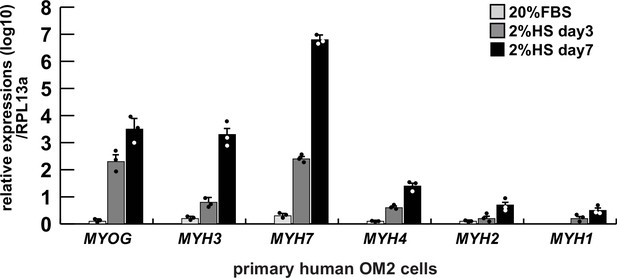
Comparative expression levels of myogenic genes in differentiated human primary hOM2 myogenic cells.
Differentiated primary human OM2 cells were examined by RT-qPCR for transcription of the myogenic differentiation markers MYOGENIN (MYOG), early differentiated myosin heavy chain 3 (MYH3), and the type 1 slow muscle marker MYH7, as well as the fast muscle markers MYH4, MYH2, and MYH1 (type 2b, type 2a, and type 2X, respectively), expressed relative to transcripts for the ribosomal protein RPL13a. FBS, fetal bovine serum, HS, horse serum.

Apoptotic assays in differentiated hOM2 myotubes under microgravity.
(A) Phase contrast (left panels) and Immunofluorescent (middle and right panels) images of caspase-3 (CASP3, green) and DAPI (blue) staining with differentiated hOM2 cells on normal (1G) or microgravity conditions (µG for 7 days). Scale bars: 100 µm. (B) Western blotting with differentiated hOM2 cells against anti-phospho-AKT (p-AKT, 60 kDa) antibody with or without the treatment of microgravity. GAPDH (whole cell, 36 kDa) and AKT (pan, 60 kDa) are used as loading controls.
-
Figure 1—figure supplement 3—source data 1
Original western blotting images of Figure 1—figure supplement 3 with anti-phospho-AKT and anti-AKT antibodies.
- https://cdn.elifesciences.org/articles/89381/elife-89381-fig1-figsupp3-data1-v1.zip
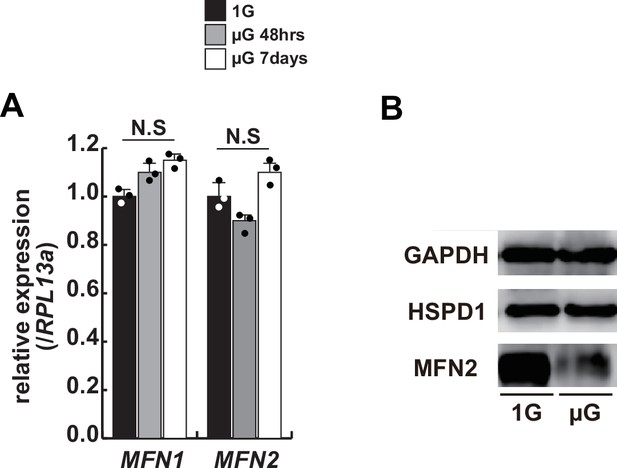
Transcription and translational levels of Mfn1/2 expression in differentiated hOM2 myotubes under microgravity.
(A) Relative transcript levels of MFN1 and MFN2 in differentiated hOM2 cells on normal (1 G) or microgravity conditions (µG 48 hr and 7 days). (B) Western blotting with differentiated hOM2 cells against anti-MFN2 antibody (80 kDa) with or without the treatment of microgravity. GAPDH (whole cell, 36 kDa) and HSPD1 (nuclear, 60 kDa) are used as loading controls.
