Atypical local and global biological motion perception in children with attention deficit hyperactivity disorder
eLife assessment
The authors use point light displays to measure biological motion (BM) perception in children (mean = 9 years) with and without ADHD, and relate it to IQ, social responsiveness scale (SRS) scores and age. They report that children with ADHD were worse at all three BM tasks, but that those tasks loading more heavily on local processing relate to social interaction skills and those loading on global processing relate to age. There are still some elements of the results that need clarification with future work, but nevertheless, the important and solid findings extend our limited knowledge of BM perception in ADHD, as well as biological motion processing mechanisms in general.
https://doi.org/10.7554/eLife.90313.5.sa0Important: Findings that have theoretical or practical implications beyond a single subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Solid: Methods, data and analyses broadly support the claims with only minor weaknesses
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Perceiving biological motion (BM) is crucial for human survival and social interaction. Many studies have reported impaired BM perception in autism spectrum disorder, which is characterised by deficits in social interaction. Children with attention deficit hyperactivity disorder (ADHD) often exhibit similar difficulties in social interaction. However, few studies have investigated BM perception in children with ADHD. Here, we compared differences in the ability to process local kinematic and global configurational cues, two fundamental abilities of BM perception, between typically developing and ADHD children. We further investigated the relationship between BM perception and social interaction skills measured using the Social Responsiveness Scale and examined the contributions of latent factors (e.g. sex, age, attention, and intelligence) to BM perception. The results revealed that children with ADHD exhibited atypical BM perception. Local and global BM processing showed distinct features. Local BM processing ability was related to social interaction skills, whereas global BM processing ability significantly improved with age. Critically, general BM perception (i.e. both local and global BM processing) may be affected by sustained attentional ability in children with ADHD. This relationship was primarily mediated by reasoning intelligence. These findings elucidate atypical BM perception in ADHD and the latent factors related to BM perception. Moreover, this study provides new evidence that BM perception is a hallmark of social cognition and advances our understanding of the potential roles of local and global processing in BM perception and social cognitive disorders.
Introduction
ADHD is a common developmental disorder with a prevalence ranging from 2 to 7% in children and adolescents, averaging approximately 5% (Sayal et al., 2018). In addition to the well-established core symptoms of ADHD (including the inability to sustain attention, hyperactivity, and impulsivity), some characteristics of autism spectrum disorder (ASD), such as dysfunction in social communication and social interaction, have also been frequently observed in children with ADHD (Grzadzinski et al., 2011; Mulligan et al., 2009; Reiersen et al., 2007). Nevertheless, experimental studies focusing on social cognition in children with ADHD are limited. Some studies have reported poor performance on social cognition tasks. Among these, impaired theory of mind (ToM) and emotion recognition are the most frequently reported (Bora and Pantelis, 2016; Nejati, 2022; Uekermann et al., 2010). It is difficult for children with ADHD to recognize the emotions and intentions of others. However, our understanding of other social cognitive processes in ADHD remains limited. Further exploration of a diverse range of social cognitions (e.g. biological motion perception) can provide a fresh perspective on the impaired social function observed in ADHD. Moreover, recent studies have indicated that social cognition in ADHD may vary depending on different factors at the cognitive, pathological, or developmental levels, such as general cognitive impairment (Bora and Pantelis, 2016), symptom severity (McKay et al., 2023), or age (Bora and Pantelis, 2016). Nevertheless, understanding how these factors relate to social cognitive dysfunction in ADHD is still in its infancy. Bridging this gap is crucial as it can help depict the developmental trajectory of social cognition and identify effective interventions for impaired social interaction in individuals with ADHD.
BM, which refers to the movement of a living creature, conveys a wealth of information beyond bodily movements (Dittrich, 1993), such as intention (Pavlova, 2012), emotion (Clarke et al., 2005), sex (Kozlowski and Cutting, 1977), and identity (Jokisch et al., 2006; Loula et al., 2005). The advent of point-light display (PLD) technology, which is used to depict human motions, (Johansson, 1973) allows researchers to separate biological motion from other characteristics such as shape and colour. Considering its seminal impact on cognitive, developmental, and clinical neuroscience, BM perception has drawn significant attention from scientists. Some researchers have attempted to deconstruct BM processing into more specific components. Our study concentrated on two fundamental abilities involved in processing BM cues (Figure 1): the ability to process local BM cues derived from major joint motion tracks, and the ability to process global BM cues of human configuration. Previous studies revealed differences between local and global BM perception. Separate neural signals for these abilities imply two independent BM processing stages (Duarte et al., 2022; Jastorff and Orban, 2009; Vangeneugden et al., 2014). Local BM cues not only help identify locomotive direction (Troje and Westhoff, 2006) but also contribute to the detection of life in a visual environment (Chang and Troje, 2008) without the observers’ explicit recognition or attention (Bertenthal and Pinto, 1994; Chang and Troje, 2009a; Chang and Troje, 2009b; Wang et al., 2010). As a result, the processing of local BM cues is less affected by attention, relatively robust to masking noise, and does not show a learning trend (Chang and Troje, 2009a; Thornton et al., 2002). In contrast, global BM processing involves top-down modulation, with attention playing a critical role in its perception (Thompson and Parasuraman, 2012; Thornton et al., 2002). Dispersed attention adversely affects performance. Compared with local BM processing, global BM processing is susceptible to learning and is heavily hindered by increased mask densities (Thornton et al., 2002). These findings suggest that local and global mechanisms play different roles in BM perception, although the exact mechanism underlying this distinction remains unclear. Exploring these two components of BM perception will enhance our understanding of the differences between local and global BM processing and shed light on the psychological processes involved in atypical BM perception.
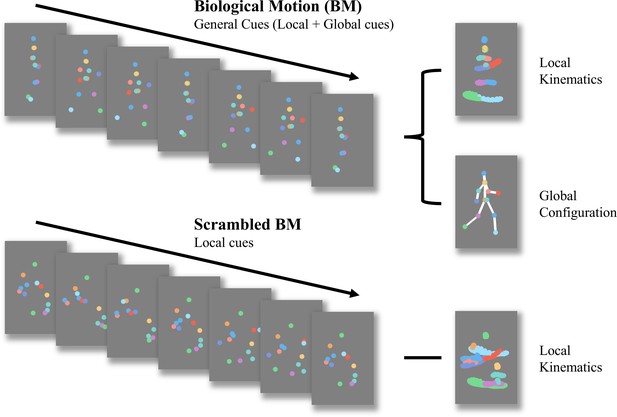
Schematic representation of biological motion (BM) and scrambled BM sequence.
Intact walker contains information of local kinematics and global configuration. Local kinematics refers to the motion tracks of each critical joint illustrated by the chromatic dot. Global configuration is composed of the relative locations of each joint. In the scrambled BM sequence, global configuration cues have been removed, but local kinematics have been retained. (Figure reconstructed from Wang et al., 2018).
In recent years, BM perception has received significant attention in studies on mental disorders (e.g. schizophrenia Kim et al., 2013) and developmental disabilities, particularly ASD, which is characterised by deficits in social communication and social interaction (Federici et al., 2020; Todorova et al., 2019). This is because BM perception is considered a hallmark of social cognition. Individuals with deficits in BM processing exhibit worse social perception in daily life (Pavlova, 2012). Another study found that participants’ ability to process BM cues correlated with their autistic traits, particularly in the subdimension of social communication (Wang et al., 2018). Therefore, examining BM perception could enhance our understanding of social dysfunction in children with ADHD. Compared with the numerous studies examining impaired BM perception in ASD, few studies have focused on BM perception in children with ADHD. An EEG study found neuroelectrophysiological changes in the processing of BM stimuli in children with ADHD (Kröger et al., 2014). Specifically, compared with the typically developing (TD) group, children with ADHD showed reduced activity of motion-sensitive components (N200) while watching biological and scrambled motions, although no behavioural differences were observed. Another study found that children with ADHD performed worse in BM detection with moderate noise ratios than the TD group (Imanipour et al., 2021). This finding may be due to the fact that BM stimuli with noise dots will increase the difficulty of identification (McKay et al., 2012), which highlights the difference in BM processing between TD and ADHD groups (Kröger et al., 2014).
