Neutrophils actively swell to potentiate rapid migration
Figures
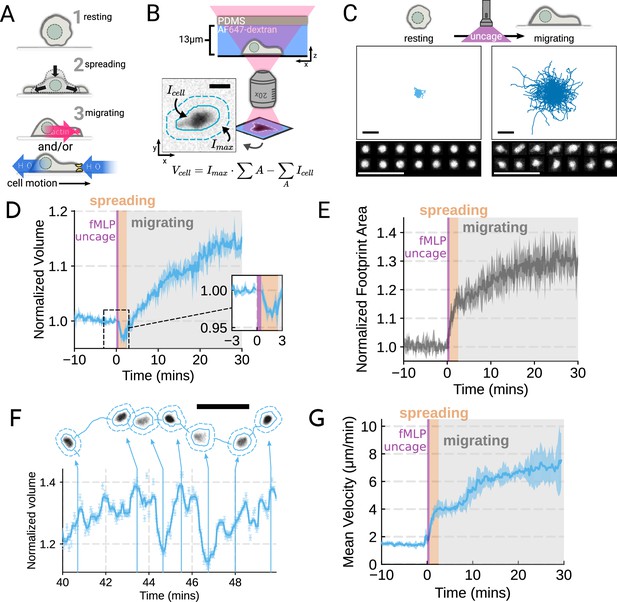
Chemoattractant stimulation elicits competing volume responses in primary human neutrophils (A) Schematic detailing the neutrophil activation process.
(B) Schematic detailing the Fluorescence Exclusion Microscopy (FxM) approach for measuring single-cell volumes, which relies on cells displacing an extracellular dye in a shallow microfluidic chamber. The inset shows an example cell with the cell footprint and local background indicated by the solid or dashed teal lines, respectively. The scale bar is 10 µm. (C) Primary human neutrophil tracks over 15 min time windows before (left) and after (right) the uncaging of the fMLP chemoattractant. Randomly selected example cells in the bottom panel show neutrophil shape before and after activation. All scale bars are 50 µm. (D) Normalized volumes of primary human neutrophils in response to chemoattractant stimulation (Volunteer n = 4, Cells = 440 total). Inset details the volume loss due to spreading immediately after uncaging. Cells initially lose volume during the spreading phase following the chemoattractant stimulation and then significantly increase in volume. The line plotted is the average of the median cell response for each volunteer, and the shaded region is the 95% CI of the mean. See Video 1 for an animated version. (E) The normalized footprint area of primary human neutrophils responding to chemoattractant. The line is the average across biological replicates of median cell footprint area at each timepoint. The footprint area shows a monotonic increase in response to activation with cell spreading prior to initiation of movement. (F) Single representative cell trace imaged with high time resolution to highlight the cell motility-related volume fluctuations. Top section depicts the cell track with the FxM images overlaid at key time points that are linked with cyan arrows to the corresponding volumes in the bottom plot. Bottom section is a scatter plot of the raw volume values, with the thick cyan line depicting the rolling median volume. See Video 2 for an animated version. Scale bar is 50 µm. (G) Mean of the per-replicate median cell velocities computed at each time point. The shaded area is the standard deviation at each time point. Cell migration begins to increase in the early spreading phase following chemoattractant stimulation and then continues to increase over the next 20 min following stimulation.
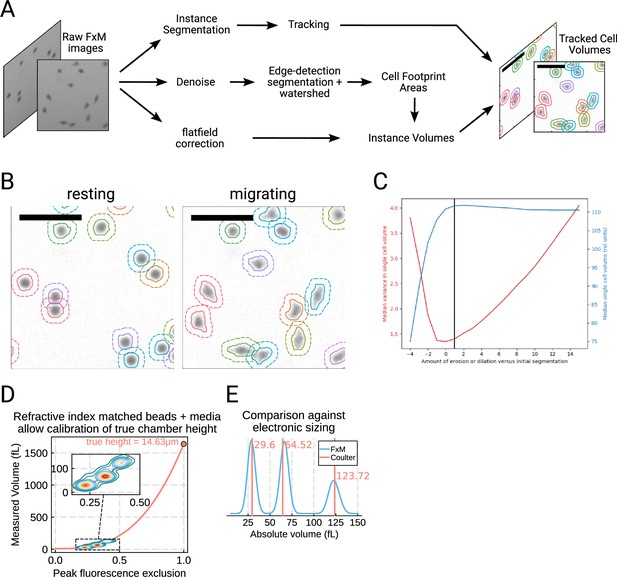
Details and validation of the Fluorescence eXclusion Microscopy pipeline.