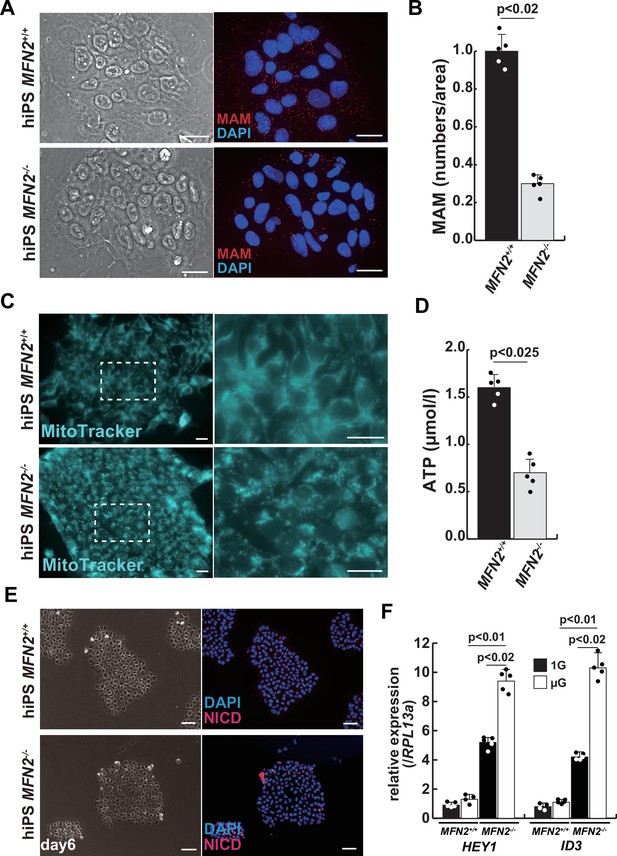
Mitochondrial abnormality and the activation of Notch in MFN2-deficient human induced pluripotent stem (iPS) cells.
(A) Phase contrast images (left panels) and mitochondrial-associated endoplasmic reticulum membrane (MAM) visualization (right panels) with or without MFN2 in human iPS cells. Red, MAM (IP3R-VDAC1 by proximity ligation assay [PLA]); blue, DAPI. Scale bars: 20 µm. (B) The quantitative analyses of MAM numbers per fixed area in human iPS cells with or without MFN2. (C) Mitochondrial morphology with MitoTracker in human iPS cells with or without MFN2 (right panels, magnified area outlined in each left panel). Scale bars: 50 µm. (D) Total adenosine triphosphate (ATP) production (µmol/l) in wildtype or MFN2-deficient human iPS cells. (E) Phase contrast (left images) and immunostaining for Notch intercellular domain (NICD; red) and DAPI (blue) on wildtype or MFN2-deficient human iPS cells (right images). Scale bars: 50 µm. (F) Relative transcription levels of HEY1 and ID3 in wildtype or MFN2-deficient human iPS cells under normal gravity (1G) or microgravity (µG). All error bars indicate ± SEM (n=5). p-Values are determined by non-parametric Wilcoxon tests for comparisons.

The generation of hMFN2-deficient human induced pluripotent stem (iPS) cells.
(A) A schematic diagram of chromosome1 where the human MFN2 gene resides, and the target site of MFN2 exon 3 containing start ATG sequences treated by the CRISPR/Cas9 system with single-strand oligodeoxynucleotide (ssODN), which have knock-in sequences (additional 1 base T or C). (B) The result of DNA sequencing showing single nucleotide addition (T/ or C, lower sequence) sequencing using knock-in human iPS cells treated with CRISPR/Cas9 and ssODN. (C) Immunofluorescent analysis with MFN2-knock-in human iPS cells (MFN2+T/+C) to check the undifferentiated state. NANOG (red), TRA-1–81 (TRA1, green), and DAPI (blue). Scale bar: 100 µm. (D) Immunofluorescent analysis with wildtype (MFN2+/+) and MFN2-knock-in human iPS cells (MFN2-/- as MFN2+T/+C) to check the expression of MFN2 protein. MFN2 (red), TRA1 (green), and DAPI (blue). Scale bars: 100 µm.
Next-generation sequencing (NGS) analyses comparing normal human induced pluripotent stem (hiPS) to deficient MFN2 (MFN2-/-) hiPS cells.
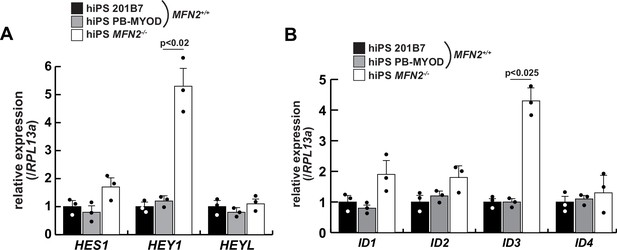
The relative expressions of Notch-related genes in MFN2-deficient human induced pluripotent stem (iPS) cells.