Despite initial findings about atypical BM perception in ADHD, previous studies on ADHD treated BM perception as a single entity, which may have led to misleading or inconsistent findings (Federici et al., 2020). Hence, it is essential to deconstruct BM processing into multiple components and motion features. To enhance our understanding of the ability to process distinct BM cues in ADHD, we employed a carefully designed behavioural paradigm, as used in our previous study (Wang et al., 2018), with slight adjustments made to adapt it for children. This paradigm comprised three tasks (Figure 2): BM-Local, BM-Global, and BM-General. BM-Local assessed the ability to process local BM cues. Scrambled BM sequences were displayed and the participants used local BM cues to judge the direction the scrambled walker was facing. BM-Global tested the ability to process the global configuration cues of the BM walker. Local cues were uninformative, and the participants used global BM cues to determine the presence of an intact walker. BM-General tested participants’ ability to process general BM cues (local +global cues). The stimulus sequences consisted of an intact walker and a mask containing similar target local cues so that the participants could use general BM cues to judge the direction the walker was facing.
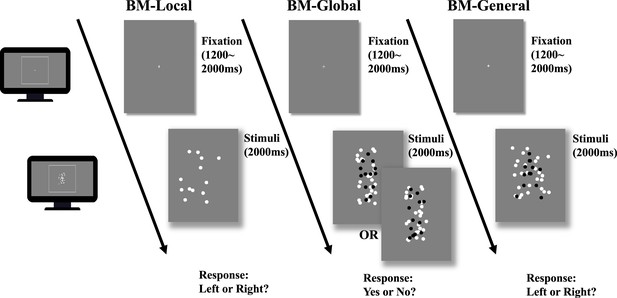
Illustration of the trial sequence.
In biological motion (BM)-Local, a monitor displayed scrambled BM sequences. Participants only judged the facing direction of the scrambled walker using local BM cues. In BM-Global, each trial only showed an intact or scrambled walker (black dots in the figure) embedded within a mask containing local BM cues. Because the two conditions contained the same local cues that were also present in the mask, the participant must rely on global BM cues to determine whether an intact walker was present in the mask. The figure shows one of five possible directions the intact walker could face (i.e. facing participants). In BM-General, the stimuli sequence consisted of an intact walker (black dots) and a mask containing similar target local cues, and children judged the direction the walker was facing using general BM cues (local +global). Dots in the figure are rendered in black for better illustration but were displayed in white in the actual experiments.
Experiment 1 examined three specific BM perception abilities in children with ADHD. Children with ADHD show impaired social interaction (Grzadzinski et al., 2011; Mulligan et al., 2009; Reiersen et al., 2007), which implies atypical social cognition. Therefore, we speculated that children with ADHD would perform worse on the three tasks than TD children. In Experiment 2, we further explored the relationship between BM perception and social interaction ability in children with ADHD and identified potential factors (e.g. intelligence quotient [IQ], age, and attention) that may affect BM perception in this population. We speculated that if the mechanisms of processing local and global BM cues are indeed distinct, as suggested by previous studies, then impairment in the ADHD population and the influential factors behind the impairment may be different.
Results
Children with ADHD exhibit atypical BM perception
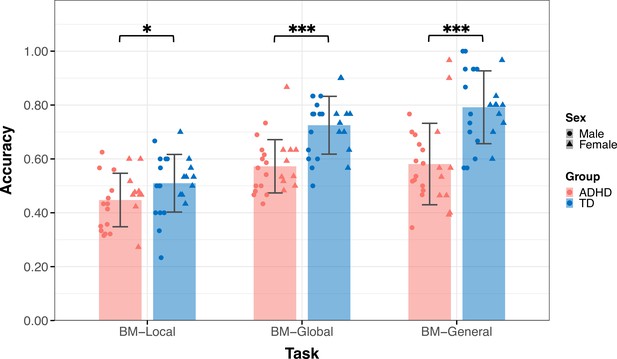
Thirty-six TD children (age = 9.09 ± 2.18, 14 male) and 39 children with ADHD (age = 9.88±2.23, 28 male) participated in Experiment 1 (Table 1). The groups did not differ by age (t73=–1.550, p=0.126) but differed in sex (χ2=8.964, p=0.004). Figure 3 displays the mean accuracies (ACC) for both the TD and ADHD groups across the three tasks in Experiment 1. We examined the difference in the ACC between the TD and ADHD groups for each task using a two-sample t-test. The results of BM-Local showed a significant difference (TD: 0.52±0.13, ADHD: 0.44±0.09, t73=3.059, p=0.003, Cohen’s d=0.71), indicating that children with ADHD exhibited impaired local BM processing ability. For BM-Global and BM-General, where children were asked to detect the presence or discriminate the direction the target walker was facing, the TD group had higher accuracies than the ADHD group (BM-Global - TD: 0.70±0.12, ADHD: 0.59±0.12, t73=3.677, p<0.001, Cohen’s d=0.85; BM-General - TD: 0.79±0.12, ADHD: 0.63±0.17, t73=4.702, p<0.001, Cohen’s d=1.09). These findings suggest the presence of impaired global and general BM perception in children with ADHD. To ensure that sex did not influence the results, we conducted a subsampling analysis with balanced data (Pirracchio et al., 2012), and the results remained consistent (see Appendix 1).
Demographic characteristics of typically developing (TD) and attention deficit hyperactivity disorder (ADHD) groups.
| Experiment 1 | p-value | Experiment 2 | p-value | |||
|---|---|---|---|---|---|---|
| TD | ADHD | TD | ADHD | |||
| Sample | 36 | 39 | 33 | 42 | ||
| Age (years) | 9.09±2.18 | 9.88±2.23 | 0.126 | 8.75±1.94 | 9.34±1.89 | 0.191 |
| Sex ratio (% male) | 38.89% | 71.79% | 0.004 | 42.42% | 64.28% | 0.059 |
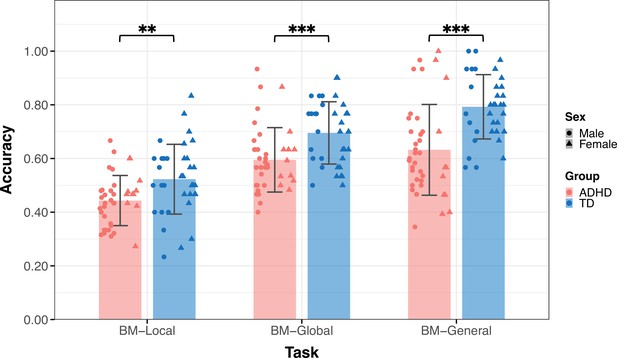
The mean accuracy of the three tasks.
Typically developing (TD) children had higher accuracies than children with attention deficit hyperactivity disorder (ADHD) in the three tasks in Experiment 1. Error bars show standard deviations. TD group: n = 36, ADHD group: n = 39; two-sample t-tests; **p<0.01, ***p<0.001.
Atypical perception of local BM information predicts impaired social interaction in ADHD
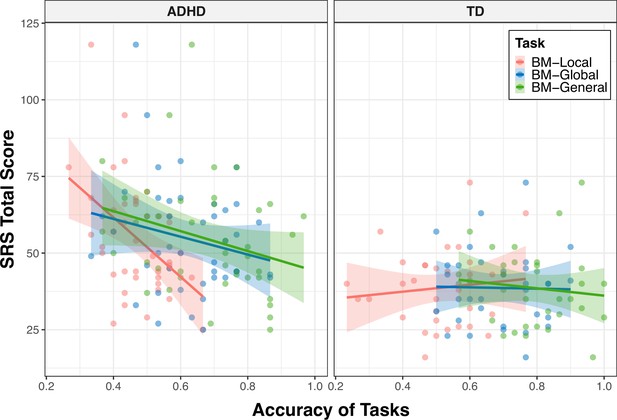
Experiment 1 provides evidence of atypical BM perception in children with ADHD. Previous studies have revealed that BM processing ability is a hallmark of social cognition (Pavlova, 2012) and is negatively correlated with social ability (Wang et al., 2018). Substantial evidence indicates that children with ADHD often experience problems with social interaction. We hypothesised that compromised social interaction in children with ADHD would be associated with BM processing. To confirm this hypothesis, we recruited 42 naïve children with ADHD (age = 9.34±1.89, 27 male) to participate in Experiment 2 and examined the relationship between their social interaction abilities and BM perception. Parents or caregivers completed the Social Responsiveness Scale (SRS). A higher SRS total score indicates worse social ability. The SRS total score of the ADHD group was higher than that of the TD group (SRS total score - ADHD: 54.64±18.42, TD: 38.64±12.47, t73=–4.277, p<0.001). We found that children with higher total SRS scores performed worse on the three tasks; that is, the abilities of BM processing were negatively correlated with SRS total score (BM-Local: r=–0.264, false discovery rate [FDR]-corrected p=0.033; BM-Global: r=–0.238, FDR-corrected p=0.039; BM-General: r=–0.359, FDR-corrected p=0.006).