(A) Overview of the modified FxM pipeline used in this work.Raw FxM images are segmented into ‘seeds,’ which are then tracked. To identify cell boundaries, we use a custom algorithm that involves denoising the raw FxM followed by edge detection and watershedding that was nucleated at the seeds. Finally, to extract the volumes we apply minimal processing with a custom flatfield correction algorithm followed by extraction using the previously-determined cell boundaries. (B) Examples of resting (left) and migrating (right) cells segmented and tracked using the aforementioned pipeline. (C) Quantifying the signal-to-noise tradeoff with the edge detection. Overly conservative or overly liberal segmentation leads to non-optimal volume measurement. The chosen cutoff (black line) balances the noise (red) and measured volume (blue). This was computed on unstimulated cells. (D) Injecting beads into the FxM chambers in refractive-index media to minimize distortions allows for true calibration of chamber height. The contour plot of the per-bead max signal depth (i.e. height of the beads from FxM) vs the measured volume using FxM is well fit by the spherical volume formula (salmon line). The intersection of this line with 1.0 on the abscissa is equivalent to a sphere that completely fills the chambers and indicates the true height of the chamber. (E) Comparing calibrated FxM volumes of the beads (cyan) against the gold standard Coulter counter (salmon) shows good agreement.
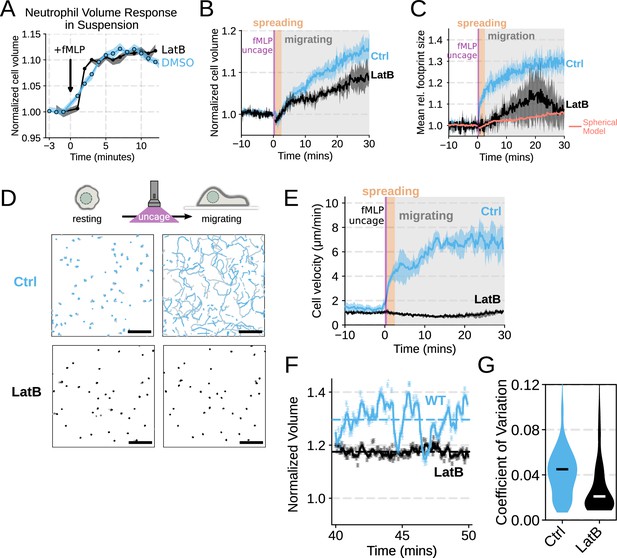
Chemoattractant-induced swelling, but not motility, is independent of actin polymerization.
(A) Human primary neutrophils were incubated with DMSO or Latrunculin B, activated with 20 nM fMLP, and then volume responses were measured using electronic sizing via a Coulter counter. Latrunculin treatment did not alter cell swelling, indicating that actin polymerization is dispensable for the chemoattractant-induced volume increase. (B) Similar results were obtained using the Fluorescence eXclusion Microscopy (FxM) assay, showing that Latrunculin-treated cells are capable of swelling after stimulation. (C) The Latrunculin-treated cells also increase their footprints, albeit less so than control cells, but this is within the range of what would be expected for this degree of chemoattractant-induced volume increase (modeled by a sphere expanding an equivalent volume). (D) Single-cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show 15 min of tracks with the tracks prior (left) and the 15 min post (right) uncaging the chemoattractant. The scale bar is 50 µm. The top panels show the large increase in motility displayed by control cells, while the Latrunculin-treated cells (bottom panels) fail to move. See Figure 1—video 1 for an animated version of this data. (E) Latrunculin-treated cells consistently fail to move in response to chemoattractant-stimulation. (F) Representative single-cell volume traces show that Latrunculin-treated cells (black) lack short-term volume fluctuations but persistently maintain an elevated volume following chemoattractant stimulation. Control cells (blue) exhibit short-term volume fluctuations. (G) The lack of short-term volume fluctuations following latrunculin treatment is borne out across the population, with the coefficient of variation in the volume for single-cells (post-swelling) being dramatically lower in Latrunculin-treated cells, suggesting that these short term volume fluctuations depend on actin-based motility. Raw and processed Fluorescence Exclusion Microscopy data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
Inhibiting actin polymerization blocks neutrophil chemokinesis.
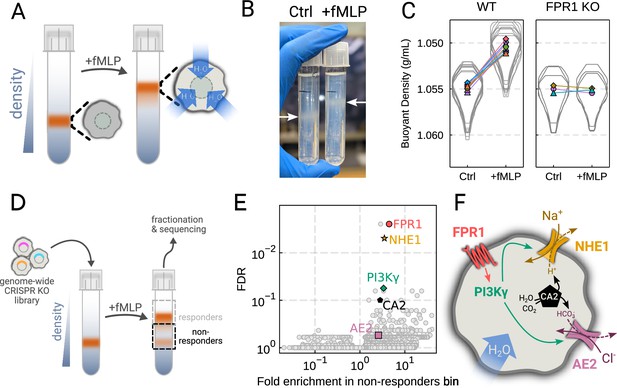
Genome-wide screen identifies regulators of chemoattractant-induced cell swelling.
