Interrogating basal ganglia circuit function in people with Parkinson’s disease and dystonia
Figures
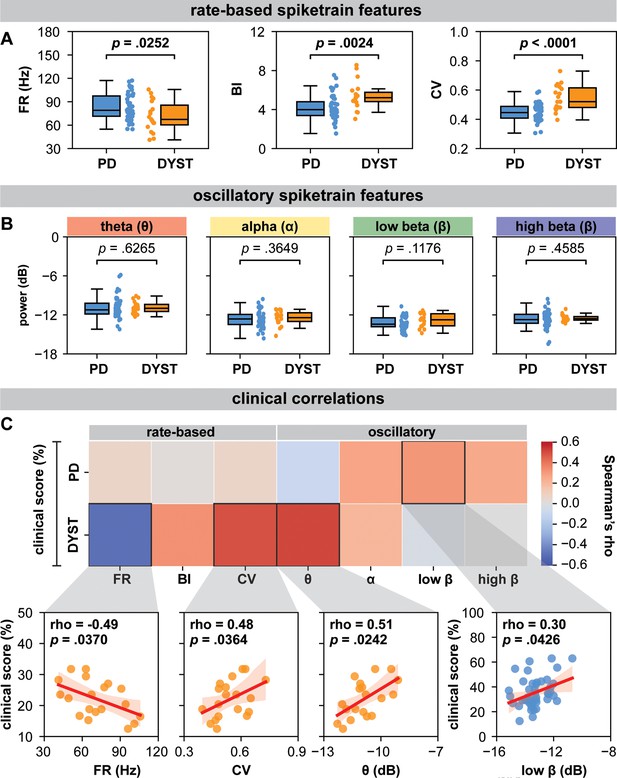
Globus pallidus internus (GPi) spiketrain feature analyses and clinical correlates of Parkinson’s disease (PD) and dystonia.
With respect to (A) rate-based spiketrain features, firing rate was greater in PD while burst index (BI) and coefficient of variation (CV) were greater in dystonia; whereas no differences were found for (B) oscillatory spiketrain features for theta, alpha, low-beta, and high-beta frequencies. Mann–Whitney U (MWU) statistical results depicted are not corrected for multiple comparisons; after correction using the Bonferroni method, only CV and BI results remain significant (see Supplementary file 3a). (C) In PD, the power of low-beta spiketrain oscillations positively correlated (Spearman correlation) with symptom severity; in dystonia, neuronal firing rate negatively correlated with symptom severity, whereas CV and the power of theta spiketrain oscillations positively correlated with symptom severity. Depicted scatterplots are results that were significant before correction for multiple comparisons; however, none of the results persist after Benjamini–Hochberg correction for false discovery rate (see Supplementary file 3b).
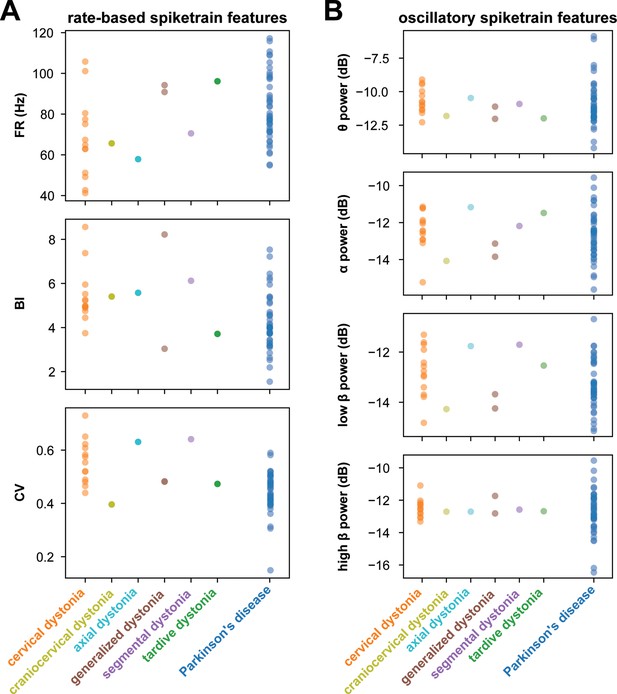
Rate-based and oscillatory spiketrain features across dystonia subtypes and Parkinson’s disease (PD).
(A) displays rate-based features, including firing rate (FR), burst index (BI), and coefficient of variation (CV), across various dystonia subtypes and PD. (B) illustrates oscillatory spiketrain features, such as theta, alpha, low-beta, and high-beta power, across different dystonia subtypes and PD.
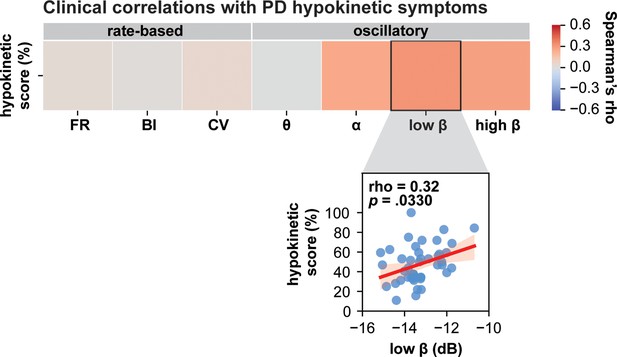
Clinical correlations with Parkinson’s disease (PD) hypokinetic symptoms.
Low-beta spiketrain oscillation power positively correlates with PD hypokinetic symptoms. The scatterplot depicts significant results before correction for multiple comparisons; however, this result did not withstand Benjamini–Hochberg correction for false discovery rate (refer to Supplementary file 3).