The correlations encompassing all data from both groups might reflect group disparities, given the significant distinction in SRS total score between TD and ADHD children, alongside their marked differences in BM processing abilities. Therefore, we conducted additional subgroup analysis to further explore the relationship between social interaction and BM processing ability in children with ADHD. As depicted in Figure 4, the correlation between the SRS total score and the ability to process local cues was only found in the ADHD group (ADHD: r=–0.461, FDR-corrected p=0.004; TD: r=0.109, FDR-corrected p=0.547), particularly on subscales of social awareness, social cognition, social communication, social motivation (seeTable 2 for detailed information). However, we did not find a statistically significant correlation between the SRS total score and global or general BM processing in either the ADHD or TD groups (global BM perception, TD: r=–0.020, FDR-corrected p=0.910, ADHD: r=–0.207, FDR-corrected p=0.374; general BM perception, TD: r=–0.118, FDR-corrected p=0.514, ADHD: r=–0.286, FDR-corrected p=0.134).
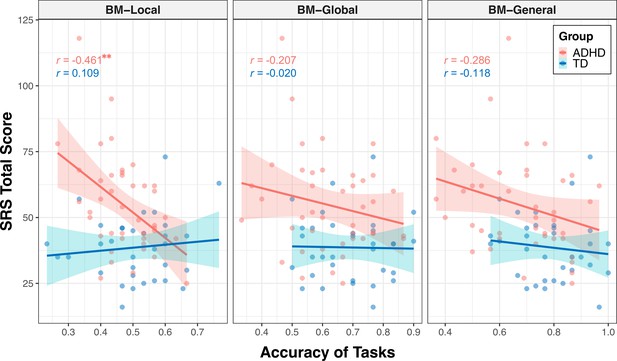
Correlations between response accuracies and social responsiveness scale (SRS) total score.
The ability to process local cues is significantly correlated with the SRS total score in the attention deficit hyperactivity disorder (ADHD) group. The shading represents the 95% confidence interval. **FDR-corrected p<0.01.
The correlation between the ability of local biological motion (BM) processing and the subdimensions of social responsiveness scale (SRS) in attention deficit hyperactivity disorder (ADHD) children.
| Correlation coefficient (r) | FDR-corrected p-value | |
|---|---|---|
| Social awareness | -0.333 | 0.039 |
| Social cognition | -0.416 | 0.020 |
| Social communication | -0.381 | 0.022 |
| Social motivation | -0.406 | 0.020 |
| Autistic mannerisms | -0.245 | 0.117 |
To determine the specificity of the correlation between local BM processing and SRS total score in the ADHD group, we constructed general linear models to further compare these correlations (Zhonglin et al., 2005) (see Appendix 2). We observed a significantly stronger correlation between SRS total score and response accuracy in the ADHD group compared to the TD group (p=0.003) for the BM-Local, but not for the BM-Global (p=0.381) or BM-General (p=0.455). Additionally, our results showed trends towards significance, indicating that the correlation between the SRS total score and response accuracy of the ADHD group in BM-Local was more negative than that in BM-Global (p=0.074) or in BM-General (p=0.073). These findings suggest that the atypical local BM processing ability may be specifically related to the impairment of social interaction in children with ADHD.
Global BM processing develops with age and is regulated by reasoning intelligence and attention function
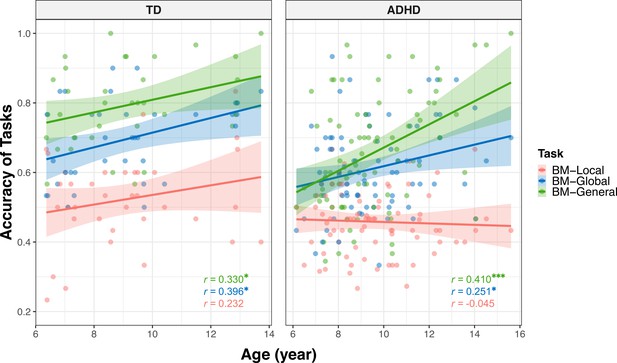
Many factors can affect social cognition in ADHD, such as general cognitive impairment (Bora and Pantelis, 2016), symptom severity (McKay et al., 2023), and age (Bora and Pantelis, 2016). To better understand their role in atypical BM processing, we examined the relationship between BM task performance and factors such as age, full-scale intellectual quotient (FIQ), and attention function in the ADHD group. Children with ADHD in Experiments 1 and 2 completed the QB test for assessing attention function (see Materials and methods). Data from the ADHD group in both Experiments 1 and 2 were integrated, resulting in 80 ADHD participants (one child did not complete the QB test). Three linear models were built to investigate the contributing factors: (a) ACCBM-Local = β0 + β1 * age + β2 * gender + β3 * FIQ + β4 * QbInattention, (b) ACCBM-Global = β0 + β1 * age + β2 * gender + β3 * FIQ + β4 * QbInattention, and (c) ACCBM-General = β0 + β1 * age + β2 * gender + β3 * FIQ + β4 * QbInattention + β5 * ACCBM-Local + β6 * ACCBM-Global. ACCBM-Local, ACCBM-Global and ACCBM-General refer to the response accuracies of the three tasks in the ADHD group, and QbInattention is the standardised score for sustained attention function. We screened the factors with the largest contribution to the models using stepwise regression. In model (a), no variable remained after stepwise regression, suggesting that local BM processing remained stable with age and was not affected by attention or IQ. In model (b), the ability to process global BM cues was enhanced with age (standardised β1=0.251, p=0.025). In model (c), higher FIQ, particularly on the subdimension of Perceptual Reasoning (standardised β3=0.271, p=0.005), and better performance in global BM processing (standardised β6=0.290, p=0.004) predicted better performance in general BM processing. Furthermore, as children aged, the ability to probe general BM information improved (standardised β1=0.365, p<0.001). It is worth noting that QbInattention showed a strong negative correlation with Perceptual Reasoning (r=–0.355, p=0.001) and general BM perception (r=–0.246, p=0.028). Owing to the potential collinearity issue, we employed a post hoc path analysis to visualise these relationships (Figure 5). The results indicated that sustained attention (i.e. QbInattention) did not directly predict performance in BM-General but was significantly indirectly predicted by Perceptual Reasoning ability. Furthermore, as children with ADHD aged, their performance in BM-General improved, both directly and through the enhanced processing of global BM cues.
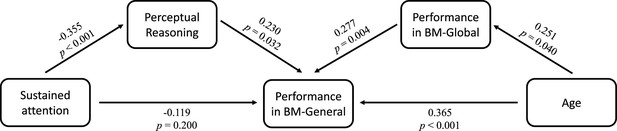
Factors influencing biological motion (BM) perception in attention deficit hyperactivity disorder (ADHD) children.
Post hoc path analysis confirmed that the effect of sustained attention on performance in BM-General was entirely mediated by Perceptual Reasoning, and the ability of global BM processing partly mediated the effect of age on performance in BM-General.
We also built three models to explore further the effects of Reasoning IQ and age on BM perception in TD children: (d) ACCBM-Local = β0 + β1 * age + β2 * gender + β3 * FIQ. (e) ACCBM-Global = β0 + β1 * age + β2 * gender + β3 * FIQ; (f) ACCBM-General = β0 + β1 * age + β2 * gender + β3 * FIQ + β4 * ACCBM-Local + β5 * ACCBM-Global. In model (d), no regressor remained significant after stepwise regression. However, in models (e) and (f), we observed positive relationships between age and performance (model e: standardized β1=0.396, p=0.017; model f: standardised β1=0.330, p=0.049). We also conducted a path analysis similar to that in the ADHD group and found no statistically significant mediator effect (Figure 5—figure supplement 1). The complete information about models a-f can be found in Table 3.