(A) Schematic detailing the buoyant density assay. The addition of fMLP causes cells to swell and decrease their density. As a result, the stimulated cells float higher in the Percoll density gradient. (B) Representative image of cell density shift following chemoattractant stimulation. Millions of cells appear as white fuzzy bands (indicated with the arrows). Cells in the right tube are stimulated with 20 nM chemoattractant (fMLP), causing them to swell and float higher in the gradient. (C) Violin plots quantifying the relative cell numbers as a function of density. Individual lines link replicate pairs. Wild-type (WT) cells shift from 1.055 g/mL to 1.050 g/mL upon stimulation, while FPR1 KO cells do not shift following fMLP stimulation. (D) Schematic detailing the buoyant density-based genome-wide CRISPR knockout screen for identifying cells that are deficient at chemoattractant-induced cell swelling. (E) Volcano plot of the results of the chemoattractant-induced cell swelling screen. Genes that showed large inhibition of cell swelling and consistent behavior across their targeting guides appear in the upper right. The genes selected for further analysis are highlighted for a more complete list see Supplementary file 1A. (F) Schematic outlining a potential pathway from chemoattractant stimulation to cell swelling.
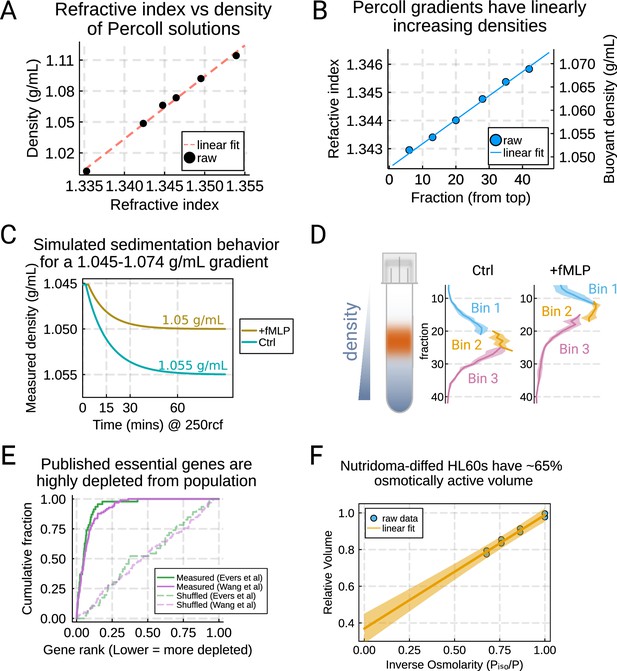
Validation of buoyant density assay and its use in CRISPR KO genome-wide screen for swelling regulators.
(A) Varying the amount of Percoll shows the linear relationship between the refractive index (as measured by a refractometer) and the density as determined by weighing solutions in a volumetric flask on an analytical balance. (B) Measuring the refractive index of different fractions from the gradient shows a high degree of linearity in buoyant density. (C) Simulation of the settling behavior of the control (teal) and stimulated cells (gold) in the Percoll gradients under a centrifugal force of 250 × g. The cells are predicted to arrive at their isopycnic point after approximately 1 hr. (D) The binning strategy for the screen for the control (left) and stimulated (right) conditions. For each replicate of each condition, the cells were split across six different tubes and these were combined into three bins to balance the minimum number of cells per bin with the resolution gained from multiple bins. (E) Previously published essential genes from Evers et al., 2016 and Wang et al., 2015, in green and purple, respectively, were highly depleted from the population, validating functionality of our CRISPR-based knockout library. (F) Ponder’s relation for dHL-60s in suspension. Increasing amounts of hyperosmolarity drives the osmotically active fraction of the volume out of the cell, and projecting out to infinite osmolarity gives 65% of the cell volume as osmotically active.
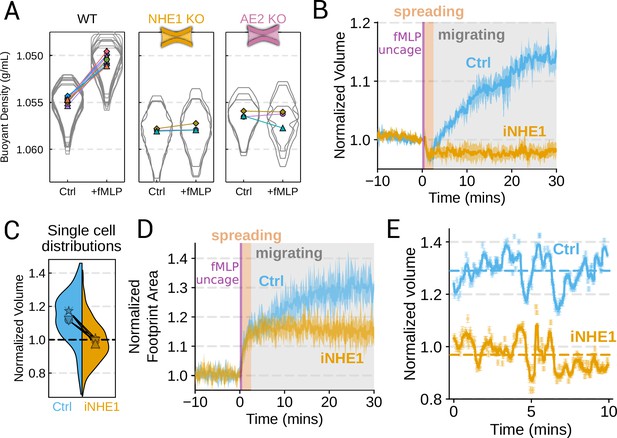
Mechanistically separating chemoattractant versus motility-based volume changes (A) Knockout of NHE1 or AE2 completely inhibits the chemoattractant-induced swelling in neutrophil-like differentiated HL-60 cells.