Transcripts of the HES family (A) and ID family (B), which are downstream of the Notch signaling pathway, were evaluated in wildtype (201B7 or PB-MYOD) and MFN2-deficient (MFN2-/-) human iPS cells. All error bars indicate ± SEM (n=3). p-Values are determined by one-way ANOVA and Tukey’s test for comparisons.
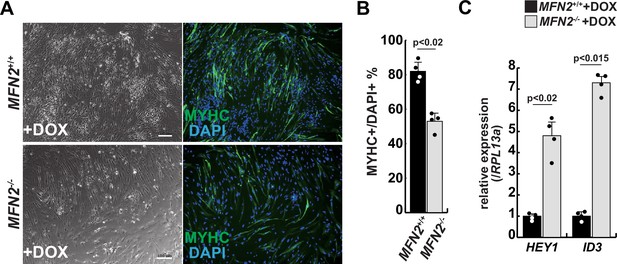
Differentiation of myogenic cells derived from MFN2-deficient human induced pluripotent stem (iPS) cells.
(A) Phase contrast (left panels) and immunofluorescent (right panels) images of myosin heavy chain (MYHC, green) and DAPI (blue) staining with control and mutant (MFN2-/-) differentiated human iPS cells after the administration of doxycycline (DOX). Scale bars: 100 µm. (B) The percentage of MYHC/DAPI-positive cells per fixed area in differentiated cells derived from wildtype or MFN2-deficient human iPS cells. (C) Relative level of HEY1 and ID3 transcripts in induced cells derived from human iPS cells with DOX treatment (MFN2+/+ or MFN2-/-+DOX). All error bars indicate ± SEM (n=4). p-Values are determined by non-parametric Wilcoxon tests for comparisons.
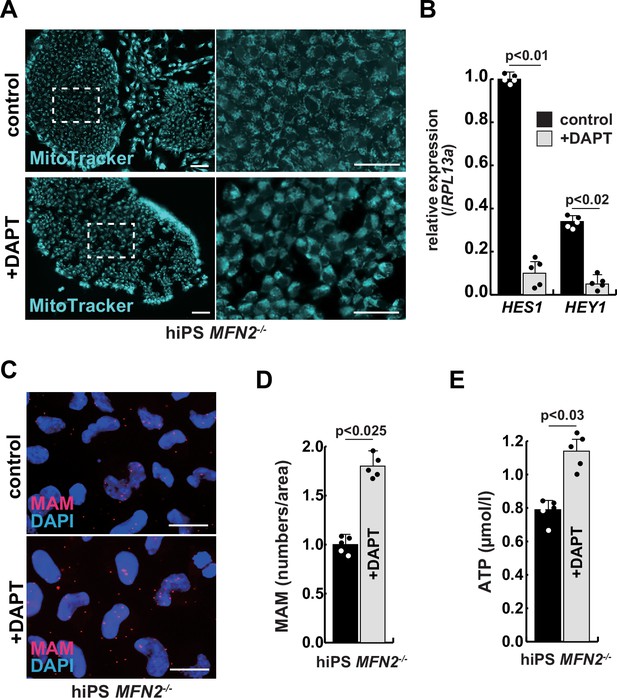
The improvement of mitochondrial abnormalities in MFN2-deficient human induced pluripotent stem (iPS) cells treated with gamma-secretase inhibitor DAPT.
(A) Mitochondrial morphology visualized by MitoTracker in MFN2-deficient human iPS cells with or without 50 µM of DAPT (right panels, magnified area outlined in right panels). Scale bars: 50 µm. (B) Relative transcription levels of HES1 and HEY1 in MFN2-deficient human iPS cells with or without DAPT. (C) Mitochondrial-associated endoplasmic reticulum membrane (MAM) visualization in MFN2-deficient human iPS cells, with or without DAPT. Red, MAM (IP3R-VDAC1 proximity ligation assay [PLA]), blue, DAPI. Scale bars: 20 µm. (D) The quantitative analyses of MAM numbers in MFN2-deficient human iPS cells with or without DAPT. (E) Total adenosine triphosphate (ATP) production in MFN2-deficient human iPS cells treated with or without DAPT. All error bars indicate ± SEM (n=5). p-Values are determined by non-parametric Wilcoxon tests for comparisons.