Coefficients and summaries of models a-f.
| Model | Predictor | Standardised coefficient | 95% confidence interval | T statistic | p-value | R square | Std. error of the estimate* |
|---|---|---|---|---|---|---|---|
| Model a | No significant variable | — | — | — | — | — | — |
| Model b | age | 0.251 | [0.033, 0.469] | 2.289 | 0.025 | 0.063 | 0.974 |
| Model c | age | 0.365 | [0.172, 0.559] | 3.759 | <0.001 | 0.339 | 0.829 |
| Perceptual Reasoning | 0.271 | [0.082, 0.459] | 2.862 | 0.005 | |||
| ACCBM-Global | 0.290 | [0.097, 0.484] | 2.987 | 0.004 | |||
| Model d | No significant variable | — | — | — | — | — | — |
| Model e | age | 0.396 | [0.076, 0.716] | 2.515 | 0.017 | 0.157 | 0.932 |
| Model f | age | 0.330 | [0.001, 0.659] | 2.039 | 0.049 | 0.109 | 0.958 |
-
*
Std. error of the estimate is the standard deviation of the error term, and is the square root of the Mean Square Residual (or Error).
In summary, our findings suggest that the ability to perceive global and general BM cues, rather than local BM cues, improves with age in both groups. We speculated that age-related improvement was different when processing different BM cues and in different groups. Therefore, we examined the differences between the age-related improvement in processing local cues and that in processing global and general cues (see Appendix 2). In the ADHD group, we observed that the ability to process general BM cues significantly improved with age compared to local cues (p<0.001) and a trend that the improvement in processing global BM cues with age was greater than that in processing local BM cues (p=0. 073). However, these patterns were not observed in the TD group. In addition, we examined the differences in the improvements in processing of BM cues with age between the two groups for each task. There was no difference in the effect of age on the response accuracy between the TD and ADHD groups for the three tasks (see Appendix 2).
Discussion
Our study contributes several promising findings concerning atypical BM perception in children with ADHD. Specifically, we observed atypical local and global BM perception in children with ADHD. Notably, local and global BM processing exhibited distinct features. The ability to process local BM cues appears to be associated with social interaction traits in children with ADHD. In contrast, global BM processing was associated with age-related development. In addition, general BM perception may be affected by factors such as attention.
BM perception is a widely studied topic in visual cognition owing to its inherent biological and social properties. BM processing has significant value in successfully navigating daily life, particularly in non-verbal communication (Clarke et al., 2005; Dittrich et al., 1996) and adaptive behaviour (Pollick et al., 2005; Thompson and Parasuraman, 2012). In TD children, there is a clear association between BM perception and social cognitive abilities (Kutsuki et al., 2009). For example, 12-month-old infants exhibit social behaviours (i.e. following gaze) elicited by BM displays (Yoon and Johnson, 2009). Therefore, BM perception plays a crucial role in the development of children’s social cognition. This ‘social interpretation’ of BM suggests that the difficulties in processing BM may serve as an indicator of impaired social interaction (Wang et al., 2018). Our results are consistent with these findings. We observed atypical BM perception in children with ADHD and a significant relationship between BM perception performance and the SRS total score. Further subgroup analysis revealed a significant negative correlation between the SRS total score and the accuracy of local BM processing in the ADHD group. This correlation was stronger in the ADHD group than in the TD group. The lack of a significant correlation may be due to the narrow range of SRS scores in the TD group. Future studies should increase the sample size to explore the correlations among diverse individuals. These findings suggest that BM processing is a distinct hallmark of social cognition in ADHD children (Pavlova, 2012; Wang et al., 2018).
BM perception is a multi-level phenomenon (Troje, 2013; Troje and Basbaum, 2008; Troje and Chang, 2013). At least in part, the processing of local and global BM information appears to involve different neural mechanisms (Duarte et al., 2022). Sensitivity to local BM cues emerges early in life (Simion et al., 2008; Vallortigara et al., 2005) and involves rapid processing in the subcortical regions (Buzzell et al., 2013; Chang et al., 2018; Duarte et al., 2022; Hirai et al., 2009). As a basic pre-attentive feature (Wang et al., 2010), local BM cues can guide visual attention spontaneously (Bosbach et al., 2004; Thornton and Vuong, 2004). In contrast, the ability to process global BM cues is related to slow cortical BM processing and is influenced by many factors such as attention (Thompson and Parasuraman, 2012; Thornton et al., 2002) and visual experience (Chang and Troje, 2009b; Troje and Basbaum, 2008). As mentioned above, we found a significant negative correlation between the SRS total score and the accuracy of local BM processing, specifically in the ADHD group. This could be due to decreased visual input related to atypical local BM processing, which further impairs global BM processing. According to the two-process theory of biological motion processing (Hirai and Senju, 2020), local BM cues guide visual attention toward BM stimuli (Bardi et al., 2011; Simion et al., 2008). Consequently, the visual input of BM stimuli increases, facilitating the development of the ability to process global BM cues through learning (Chang and Troje, 2009a; Grossman et al., 2004). The latter is a prerequisite for attributing intentions to others and facilitating social interactions with other individuals (Chang and Troje, 2008; Frith and Frith, 1999; Troje and Chang, 2023). Thus, atypical local BM processing may contribute to impaired social interactions through altered visual input. Further empirical studies are required to confirm these hypotheses.
The ability to process global BM cues develops with age. Previous studies indicated that global BM perception is enhanced with age in TD children (Annaz et al., 2010; Ghanouni et al., 2015). This developmental trend in global BM processing is also evident in individuals with impaired BM perception (e.g. children with ASD). BM processing performance in children with ASD becomes more aligned with that of TD children as they age (Hubert et al., 2007; Murphy et al., 2009; Saygin et al., 2010; Todorova et al., 2019). Our study contributes new evidence to the understanding of the development of global BM processing. We found that the ability to process global and general BM cues improved significantly with age in both the TD and ADHD groups, implying that the processing module for global BM cues tends to mature with development. This finding is akin to the potential age-related improvements observed in certain aspects of social cognitive deficits in individuals with ADHD (Bora and Pantelis, 2016). Interestingly, in the ADHD group, the improvement in processing general and global BM cues was greater than in processing local BM cues. Few developmental studies have been conducted on local BM processing. The ability to process local BM cues remained stable and did not exhibit a learning trend (Chang and Troje, 2009a; Thornton et al., 2002). A reasonable interpretation may be that local BM processing is a low-level mechanism, probably performed by the primary visual cortex and subcortical regions such as the superior colliculus, pulvinar, and ventral lateral nucleus (Chang et al., 2018; Hirai and Senju, 2020; Loula et al., 2005), which are not malleable. Although there was no statistical difference between the improvements in processing local and global BM cues in the TD group, this may be due to the relatively small sample size or the relatively higher baseline abilities of BM perception in TD children, resulting in a relatively milder improvement. It is worth noting that the ability to process global BM cues was positively correlated with the performance in processing general BM cues in the ADHD group, whereas no such correlation was found in the TD group. This suggests that TD children are able to extract and integrate both local and global cues, whereas children with ADHD may rely more on global BM cues to judge the direction the walker is facing when presented with both local and global BM cues, which correspond to a hierarchical model (Troje and Basbaum, 2008). Once a living creature is detected, an agent (i.e. is it a human?) can be recognised by a coherent, articulated body structure that is perceptually organised based on its motions (i.e. local BM cues) (Rosch, 1988). This involves top-down processing and probably requires attention (Cavanagh et al., 2001; Thornton et al., 2002), particularly in the presence of competing information (Thompson and Parasuraman, 2012). Our findings are consistent with those of previous studies on the cortical processing of BM (Safford et al., 2010), as we found that the severity of inattention in children with ADHD was negatively correlated with their performance in global BM processing, whereas this significant correlation was not found in local BM processing, which may involve bottom-up processing (Hirai and Senju, 2020; Troje and Chang, 2023) and might not need participants’ explicit attention (Chang and Troje, 2009a; Hirai et al., 2011; Thompson et al., 2007; Wang et al., 2010). However, further studies are needed to verify this hypothesis.