(B) NHE1 inhibition in human primary neutrophils blocks fMLP-induced swelling but does not inhibit the spreading-induced volume loss. An animated version is available as Video 4. (Ctrl: Volunteer N = 4, iNHE1: Volunteer n = 6). (C) The distributions of single-cell volumes 30 min post-chemoattractant stimulation demonstrates that the NHE1-inhibited neutrophils remain close to the pre-stimulation volumes, i.e., 1.0 on the ordinate. (D) NHE1-inhibited neutrophils have similar increases in their footprint area when they spread and begin moving following fMLP stimulation. (E) High temporal resolution imaging of the motility-induced volume fluctuations starting at 30 min post-stimulation demonstrate that both control and NHE1-inhibited neutrophils show similar short-term volume fluctuations around significantly different baselines (dashed lines). For an animated version, see Videos 2 and 3.
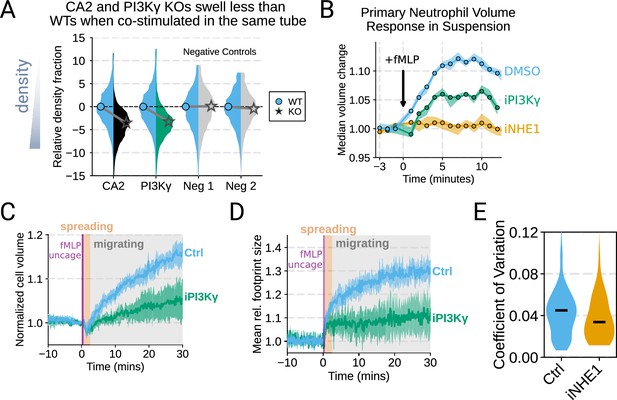
Additional validation of swelling screen hits.
(A) Mixed wild-type (WT) and CRISPR KO dHL-60 populations post-stimulation show that CA2 (black) and PI3Ky (green) KO both fail to decrease their densities as much as the WT (cyan) population following chemoattractant stimulation. Cells with negative control guides (light gray) have normal volume responses. All tubes were fractionated and aligned on the fraction containing the median of the WT population. Negative values indicate a fraction with a higher density than WT. (B) To validate the perturbations to cell swelling observed with Fluorescence eXclusion Microscopy (FxM), primary human neutrophils were stimulated in suspension, and their volumes were measured using a Coulter counter. 20 nM fMLP was added at the 0 min mark. Shaded regions represent the 95% confidence intervals. (C) PI3Kγ inhibition blocks the chemoattractant-induced volume change in primary human neutrophils, as assayed by FxM. (D) PI3Kγ inhibition also blocked the chemoattractant-drive shape change in human primary neutrophils, as measured by the change in footprint area in FxM. (E) The coefficient of variation in volume for control (cyan) and iNHE1 (gold) inhibited human primary neutrophils undergoing chemokinesis are comparable, suggesting that the volume fluctuations are unchanged in moving cells upon NHE1 and PI3Kγ inhibition despite the different baseline volumes. Raw and processed FxM and Coulter data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
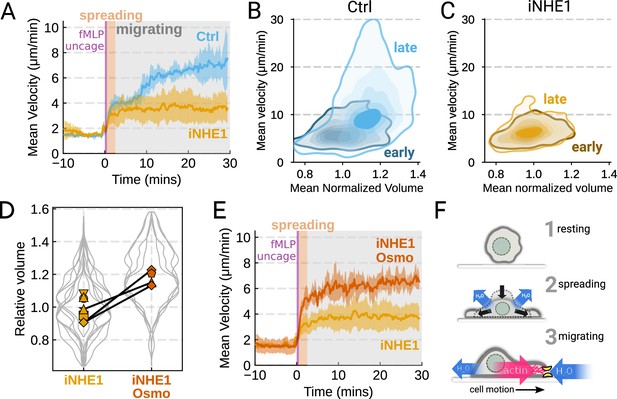
The chemoattractant-driven volume gain is necessary and sufficient for rapid cell migration following stimulation (A) Comparison of control (blue) or NHE1-inhibited (yellow) primary human neutrophil migration following chemoattractant stimulation.