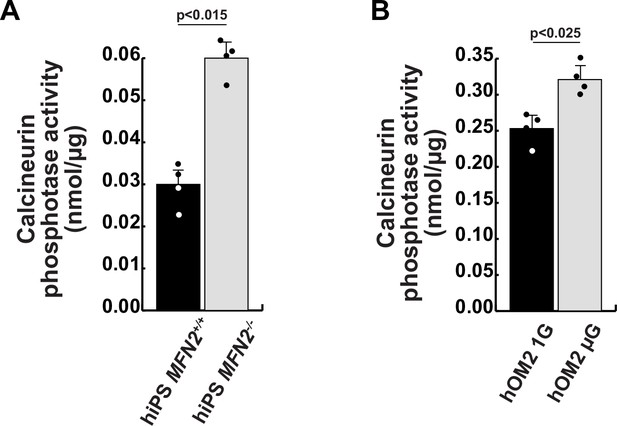
Calcineurin activity in human induced pluripotent stem (iPS) cells and differentiated muscle cell cultures.
(A) Calcineurin activity was monitored in wildtype (MFN+/+) and MFN2-deficient (MFN2-/-) human iPS cells. (B) Differentiated human hOM2 cells were utilized in both normal gravity (1G) and microgravity (µG) conditions. All error bars indicate ± SEM (n=4). p-Values are determined by non-parametric Wilcoxon tests for comparisons. *p<0.05.
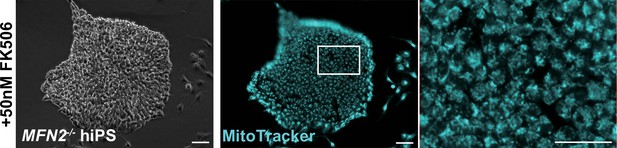
Mitochondrial morphological changes in MFN2-deficient human induced pluripotent stem (iPS) cells remained unaltered in the presence of FK506, a calcineurin inhibitor.
MFN2-deficient human iPS cells were treated with FK506, and the mitochondrial morphology was monitored using MitoTracker (blue, right panels). Phase contrast is shown on the left panels. Scale bars: 100 µm.
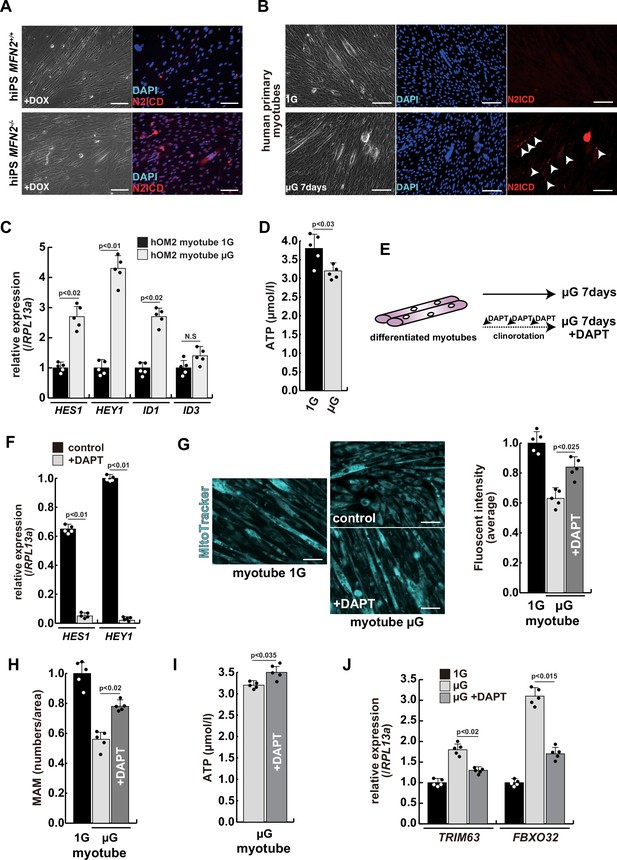
The atrophic phenotype of human myotubes under microgravity is alleviated by the gamma-secretase inhibitor DAPT.