Interestingly, children with impaired BM perception may employ a compensatory strategy (Rutherford and Troje, 2012). Previous studies found no impairment in BM recognition in individuals with autism with high IQ (Rutherford and Troje, 2012), but children with ASD exhibited weaker adaptation effects for biological motion than TD children (van Boxtel et al., 2016). One possibility is that individuals with a high IQ and impaired BM perception can develop or employ reliable strategies for BM recognition, compensating for the lack of intuitive social perceptual processing (Atkinson, 2009; Koldewyn et al., 2010). The current study supports this assumption, as children with a higher IQ, particularly in Perceptual Reasoning, demonstrated better performance. Owing to the impact of attention deficits on Perceptual Reasoning, the performance of children with ADHD did not align with that of TD children.
Overall, our study reveals two distinct and atypical fundamental abilities underlying BM perception in children with ADHD. Notably, anomalous local BM processing may predict impaired social interactions in children with ADHD. Moreover, these results revealed the potential contributions of age, IQ, and attention to BM information processing. These findings also shed new light for future studies. First, different developmental trends appear in local and global BM processing. Further studies are required to explore the relationship between the two fundamental BM processing abilities, which will contribute to understanding the respective and mutual neural mechanisms underlying the two types of BM processing. Second, exploring the performance of more advanced BM processing in children with ADHD, such as emotion and identity recognition in BM tasks, is necessary to delineate the neural profiles involved in processing BM in ADHD. Finally, a comparative study between ADHD and ASD is warranted to identify common neuropsychological traits and biomarkers of social cognition impairment.
Materials and methods
Participants
One hundred seventeen children with and without ADHD were recruited for this study. Eighty-one children met the ADHD diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013). The clinical diagnosis was first made by an experienced child and adolescent psychiatrist in the Child and Adolescent Psychiatric Outpatient Department of Peking University Sixth Hospital, based on the ADHD Rating Scale. The Chinese version of the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version DSM-5 (K-SADS-PL-C DSM-5) (Dun et al., 2022; Kaufman et al., 2016), a semi-structured interview instrument, was then implemented to confirm the diagnosis. Thirty-six TD children from ordinary primary schools in Beijing were screened for the presence of ADHD, ASD, affective disorders, and behavioural disorders by a trained psychiatrist. All participants in the ADHD group had a full-scale IQ >75 (fifth upper percentile) on the Wechsler Intelligence Scale for Children-Fourth Edition, and all TD children had a full-scale IQ above the fifth percentile on Raven’s Standard Progressive Matrices (Pind et al., 2003), which is used to measure reasoning ability and is regarded as a non-verbal estimate of intelligence. The exclusion criteria for both groups were as follows: (a) neurological diseases; (b) other neurodevelopmental disorders (e.g. ASD, mental retardation, and tic disorders), affective disorders, and schizophrenia; (c) disorders that would impact the completion of the experiment; (d) taking psychotropic drugs or stimulants within the past 30 days; and (e) previous head trauma or neurosurgery.
Thirty-six TD children (age = 9.09±2.18, 14 male) and 39 children with ADHD (age = 9.88±2.23, 28 male) participated in Experiment 1. The groups did not differ by age (t73=–1.550, p=0.126) but differed in sex (χ2=8.964, p=0.004). Forty-two ADHD children (age = 9.34±1.89, 27 male) participated in Experiment 2. The participants did not participate in Experiment 1. The participants’ demographic characteristics are presented in Table 1. Currently, there is no comparable study on ADHD that indicates effect size as a reference. Studies investigating BM perception in children with ASD typically have sample sizes ranging from 15 to 35 participants per group (Todorova et al., 2019). Considering the mild impairment of social function in children with ADHD, we determined that a sample size of 35–40 participants per group was reasonable for this study. All individuals in each group had normal or corrected-to-normal vision and were naïve to the experimental objectives. Written informed consent, and consent to publish, was obtained from the parents of all the children before testing. This study was approved by the Institutional Review Boards of Peking University Sixth Hospital and the Institute of Psychology, Chinese Academy of Sciences.
Assessment
K-SADS-PL-C DSM-5
Request a detailed protocolThe K-SADS-PL-C DSM-5 is a semi-structured interview instrument used to evaluate mental disorders in children and adolescents aged 6–18 years (Kaufman et al., 2016). It involves 35 diagnoses based on the diagnostic criteria of the DSM-5. A trained psychiatrist confirmed the diagnosis by interviewing the parents and children. The Chinese version has demonstrated great psychometric properties (Dun et al., 2022).
ADHD rating scale
Request a detailed protocolThe ADHD Rating Scale was adapted from the ADHD diagnostic criteria of the DSM (Reid et al., 1998), which requires parents or teachers to complete the scale independently. Its Chinese version has excellent psychometric properties and consists of two subscales (Su et al., 2015): inattention (IA, nine items) and hyperactivity-impulsivity (HI, nine items). Each item is rated on a four-point Likert scale ranging from 1 (the symptom appears ‘never or rarely’) to 4 (the symptom appears ‘very often’). The final results create three scores: (1) IA dimension score, (2) HI dimension score, and (3) total score. Higher scores indicate more severe ADHD symptoms.
Social responsiveness scale
Request a detailed protocolThe social responsiveness scale (SRS) is a widely used quantitative measure with 65 items used to assess the severity of social impairment in many mental disorders (Constantino and Gruber, 2005), and the psychometric properties of the Chinese version are reliable (Cen et al., 2017). It includes five sub-dimensions: social awareness, social cognition, social communication, social motivation, and autistic mannerisms. Each item is rated on a scale from 0 (never true) to 3 (almost always true), with higher scores indicating worse social ability.
Wechsler intelligence scale for children-fourth edition
Request a detailed protocolThe Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) is widely used to test comprehensive intelligence in individuals aged 6–17 years. It contains 15 subtests comprising four broad areas of intellectual functioning: Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed. The scores in the four broad areas constitute the full-scale intellectual quotient (FIQ).
QB test
Request a detailed protocolThe QB test is a 15 min continuous performance test (CPT) for assessing inattention and impulsivity, with a high-resolution infrared camera monitoring the participant’s activity (QbTech, 2010). Previous psychometric studies have validated its good measurement properties (Hult et al., 2018). After the test is completed, several Q scores are calculated to summarise the participants’ performances. The Q scores are standardised based on normative data matched for sex and age. A higher Q score implies more abnormal performance. In this study, we focused on QbInattention, the Q score indicating sustained attention, particularly when children are focused on tasks.
Stimuli and procedure
Request a detailed protocolPoint-light BM stimuli sequences adopted in this study have been used in previous studies (Vanrie and Verfaillie, 2004), which were derived from the configurations of individual walking motions on a treadmill and did not contain an overall translation. Each frame of BM sequences consisted of 13 white dots representing the human head and major joints and was displayed on a grey background (see Video 1). Each walking cycle lasted 1 s with 30 frames. For each trial, the initial frame of BM sequences was randomised. The entire point-lighted BM walker was presented at approximately a 5.7° vertical visual angle. Stimuli were presented on a 14-inch monitor, and responses were evaluated using MATLAB together with PsychToolbox extensions. All subjects completed the experiments in a dimly lit room with their heads on a chinrest to ensure that their eyes were 50 cm away from the monitor.
An intact walker without a mask.
The dots in the video are rendered in chromatic colors for better illustration and displayed in white in the actual experiments.
In Experiment 1, the children were required to complete three tasks that were similar to but slightly modified from the versions implemented in our previous study (Wang et al., 2018; Figure 2). Each trial began with a fixation cross (0.6 ° × 0.6°). Following a random interval of 1200–1500 ms, the monitor displayed a task-specific BM sequence lasting for 2 s (60 frames).