Mean of the per-replicate median cell velocities is shown, with the shaded area indicating standard deviation at each time point. (Ctrl: Volunteer n = 4, iNHE1: Volunteer n = 6). (B) Contour plots of the average velocity versus average normalized volume for single unperturbed neutrophils for the initial 10 min window following stimulation (early) and from 20 to 30 min following stimulation (late). (C) Contour plots of the average velocity versus average normalized volume for single NHE1-inhibited neutrophils for the initial 10 min window following stimulation (early) and from 20 to 30 min following stimulation (late). (D) Dilution of imaging media with 20% water led to a ~15% increase in the median cell volumes of iNHE1 cells (iNHE1 Osmo; red) versus iNHE1 cells in normal media (yellow). Volumes are normalized relative to the median iNHE1 cell volume. This is similar to the magnitude of chemoattractant-induced swelling in control cells. The black lines connect conditions where both conditions were measured for the same volunteer. (iNHE1 Osmo: Volunteer n = 3, 4 total replicates; iNHE1: Volunteer n = 6) (E) Testing the ability of cell swelling to rescue the migration defect in NHE1-inhibited neutrophils. Mean of the pre-replicate median cell velocities computed at each time point for NHE1 inhibited cells (yellow) versus mildly hypoosmotically swollen NHE1 inhibited cells (red). Shaded area is the standard deviation at each time point. (iNHE1 Osmo: Volunteer n = 3, 4 total replicates; iNHE1: Volunteer N = 6). See Video 5 for representative chemokinetic behavior. (F) Summary schematic. Cell swelling collaborates with actin polymerization to potentiate chemoattractant-induced cell migration.
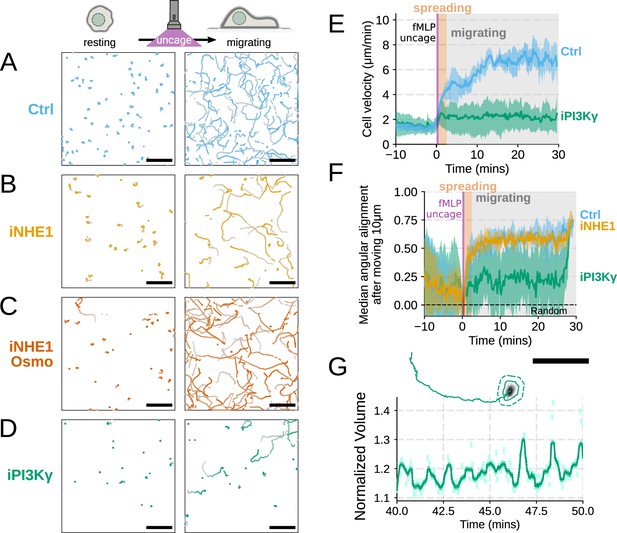
Additional validation of motility phenotypes.
(A–D) Single-cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show tracks of cells 15 min prior (left) versus 15 min post (right) uncaging the chemoattractant. The scale bar is 50 µm. Color saturation indicates time with tracks progressing from gray to full color. Animated versions are available as Video 5 and Figure 4—video 1. (A) Control cells show a large increase in movement upon uncaging, (B) NHE1 inhibited cells also initiate movement but to a lesser degree, (C) hypo-osmotic shock rescues the NHE1 motility defect. (D) PI3Kγ leads to a large fraction of cells failing to initiate movement. (E) PI3Kγ inhibition showed near complete blockage of the chemoattractant-induced motility increase in primary human neutrophils. (F) Control neutrophils (blue) show an increased angular alignment upon stimulation as their motility becomes directional. NHE1-inhibition (gold, iNHE1) has very little effect on this process, while PI3Kγ inhibition (green) leads to a reduction in this alignment at the population level. (G) For the PI3Kγ inhibited cells that start migrating, the migration-induced volume fluctuations are comparable to iNHE1 and control cells. The top panel shows the track of a representative migrating PI3Kγ inhibited cell and the bottom panel, its corresponding volume normalized to the pre-stimulation volume. The scale bar is 50 µm. Raw and processed FxM data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
Inhibiting PI3K signaling interferes with neutrophil chemokinesis.
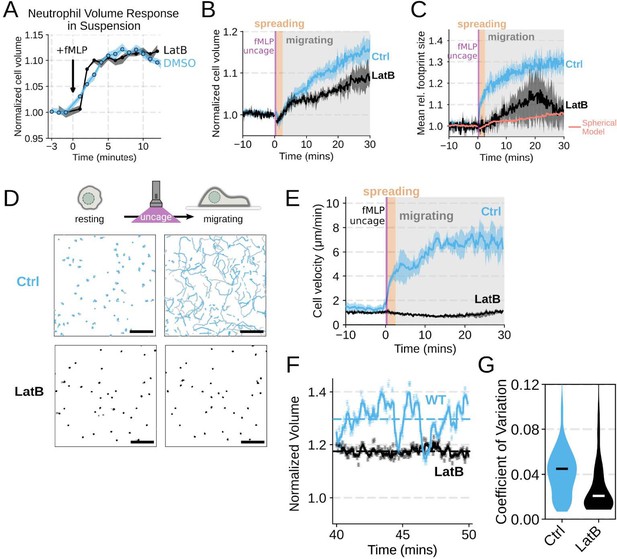
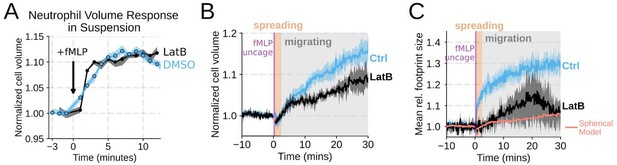
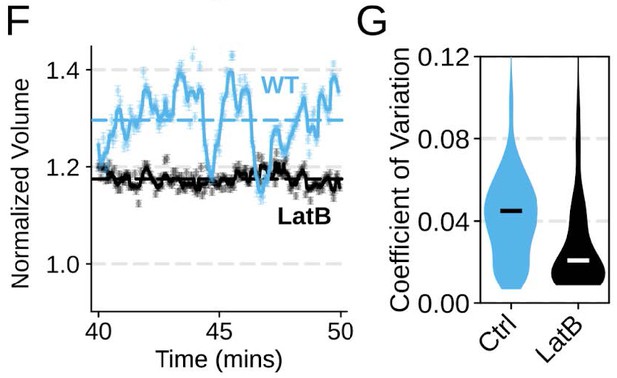
Chemoattractant-induced swelling, but not motility, is independent of actin polymerization.