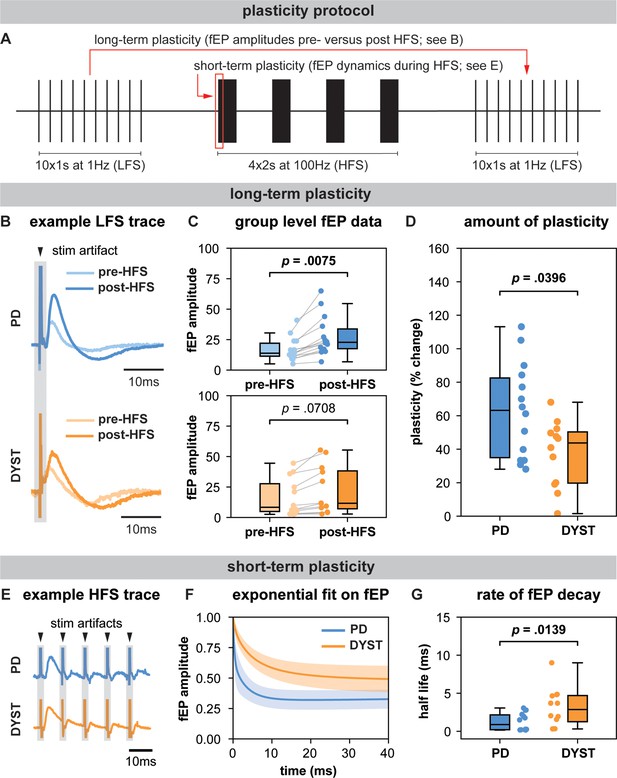
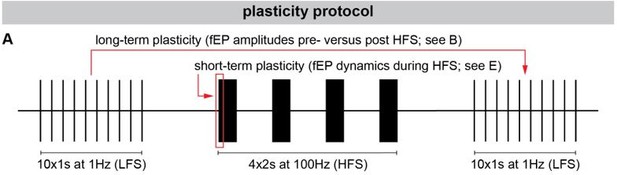
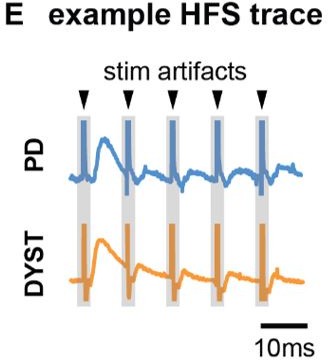
Long-term and short-term effects of high-frequency stimulation (HFS) on striato-pallidal plasticity in Parkinson’s disease (PD) and dystonia.
(A) Schematic of the plasticity protocol to assess long-term plasticity via field-evoked potential (fEP) amplitude comparisons pre- versus post-HFS and short-term plasticity via fEP dynamics during HFS. (B) highlights example fEP traces for measuring long-term plasticity pre- versus post-HFS, with (C) displaying group-level fEP amplitudes pre- versus post-HFS across diseases. (D) illustrates the amount of plasticity (i.e., percentage change in fEP amplitudes pre- versus post-HFS) in both PD and dystonia, with PD showing higher levels of plasticity. (E) provides an example of fEP traces during HFS for assessing short-term plasticity, with (F) depicting group-level decay rates of fEP amplitudes using an exponential fit on the fEP amplitudes over the first five stimulus pulses across diseases. (G) shows the half-life of the fitted exponential (i.e., rate of attenuation of fEP amplitudes) between PD and dystonia, with PD demonstrating faster fEP attenuation.
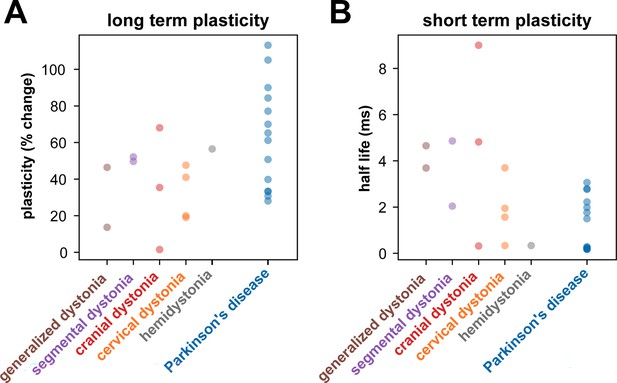
Long- and short-term plasticity across dystonia subtypes and Parkinson’s disease (PD).
(A) shows the amount of plasticity (i.e., percentage change in field-evoked potential [fEP] amplitudes pre- versus post-high-frequency stimulation [HFS]) across different dystonia subtypes and PD. (B) shows the half-life of the fitted exponential (i.e., rate of attenuation of fEP amplitudes) across different dystonia subtypes and PD.
Additional files
-
Supplementary file 1
Data summary.
The table provides a summary of the patient data included in the study. It details the diseases studied, the types of data collected (e.g., neuronal, plasticity), and the corresponding clinical scores.
- https://cdn.elifesciences.org/articles/90454/elife-90454-supp1-v2.docx
-
Supplementary file 2
Neuronal feature summary.
The table compares neuronal features such as firing rates, patterns, and oscillations between PD and dystonia patients.
- https://cdn.elifesciences.org/articles/90454/elife-90454-supp2-v2.docx
-
Supplementary file 3
Multiple comparisons.
(a) The table provides Bonferroni-corrected statistics for comparing neuronal features across different diseases, and (b) reports the Benjamini–Hochberg false discovery rate-corrected statistics for correlating neuronal features with clinical scores.
- https://cdn.elifesciences.org/articles/90454/elife-90454-supp3-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/90454/elife-90454-mdarchecklist1-v2.pdf