BM-Local assessed participants’ ability to process local BM cues. During the task, the monitor displayed only a scrambled walker facing either the left or right (Video 2). Specifically, the 13 dots constituting the intact walker were randomly relocated within the original range of the BM walker (randomly presented in 2D). This manipulation disrupted the global configuration of the intact walker while retaining local kinematics. After the display, we required the children to press a left or right button to indicate the direction of motion of the unidentified creature (i.e. the scrambled BM walker) as accurately as possible. Children did not receive feedback on the accuracy of each response. Thirty trials were conducted, with 15 trials for each condition (left and right).
An example of biological motion (BM)-Local (a scrambled walker without a mask).
The chromatic dots in this video correspond to the major joints of the intact walker in Video 1 and are displayed in white in the actual experiments.
BM-Global tested the ability to process the global configuration cues of the BM walker. A target walker (scrambled or intact) was displayed within the mask (Video 3) during this task. The mask consisted of two scrambled target walkers (26 dots) with the same locomotion direction as the target walker, displayed within a boundary approximately 1.44 times larger than the intact walker. A scrambled or intact version of the target walker was randomly embedded in the mask and entirely overlaid. Thus, the global BM component could be isolated as two conditions (i.e. scrambled and intact walkers) containing the same local kinematics information, rendering the local motion cues uninformative. The children were required to judge whether there was an intact walker on the mask. A correct response relied on the extraction of global cues from an intact walker. To prevent children from learning the shape of the walker (Chang and Troje, 2009a), we set target walkers that possibly faced one of five equally spaced directions from left to right. Of the five walkers used, two-faced straight to the left or right, orthogonal to the viewing direction. Two walked with their bodies oriented at a 45 degree angle to the left or right of the observer. The last one walked towards the observer. Video 4 shows the five-facing directions of the walker. Thirty trials were conducted consisting of two conditions (intact or scrambled target).
An example of biological motion (BM)-Global (an intact or scrambled walker with a mask).
The chromatic dots in this video correspond to the major joints of the intact walker in Video 1 and are displayed in white in the actual experiments.
Intact walkers facing five directions in biological motion (BM)-Global.
The dots in this video are rendered in black for better illustration and displayed in white in the actual experiments.
BM-General tested participants’ ability to process general BM cues (local and global). In BM-General, the monitor displayed an intact walker (facing either the left or right) embedded within a mask (see Video 5). The mask used in this task was similar to that used in BM-Global. The children were required to judge the direction the target walker was facing (left or right). Because the mask and target walker contained the same local BM cues and the target walker was presented with additional global configuration cues, children could rely on general BM information (i.e. a combination of local and global cues) to perform the task. BM-General consisted of 30 trials, with 15 trials for each facing direction. The other parameters of BM-Global and BM-General were similar to those of BM-Local. Before each task, the children practiced for five trials to ensure a good understanding. We performed the three tasks in a fixed order so that the participants were naïve to the nature of the local BM cues in BM-Local.
The example of biological motion (BM)-General (an intact walker with a mask).
The chromatic dots in this video correspond to the major joints of the intact walker in Video 1 and are displayed in white in the actual experiments.
In Experiment 2, 42 children with ADHD completed the same procedure as in Experiment 1. In addition, the parents completed the SRS to assess social interaction.
Statistics
Two-sample t-tests were used to examine the difference in BM perception abilities between TD and ADHD children (Fagerland, 2012; Rochon et al., 2012), and Pearson’s correlation analyses were used to assess the relationship between the accuracy of each task and the SRS score. Additionally, general linear models and path analyses were used to explore potential factors influencing BM perception. A p-value <0.05 was considered statistically significant. Path analyses were conducted using AMOS, whereas other analyses were conducted using SPSS.
Appendix 1
Children with ADHD exhibit atypical BM perception (subsampled balanced data)
To address potential gender-related confounds, we applied a statistical matching technique, Propensity Score Matching (PSM), to obtain a sub-dataset (1:1 matching) using the Matching package in R 4.3.0. We matched the characteristics, namely age and gender, to create more similar groups. After matching, 24 TD children (age = 9.70±2.08, 14 male) and 24 children with ADHD (age = 9.67±1.93, 14 male) were included in the following analysis. The two groups did not differ significantly in age (t46=0.041, p=0.967) and had an identical gender ratio. Our findings, as reproduced in Appendix 1—figure 1, confirmed that the TD group exhibited higher response accuracy than the ADHD group in all three tasks (BM-Local - TD: 0.51±0.11, ADHD: 0.45±0.10, t46=2.092, p=0.042, Cohen’s d=0.60; BM-Global - TD: 0.73±0.11, ADHD: 0.57±0.10, t46=5.116, p<0.001, Cohen’s d=1.48; BM-General - TD: 0.79±0.14, ADHD: 0.58±0.15, t46 = 5.088, p<0.001, Cohen’s d=1.47).
The mean accuracy of the three tasks (subsampled balanced data).
Typically developing (TD) children had higher accuracies than children with attention deficit hyperactivity disorder (ADHD) in three tasks in Experiment 1. Error bars show standard deviations. TD group: n = 24, ADHD group: n = 24; two-sample t-tests; *p<0.05; ***p<0.001.
Appendix 2
The correlation between SRS total scores and the ability to process local BM cues in the ADHD group is significantly stronger than in the TD group, and the age-related improvement in processing general BM cues is significantly greater than in processing local BM cues in ADHD group
To further determine the specificity of the correlation between local BM processing and SRS total score in the ADHD group. We constructed general linear models to compare these correlations. We first examined the difference in the correlations between the response accuracy for each task and SRS total score between the TD and ADHD groups. The differences between correlations were tested by testing interaction terms in general linear models. Specifically, we recoded the categorical variable (i.e. group) into a dummy variable (D) using the TD group as a reference and constructed a general linear model for each task (Appendix 2—table 1, Model 1–3): SRS = β0 + β1 * ACC + β2 * d + β3 * (ACC * D). ACC refers to the response accuracy, and SRS refers to the SRS total score. If the effect of the interaction term (i.e. β3) is statistically significant, it indicates that the correlations between response accuracy and SRS total score for the TD and ADHD groups are significantly different. For BM-Local, we observed that the correlation between SRS total score and ACC in the ADHD group was significantly stronger than in the TD group (standardised β3=–0.629, p=0.003). However, no significant difference was identified with regard to BM-Global (standardised β3=–0.195 p=0.381) or BM-General (standardised β3=–0.179, p=0.455).
In addition, we further examined the relative differences in correlations with SRS total score between BM-Local and BM-Global or BM-General in the ADHD group (Appendix 2—figure 1). Similarly, we recoded three task types to two dummy variables, D1 and D2, using BM-Local as a reference. The coefficient of D1 represents the difference in relationship to SRS total score between BM-Local and BM-Global, and the coefficient of D2 represents the difference in relationship to SRS total score between BM-Local and BM-General. A general linear model was constructed (Appendix 2—table 1, Model 4): SRS = β0 + β1 * ACC + β2 * D1 + β3 * D2 + β4 * (ACC * D1) + β5 * (ACC * D2). If the effect of the interaction term (i.e. β4 or β5) is statistically significant, it indicates a difference in correlations with SRS total score between BM-Local and BM-Global (or BM-General). The results suggested trends where the correlations with SRS total score were more negative for BM-Local relative to BM-Global (standardised β4=0.580 p=0.074) and BM-General (standardised β5=0.550 p=0.073).
To further examined the differences between the age-related improvement in processing local cues and that in processing global and general cues (Appendix 2—figure 2), we employed similar analyses as described earlier. We recoded task types into two dummy variables, D1 and D2, using BM-Local as a reference. The coefficient of D1 represents the difference in relationship to age between BM-Local and BM-Global, and the coefficient of D2 represents the difference in relationship to age between BM-Local and BM-General. The following model was created for each group (Appendix 2—table 2, Model 5–6): ACC = β0 + β1 * age + β2 * D1 + β3 * D2 + β4 * (age * D1) + β5 * (age * D2). If the effect of the interaction term (i.e. β4 or β5) is statistically significant, it indicates a difference in the effect of age on ACC between BM-Local and BM-Global (or BM-General). In the ADHD group, we observed a significant difference in the effect of age on ACC between BM-Local and BM-General (standardised β5=0.462, p<0.001) and marginally significant differences in the effect of age on ACC between BM-Local and BM-Global (standardised β4=0.228, p=0.073). However, there was no difference in the effect of age between BM-Local and BM-Global or BM-General in the TD group (standardised β4=0.095, p=0. 575; standardised β5=0.056, p=0.739).