(A) Human primary neutrophils were incubated with DMSO or Latrunculin B, activated with 20 nM fMLP, and then volume responses were measured using electronic sizing via a Coulter counter. Latrunculin treatment did not alter cell swelling, indicating that actin polymerization is dispensable for the chemoattractant-induced volume increase. (B) Similar results were obtained using the FxM assay, showing that Latrunculin-treated cells are capable of swelling after stimulation. (C) The Latrunculin-treated cells also increase their footprints, albeit less so than control cells, but this is within the range of what would be expected for this degree of chemoattractant-induced volume increase (modeled by a sphere expanding an equivalent volume). (D) Single cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show 15 minutes of tracks with the tracks prior (left) and the 15 minutes post (right) uncaging the chemoattractant. The scale bar is 50 microns. The top panels show the large increase in motility displayed by control cells, while the Latrunculin-treated cells (bottom panels) fail to move. (E) Latrunculin-treated cells consistently fail to move in response to chemoattractant-stimulation. (F) Representative single cell volume traces show that Latrunculin-treated cells (black) lack short-term volume fluctuations but persistently maintain an elevated volume following chemoattractant stimulation. Control cells (blue) exhibit short-term volume fluctuations. (G) The lack of short-term volume fluctuations following latrunculin treatment is borne out across the population, with the coefficient of variation in the volume for single cells (post-swelling) being dramatically lower in Latrunculin-treated cells, suggesting that these short term volume fluctuations depend on actin-based motility.
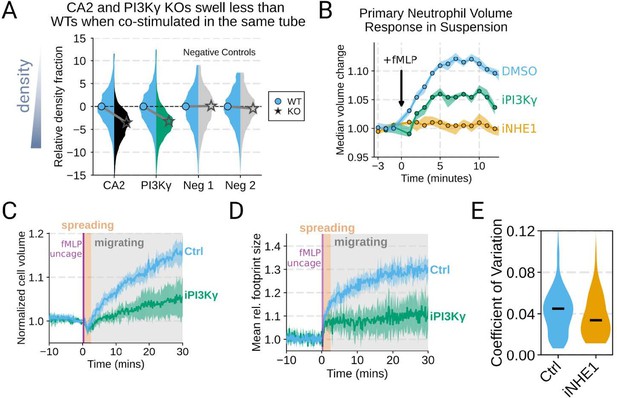
Additional validation of swelling screen hits.
(A) Mixed WT and CRISPR KO dHL-60 populations post-stimulation show that CA2 (black) and PI3Ky (green) KO both fail to decrease their densities as much as the WT (cyan) population following chemoattractant stimulation. Cells with negative control guides (light gray) have normal volume responses. All tubes were fractionated and aligned on the fraction containing the median of the WT population. Negative values indicate a fraction with a higher density than WT. (B) To validate the perturbations to cell swelling observed with FxM, primary human neutrophils were stimulated in suspension, and their volumes were measured using a Coulter counter. 20 nM fMLP was added at the 0 minute mark. Shaded regions represent the 95% confidence intervals. (C) PI3Kγ inhibition blocks the chemoattractant-induced volume change in primary human neutrophils, as assayed by FxM. (D) PI3Kγ inhibition also blocked the chemoattractant-drive shape change in human primary neutrophils, as measured by the change in footprint area in FxM (E) The coefficient of variation in volume for control (cyan) and iNHE1 (gold) inhibited human primary neutrophils undergoing chemokinesis are comparable, suggesting that the volume fluctuations are unchanged in moving cells upon NHE1 and PI3Kγ inhibition despite the different baseline volumes.