(A) Phase contrast (left panels) and immunostaining (right panels) for NOTCH2 intercellular domain (N2ICD; red) and DAPI (blue) on differentiated myotubes derived from wildtype, or MFN2-deficient human induced pluripotent stem (iPS) cells by doxycycline (DOX) treatment. Scale bars: 50 µm. (B) Phase contrast images (left panels) and immunostaining for NOTCH2 intercellular domain (right panels, N2ICD; red) and DAPI (middle panels, blue) on differentiated myotubes under normal gravity (1G) or microgravity (µG) for 7 days. Scale bars: 50 µm. (C) Relative transcription levels of HES family genes (HES1, HEY1) and ID family genes (ID1, ID3) in differentiated myotubes derived from wildtype or MFN2-deficient human iPS cells after DOX treatment. (D) Total adenosine triphosphate (ATP) production in differentiated human primary myotube under normal gravity (1G) or microgravity (µG) for 7 days. (E) The schematic representation of differentiated human primary myotubes under microgravity (µG) for 7 days with or without DAPT. (F) Relative transcription levels of HES1 or HEY1 in differentiated human primary myotubes under microgravity for 7 days with or without DAPT. (G) Mitochondrial morphology with MitoTracker in differentiated human primary myotubes under normal gravity (1G; left panel) and microgravity with or without DAPT (µG; upper and lower left panels). The average fluorescent intensity of total cells treated with MitoTracker is indicated on the right. Scale bars: 50 µm. (H) Quantitative analyses of mitochondrial-associated endoplasmic reticulum membrane (MAM) numbers in differentiated human myotubes under microgravity with or without DAPT. (I) Total ATP production in differentiated human myotubes under microgravity with or without DAPT. (J) Relative transcription levels of TRIM63 and FBXO32 (muscle atrophy) in differentiated human primary myotubes under normal gravity (1G) and microgravity (µG) with or without DAPT for 7 days. All error bars indicate ± SEM (n=5). p-Values are determined by non-parametric Wilcoxon tests or one-way ANOVA and Tukey’s test for comparisons. N.S., not significant.

Notch2 was expressed at the highest level compared to other Notch family members in human myogenic cells.
Relative transcript levels of Notch1–4 genes in growing (20% FBS) and differentiated (2% HS for 7 days) human primary hOM2 cells. FBS, fetal bovine serum; HS, horse serum.

Myosin heavy chain expressions in differentiated hOM2 myogenic cells with or without DAPT under microgravity.
Relative transcript levels of MYH3, MYH7, and MYH1 genes in differentiated human primary hOM2 cells. 1G, under normal gravity; µG, under microgravity, µG+DAPT; with DAPT under microgravity, N.S.; not significant.
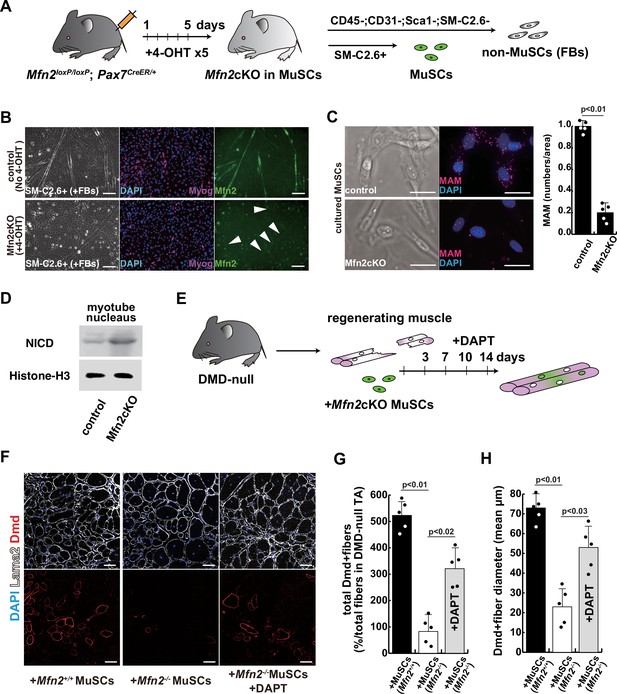
The regenerative capacity of Mfn2-deficient mouse muscle is reduced and that of Mfn2-deficient muscle stem cells (MuSCs) when transplanted into dystrophic muscle in vivo is improved by DAPT treatment of the muscle.