We also examined the differences in age-related improvements in processing BM cues between two groups for each task (Appendix 2—figure 3). We recoded the variable group into a dummy variable (D) using the TD group as a reference, and established a general linear model for each task (Appendix 2—table 2, Model 7–9): ACC = β0 + β1 * age + β2 * D + β3 * (age * D). If the effect of the interaction term (i.e. β3) is statistically significant, it indicates a difference in the effect of age on ACC between the TD group and the ADHD group. The results showed no difference in the effect of age on ACC between the TD and the ADHD group in three tasks (BM-Local: standardised β3=–0.306, p=0.112; BM-Global: standardised β3=–0.091, p=0.621; BM-General: standardised β3=0.192, p=0.263).
Correlations between the response accuracies and social responsiveness scale (SRS) total score.
The shading represents the 95% confidence interval.
Correlations between the response accuracies and age (showed by group).
The shading represents the 95% confidence interval. * non-corrected p<0.05, *** non-corrected p<0.001.
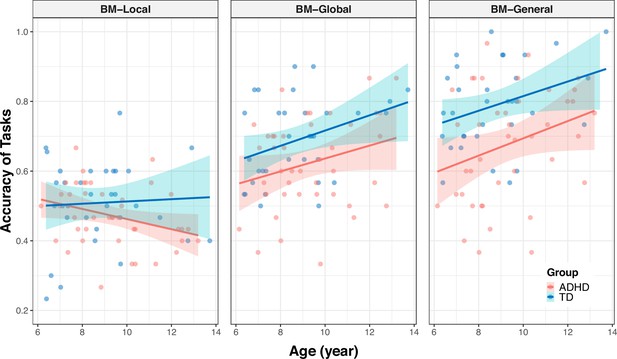
Correlations between the response accuracies and age (showed by task).
The shading represents the 95% confidence interval.
Coefficients and summaries of models for the relationship between social responsiveness scale (SRS) total score and biological motion (BM) processing.
| Model | Predictor | Standardised coefficient | 95% confidence interval | T statistic | p-value | R square | Std. error of the estimate* |
|---|---|---|---|---|---|---|---|
| Model 1†: SRS = β0 + β1 * ACCBM-Local + β2 * D + β3 * (ACCBM-Local * D) | ACCBM-Local | 0.066 | [–0.190, 0.322] | 0.513 | 0.609 | 0.328 | 0.837 |
| D | 0.821 | [0.427, 1.215] | 4.151 | <0.001 | |||
| ACCBM-Local * D | –0.629 | [-1.030,–0.228] | –3.127 | 0.003 | |||
| Model 2†: SRS = β0 + β1 * ACCBM-Global + β2 * D + β3 * (ACCBM-Global * D) | ACCBM-Global | –0.015 | [–0.360, 0.329] | –0.090 | 0.929 | 0.226 | 0.898 |
| D | 0.845 | [0.413, 1.277] | 3.898 | <0.001 | |||
| ACCBM-Global * D | –0.195 | [–0.636, 0.246] | –0.881 | 0.381 | |||
| Model 3†: SRS = β0 + β1 * ACCBM-General + β2 * D + β3 * (ACCBM-General * D) | ACCBM-General | –0.104 | [–0.498, 0.290] | –0.526 | 0.600 | 0.251 | 0.883 |
| D | 0.765 | [0.318, 1.212] | 3.411 | 0.001 | |||
| ACCBM-General * D | –0.179 | [–0.653, 0.296] | –0.751 | 0.455 | |||
| Model 4 ‡: SRSADHD = β0 + β1 * ACC + β2 * D1 + β3 * D2 + β4 * (ACC * D1) + β5 * (ACC * D2) | ACC | –0.828 | [-1.357,–0.299] | –3.098 | 0.002 | 0.112 | 0.961 |
| D1 | 0.683 | [0.100, 1.267] | 2.317 | 0.022 | |||
| D2 | 0.785 | [0.185, 1.386] | 2.589 | 0.011 | |||
| ACC * D1 | 0.580 | [–0.057, 1.216] | 1.803 | 0.074 | |||
| ACC * D2 | 0.550 | [–0.052, 1.152] | 1.808 | 0.073 | |||
-
*
Std. error of the estimate is the standard deviation of the error term, and is the square root of the Mean Square Residual (or Error).
-
†
The variable D was a dummy variable (TD group: D=0, ADHD group: D=1, i.e. TD group as a reference).
-
‡
The variable D1 and D2 were dummy variables (BM-Local: D1=0, D2=0; BM-Global: D1=1, D2=0; BM-General: D1=0, D2=1, i.e. BM-Local as a reference).
Coefficients and summaries of models for the relationship between age and biological motion (BM) processing.
| Model | Predictor | Standardised coefficient | 95% confidence interval | T statistic | p- value | R square | Std. error of the estimate* |
|---|---|---|---|---|---|---|---|
| Model 5†: ACCADHD = β0 + β1 * age + β2 * D1 + β3 * D2 + β4 * (age * D1) + β5 * (age * D2) | age | –0.027 | [–0.203, 0.150] | –0.296 | 0.768 | 0.373 | 0.801 |
| D1 | 0.977 | [0.728, 1.227] | 7.722 | 0.000 | |||
| D2 | 1.269 | [1.019, 1.518] | 10.023 | 0.000 | |||
| age * D1 | 0.228 | [–0.022, 0.478] | 1.800 | 0.073 | |||
| age * D2 | 0.462 | [0.212, 0.712] | 3.639 | <0.001 | |||
| Model 6†: ACCTD = β0 + β1 * age + β2 * D1 + β3 * D2 + β4 * (age * D1) + β5 * (age * D2) | age | 0.181 | [–0.055, 0.418] | 1.521 | 0.131 | 0.517 | 0.712 |
| D1 | 1.046 | [0.714, 1.379] | 6.236 | <0.001 | |||
| D2 | 1.636 | [1.303, 1.969] | 9.751 | <0.001 | |||
| age * D1 | 0.095 | [–0.240, 0.429] | 0.562 | 0.575 | |||
| age * D2 | 0.056 | [–0.278, 0.391] | 0.333 | 0.739 | |||
| Model 7 ‡: ACCBM-Local = β0 + β1 * age + β2 * D + β3 * (age * D) | age | 0.266 | [–0.042, 0.575] | 1.709 | 0.090 | 0.100 | 0.961 |
| D | –0.631 | [-1.016,–0.245] | –3.242 | 0.002 | |||
| age * D | –0.306 | [–0.684, 0.073] | –1.600 | 0.112 | |||
| Model 8 ‡: ACCBM-Global = β0 + β1 * age + β2 * D + β3 * (age * D) | age | 0.342 | [0.046, 0.638] | 2.290 | 0.024 | 0.172 | 0.922 |
| D | –0.723 | [-1.093,–0.353] | –3.874 | <0.001 | |||
| age * D | –0.091 | [–0.454, 0.272] | –0.496 | 0.621 | |||
| Model 9 ‡: ACCBM-General = β0 + β1 * age + β2 * D + β3 * (age * D) | age | 0.229 | [–0.047, 0.505] | 1.643 | 0.103 | 0.280 | 0.860 |
| D | –0.884 | [-1.229,–0.540] | –5.081 | <0.001 | |||
| age * D | 0.192 | [–0.146, 0.531] | 1.126 | 0.263 | |||
-
*
Std. error of the estimate is the standard deviation of the error term, and is the square root of the Mean Square Residual (or Error).
-
†
The variable D1 and D2 were dummy variables (BM-Local: D1=0, D2=0; BM-Global: D1=1, D2=0; BM-General: D1=0, D2=1, i.e. BM-Local as a reference).
-
‡
The variable D was a dummy variable (TD group: D=0, ADHD group: D=1, i.e. TD group as a reference).
Data availability
The data analyzed during the study is available at https://osf.io/37p5s/.