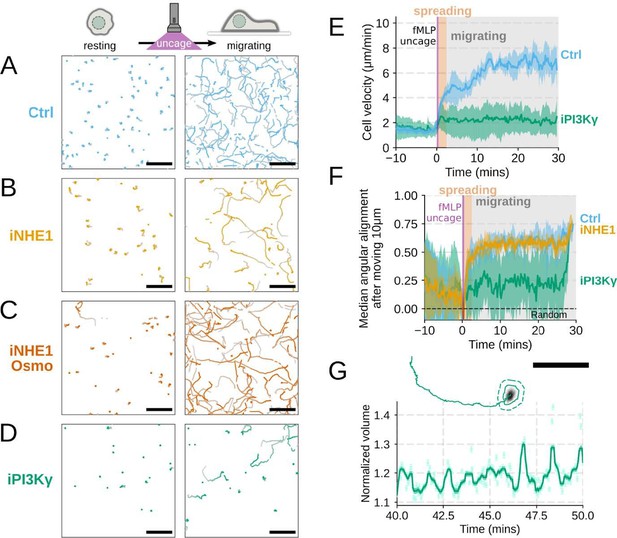
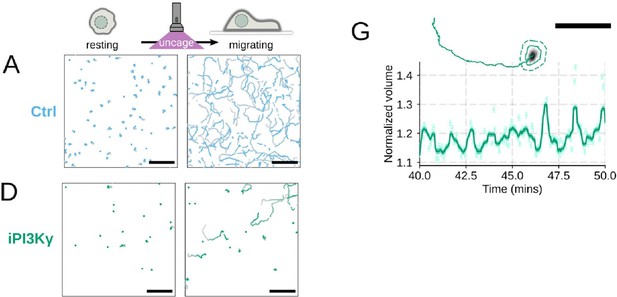
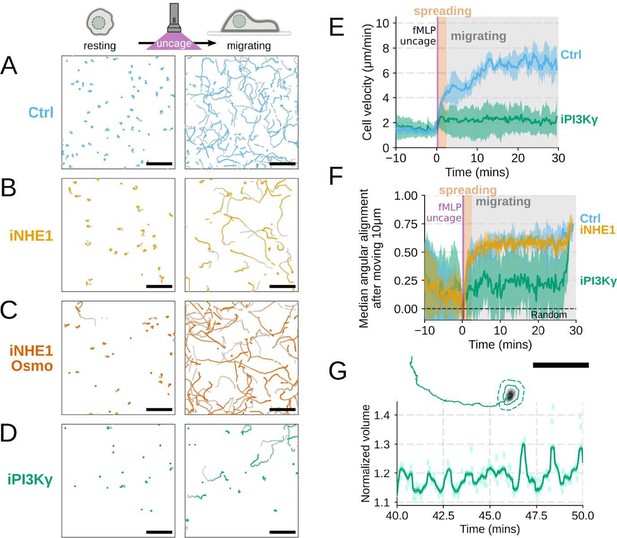
Additional validation of motility phenotypes.
(A-D) Single cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show tracks of cells 15 minutes prior (left) versus 15 minutes post (right) uncaging the chemoattractant. The scale bar is 50 microns. Color saturation indicates time with tracks progressing from gray to full color. (A) Control cells show a large increase in movement upon uncaging, (B) NHE1 inhibited cells also initiate movement but to a lesser degree, (C) hypo-osmotic shock rescues the NHE1 motility defect. (D) PI3Kγ leads to a large fraction of cells failing to initiate movement. (E) PI3Kγ inhibition showed near complete blockage of the chemoattractant-induced motility increase in primary human neutrophils. (F) Control neutrophils (blue) show an increased angular alignment upon stimulation as their motility becomes directional. NHE1-inhibition (gold, iNHE1) has very little effect on this process, while PI3Kγ inhibition (green) leads to a reduction in this alignment at the population level. (G) For the PI3Kγ inhibited cells that start migrating, the migration-induced volume fluctuations are comparable to iNHE1 and control cells. The top panel shows the track of a representative migrating PI3Kγ inhibited cell and the bottom panel, its corresponding volume normalized to the pre-stimulation volume. The scale bar is 50 microns.
Chemoattractant-induced swelling, but not motility, is independent of actin polymerization.
(A) Human primary eutrophils were incubated with DMSO or Latrunculin B, activated with 20 nM fMLP, and then volume responses were measured using electronic sizing via a Coulter counter. Latrunculin treatment did not alter cell swelling, indicating that actin polymerization is dispensable for the chemoattractant-induced volume increase. (B) Similar results were obtained using the FxM assay, showing that Latrunculin-treated cells are capable of swelling after stimulation. (C) The Latrunculin-treated cells also increase their footprints, albeit less so than control cells, but this is within the range of what would be expected for this degree of chemoattractant-induced volume increase (modeled by a sphere expanding an equivalent volume).

Additional validation of swelling screen hits.
(B) To validate the perturbations to cell swelling observed with FxM, primary human neutrophils were stimulated in suspension, and their volumes were measured using a Coulter counter. 20 nM fMLP was added at the 0 minute mark. Shaded regions represent the 95% confidence intervals.
Additional validation of motility phenotypes.