(A) The flowchart to isolate MuSCs (SM-C/2.6+) and non-myogenic fibroblasts (FBs) derived from conditionally Mfn2-knockout mice after 4-OH tamoxifen (4-OHT) injection. (B) Immunostaining for Mfn2 (green), myogenin (Myog; red), and DAPI (blue) on differentiated myotubes derived from wildtype or Mfn2-deficient mouse MuSCs sorted as SM-C/2.6-positive cells, co-cultured with non-myogenic FBs. Scale bars: 50 µm. (C) Phase contrast images (left panels) and mitochondrial-associated endoplasmic reticulum membranes (MAMs) visualization (right panels) and quantitative analyses of MAM numbers (right) on cultured MuSCs. Red, MAM (IP3R-VDAC1 proximity ligation assay [PLA]), blue, DAPI. Scale bars: 20 µm. (D) Western blotting analyses of lysates from control and Mfn2-mutant cultured MuSCs. Nuclear lysates were analyzed with antibodies against Notch intercellular domain (NICD, 80 kDa). Histone H3 was used as a loading control (15 kDa). (E) The flowchart for the transplantation into tibialis anterior (TA) muscles of DMD-/y mice (12 weeks of age) with Mfn2-deficient MuSCs (1.0×104 cells) and the treatment with DAPT every 3–4 days after the transplantation. (F) Transverse sectional images of TA muscles 14 days after the transplantation with the same number of MuSCs sorted as SM/C-2.6-positive cells derived from wildtype or conditional Mfn2-knockout mice. Immunostaining for Dystrophin (Dmd, red as transplanted areas), laminin-a2 (Lama2, white to show the outline of myofibers), and DAPI (blue) on engrafted TA muscle after the transplantation. Scale bars: 50 µm. (G) The quantification of the total number of Dystrophin-positive (Dmd+) regenerated myofibers on the section transplanted with an equivalent number of normal or Mfn2-deficient MuSCs, with or without the treatment of the transplanted muscle with DAPT. (H) The average diameter of Dystrophin-positive (Dmd+) myofibers that are contributed by the transplanted MuSCs, as described in (G). All error bars indicate ± SEM (n=5). p-Values are determined by non-parametric Wilcoxon tests or one-way ANOVA and Tukey’s test for comparisons.
-
Figure 5—source data 1
Original western blotting images of Figure 5D with anti-Histone H3 and anti-Notch intercellular domain (NICD) antibodies.
- https://cdn.elifesciences.org/articles/89381/elife-89381-fig5-data1-v1.zip
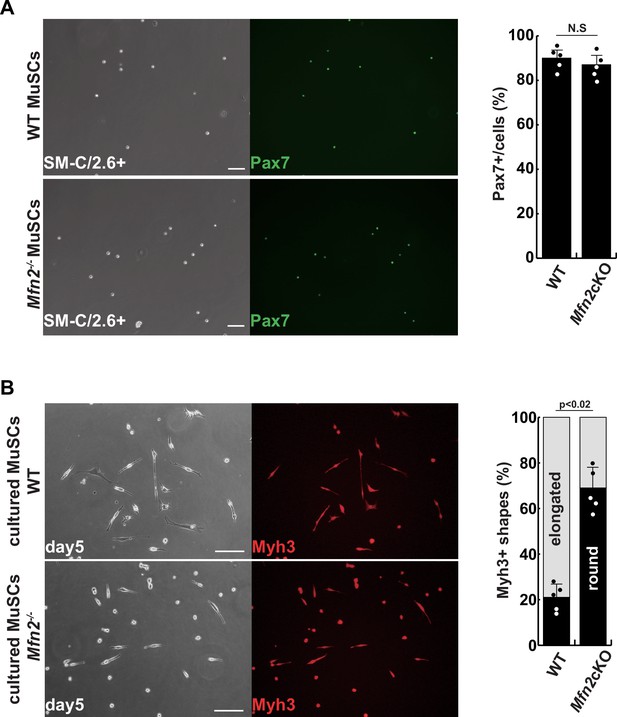
Isolated muscle stem cells (MuSCs) derived from conditional Mfn2-knockout mice.