References
-
BookDiagnostic and Statistical Manual of Mental Disorders: DSM-5American Psychiatric Association.https://doi.org/10.1176/appi.books.9780890425596
-
Development of motion processing in children with autismDevelopmental Science 13:826–838.https://doi.org/10.1111/j.1467-7687.2009.00939.x
-
Global processing of biological motionsPsychological Science 5:221–225.https://doi.org/10.1111/j.1467-9280.1994.tb00504.x
-
A Simon effect with stationary moving stimuliJournal of Experimental Psychology. Human Perception and Performance 30:39–55.https://doi.org/10.1037/0096-1523.30.1.39
-
t-tests, non-parametric tests, and large studies--a paradox of statistical practice?BMC Medical Research Methodology 12:78.https://doi.org/10.1186/1471-2288-12-78
-
Interacting minds--a biological basisScience 286:1692–1695.https://doi.org/10.1126/science.286.5445.1692
-
Biological motion perception is affected by age and cognitive style in children aged 8-15Neurology Research International 2015:594042.https://doi.org/10.1155/2015/594042
-
Learning to see biological motion: brain activity parallels behaviorJournal of Cognitive Neuroscience 16:1669–1679.https://doi.org/10.1162/0898929042568569
-
Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD?Journal of Autism and Developmental Disorders 41:1178–1191.https://doi.org/10.1007/s10803-010-1135-3
-
The two-process theory of biological motion processingNeuroscience and Biobehavioral Reviews 111:114–124.https://doi.org/10.1016/j.neubiorev.2020.01.010
-
Brief report: recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disordersJournal of Autism and Developmental Disorders 37:1386–1392.https://doi.org/10.1007/s10803-006-0275-y
-
ADHD and the QbTest: Diagnostic Validity of QbTestJournal of Attention Disorders 22:1074–1080.https://doi.org/10.1177/1087054715595697
-
Deficits in working memory and theory of mind may underlie difficulties in social perception of children with ADHDNeurology Research International 2021:3793750.https://doi.org/10.1155/2021/3793750
-
Visual perception of biological motion and a model for its analysisPerception & Psychophysics 14:201–211.https://doi.org/10.3758/BF03212378
-
ReportK-SADS-PL DSM-5 November 2016Advanced Center for Intervention and Services Research (ACISR) for Early Onset Mood and Anxiety Disorders-Western Psychiatric Institute and Clinic; Child and Adolescent Research and Education (CARE) Program, Yale University.
-
Recognizing the sex of a walker from a dynamic point-light displayPerception & Psychophysics 21:575–580.https://doi.org/10.3758/BF03198740
-
Individual differences in changes in infants’ interest in social signals in relation to developmental indexInfant Behavior & Development 32:381–391.https://doi.org/10.1016/j.infbeh.2009.06.004
-
Recognizing people from their movementJournal of Experimental Psychology. Human Perception and Performance 31:210–220.https://doi.org/10.1037/0096-1523.31.1.210
-
Autism symptoms in Attention-Deficit/Hyperactivity Disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disordersJournal of Autism and Developmental Disorders 39:197–209.https://doi.org/10.1007/s10803-008-0621-3
-
Reading mind from the eyes in individuals with attention deficit-hyperactivity disorder (ADHD): A meta-analysisExpert Review of Neurotherapeutics 22:889–896.https://doi.org/10.1080/14737175.2022.2151899
-
Biological motion processing as a hallmark of social cognitionCerebral Cortex 22:981–995.https://doi.org/10.1093/cercor/bhr156
-
Raven’s Standard Progressive Matrices: new school age norms and a study of the test’s validityPersonality and Individual Differences 34:375–386.https://doi.org/10.1016/S0191-8869(02)00058-2
-
Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample sizeBMC Medical Research Methodology 12:70.https://doi.org/10.1186/1471-2288-12-70
-
Gender recognition from point-light walkersJournal of Experimental Psychology. Human Perception and Performance 31:1247–1265.https://doi.org/10.1037/0096-1523.31.6.1247
-
Assessing culturally different students for attention deficit hyperactivity disorder using behavior rating scalesJournal of Abnormal Child Psychology 26:187–198.https://doi.org/10.1023/a:1022620217886
-
Autistic traits in a population-based ADHD twin sampleJournal of Child Psychology and Psychiatry, and Allied Disciplines 48:464–472.https://doi.org/10.1111/j.1469-7610.2006.01720.x
-
To test or not to test: Preliminary assessment of normality when comparing two independent samplesBMC Medical Research Methodology 12:81.https://doi.org/10.1186/1471-2288-12-81
-
IQ predicts biological motion perception in autism spectrum disordersJournal of Autism and Developmental Disorders 42:557–565.https://doi.org/10.1007/s10803-011-1267-0
-
ADHD in children and young people: prevalence, care pathways, and service provisionThe Lancet. Psychiatry 5:175–186.https://doi.org/10.1016/S2215-0366(17)30167-0
-
Parent ratings of ADHD symptoms in chinese urban schoolchildren: assessment with the chinese ADHD rating scale-IV: home versionJournal of Attention Disorders 19:1022–1033.https://doi.org/10.1177/1087054712461177
-
Active versus passive processing of biological motionPerception 31:837–853.https://doi.org/10.1068/p3072
-
Incidental processing of biological motionCurrent Biology 14:1084–1089.https://doi.org/10.1016/j.cub.2004.06.025
-
BookWhat is biological motion? definition, stimuli and paradigmsIn: Rutherford MD, Kuhlmeier VA, editors. Social Perception: Detection and Interpretation of Animacy, Agency, and Intention. Boston Review. pp. 13–36.https://doi.org/10.7551/mitpress/9780262019279.003.0002
-
BookShape-independent processing of biological motionIn: Johnson K, Shiffrar M, editors. People Watching: Social, Perceptual, and Neurophysiological Studies of Body Perception. Oxford Series in Visual Cognition. pp. 82–100.https://doi.org/10.1093/acprof:oso/9780195393705.003.0006
-
Life detection from biological motionCurrent Directions in Psychological Science 32:26–32.https://doi.org/10.1177/09637214221128252
-
Social cognition in attention-deficit hyperactivity disorder (ADHD)Neuroscience and Biobehavioral Reviews 34:734–743.https://doi.org/10.1016/j.neubiorev.2009.10.009
-
Distinct neural mechanisms for body form and body motion discriminationsThe Journal of Neuroscience 34:574–585.https://doi.org/10.1523/JNEUROSCI.4032-13.2014
-
Perception of biological motion: a stimulus set of human point-light actionsBehavior Research Methods, Instruments, & Computers 36:625–629.https://doi.org/10.3758/bf03206542
-
Biological motion displays elicit social behavior in 12-month-oldsChild Development 80:1069–1075.https://doi.org/10.1111/j.1467-8624.2009.01317.x
-
A comparison of moderator and mediator and their applicationsScience Press 37:268–274.
Article and author information
Author details
Funding
Beijing Municipal Science and Technology Commission (Z181100001518005)
- Li Yang
Ministry of Science and Technology of the People's Republic of China (2021ZD0203800)
- Yi Jiang
National Natural Science Foundation of China (31830037)
- Yi Jiang
Interdisciplinary Innovation Team (JCTD-2021-06)
- Yi Jiang
Fundamental Research Funds for the Central Universities
- Yi Jiang
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank the three reviewers for their constructive comments, and Dr. Shuo Gao and Dr. Li Shen for assistance with manuscript revision. Special thanks to the parents and the children who took part in the study. This research was supported by grants from the Beijing Municipal Science and Technology Commission (Z181100001518005), the Ministry of Science and Technology of China (2021ZD0203800), the National Natural Science Foundation of China (31830037), the Interdisciplinary Innovation Team (JCTD-2021–06), and Fundamental Research Funds for the Central Universities.
Ethics
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent, and consent to publish, was obtained from the parents of all children who participated in the study. The institutional review boards of the Peking University Sixth Hospital and the Institute of Psychology, Chinese Academy of Sciences have approved this study (reference number for approval: (2020) Ethics Review No.9 and H23030).
Version history
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.90313. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Tian et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,777
- views
-
- 141
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 1
- citation for umbrella DOI https://doi.org/10.7554/eLife.90313
-
- 1
- citation for Reviewed Preprint v1 https://doi.org/10.7554/eLife.90313.1