(A) Phase contrast (left panels) and immunofluorescent (right panels) images of isolated MuSCs as SM-C/2.6-positive cells derived from each tibialis anterior (TA) muscle, with anti-Pax7 (green) as a marker of MuSCs, and the proportion of Pax7-positive cells among all isolated cells (right). Scale bars: 50 µm (B) Phase contrast (left panels) and immunofluorescent (right panels) images of cultured MuSCs for 5 days with anti-Myh3 (red) as a marker of differentiated muscle cells (left), and the proportion of Myh3-positive, non-elongated, round-type myogenic cells (right). Scale bars: 50 µm. All error bars indicate ± SEM (n=4). p-Values are determined by non-parametric Wilcoxon tests for comparisons. N.S., not significant.
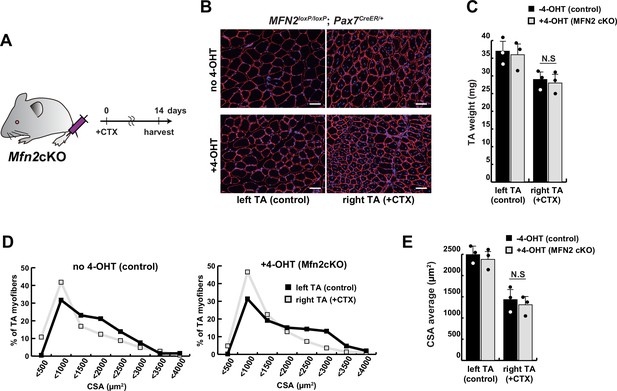
The regenerative capacity of conditional Mfn2-knockout mice, specifically in muscle stem cells, did not exhibit any significant alteration following a single muscle injury.
(A) Experimental design with Mfn2 conditional knockout mice (Mfn2cKO) for cardiotoxin (CTX) injection and sample collection after 14 days. (B) Immunofluorescence of laminin-2a (red) and DAPI (blue) on transverse sections in the tibialis anterior (TA) muscle of conditional Mfn2cKO (Mfn2loxP/loxP; Pax7CreERT2/+) mice with or without 4-hydroxytamoxifen (4-OHT) at 12 weeks of age. Scale bars: 50 µm. (C) TA weight of control left legs and CTX-injected right legs with or without 4-OHT. (D) Distribution of cross-sectional areas (CSA) of myofibers in each TA muscle. (E) Average CSA of each TA sample. All error bars indicate ± SEM (n=3). N.S., not significant.
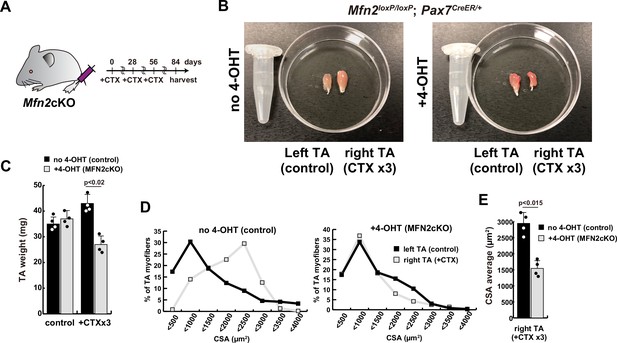
Reduced muscle hypertrophy in conditional Mfn2-knockout mice after several cardiotoxin (CTX)-induced muscle injuries.
(A) Experimental design utilizing Mfn2 conditional Mfn2cKO (Mfn2loxP/loxP; Pax7CreERT2/+) mice, involving three rounds of CTX injection to each right tibialis anterior (TA) muscle at 28-day intervals, with sample collection conducted 84 days after the initial CTX injection. (B) Photographs of TA muscles after the dissection. (C) TA weight of control left legs and CTX-injected right legs with or without 4-OHT. (D) Distribution of cross-sectional areas (CSA) of myofibers in each TA muscle. (E) Average CSA of each TA sample. All error bars indicate ± SEM (n=4). p-Values are determined by non-parametric Wilcoxon tests for comparisons.
Additional files
-
Supplementary file 1
Primers for the expression analysis by RT-qPCR of the mRNAs are indicated.
- https://cdn.elifesciences.org/articles/89381/elife-89381-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/89381/elife-89381-mdarchecklist1-v1.docx