(A-D) Single cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show tracks of cells 15 minutes prior (left) versus 15 minutes post (right) uncaging the chemoattractant. The scale bar is 50 microns. Color saturation indicates time with tracks progressing from gray to full color. (A) Control cells show a large increase in movement upon uncaging. (D) PI3Kγ leads to a large fraction of cells failing to initiate movement. (E) PI3Kγ inhibition showed near complete blockage of the chemoattractant-induced motility increase in primary human neutrophils. (G) For the PI3Kγ inhibited cells that start migrating, the migration-induced volume fluctuations are comparable to iNHE1 and control cells. The top panel shows the track of a representative migrating PI3Kγ inhibited cell and the bottom panel, its corresponding volume normalized to the pre-stimulation volume. The scale bar is 50 microns.

Additional validation of motility phenotypes.
(G) For the PI3Kγ inhibited cells that start migrating, the migration-induced volume fluctuations are comparable to iNHE1 and control cells. The top panel shows the track of a representative migrating PI3Kγ inhibited cell and the bottom panel, its corresponding volume normalized to the pre-stimulation volume. The scale bar is 50 microns.
Chemoattractant-induced swelling, but not motility, is independent of actin polymerization.
(F) Representative single cell volume traces show that Latrunculin-treated cells (black) lack short-term volume fluctuations but persistently maintain an elevated volume following chemoattractant stimulation. Control cells (blue) exhibit short-term volume fluctuations. (G) The lack of short-term volume fluctuations following latrunculin treatment is borne out across the population, with the coefficient of variation in the volume for single cells (post-swelling) being dramatically lower in Latrunculin-treated cells, suggesting that these short term volume fluctuations depend on actin-based motility.
Additional validation of motility phenotypes.
(A-D) Single cell tracks of primary human neutrophils responding to acute chemoattractant stimulation. Both panels show tracks of cells 15 minutes prior (left) versus 15 minutes post (right) uncaging the chemoattractant. The scale bar is 50 microns. Color saturation indicates time with tracks progressing from gray to full color. (A) Control cells show a large increase in movement upon uncaging, (B) NHE1 inhibited cells also initiate movement but to a lesser degree, (C) hypo-osmotic shock rescues the NHE1 motility defect. (D) PI3Kγ leads to a large fraction of cells failing to initiate movement. (E) PI3Kγ inhibition showed near complete blockage of the chemoattractant-induced motility increase in primary human neutrophils. (F) Control neutrophils (blue) show an increased angular alignment upon stimulation as their motility becomes directional. NHE1-inhibition (gold, iNHE1) has very little effect on this process, while PI3Kγ inhibition (green) leads to a reduction in this alignment at the population level. (G) For the PI3Kγ inhibited cells that start migrating, the migration-induced volume fluctuations are comparable to iNHE1 and control cells. The top panel shows the track of a representative migrating PI3Kγ inhibited cell and the bottom panel, its corresponding volume normalized to the pre-stimulation volume. The scale bar is 50 microns.
Videos
Primary human neutrophils show a biphasic volume response to chemoattractant stimulation.
Left panel shows the individual cells (cyan solid lines) with their individual localities (dashed lines). The actual Fluorescence eXclusion Microscopy (FxM) signal is visible within the cell footprints. The video is 40 min long with chemoattractant uncaging after the first 10 min. The scale bar is 50 µm. The right panel shows the corresponding median volume of the population (cyan) with the 95% confidence interval (light cyan). The volume is relative to the average over the 2 min window prior to uncaging. Data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
Single-cell volume tracking reveals motility-associated volume changes in migrating primary human neutrophils.
Top panel shows a representative cell migrating starting at 40 min following chemoattractant stimulation. The solid cyan line is the cell track. The cell’s footprint is denoted with a solid cyan line while its local background is encapsulated by the dashed cyan line. The scale bar is 50 µm. The bottom panel shows the concurrent volume changes with the raw Fluorescence eXclusion Microscopy (FxM) measurements displayed in light cyan and the rolling median is shown in cyan. The volume is normalized to the median cell volume in the 2 min window prior to stimulation. Data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
NHE1-inhibited primary human neutrophils retain the motility-related volume fluctuations but at much lower baseline volumes.
Top panel shows a representative cell migrating starting at 30 min following chemoattractant stimulation. The solid gold line is the cell track and the cell’s footprint is denoted with a solid gold line while its local background is encapsulated by the dashed gold line. The scale bar is 50 µm. The bottom panel shows the concurrent volume changes with the raw Fluorescence eXclusion Microscopy (FxM) measurements displayed in light gold and the rolling median is shown in gold. The volume is normalized to the median cell volume in the 2 min window prior to stimulation. Data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
NHE1 inhibition blocks chemoattractant-induced cell swelling and impairs chemokinesis.
Left panel shows the individual cells (cyan or gold solid lines) with their individual localities (dashed lines). The top left panel are control cells (cyan) and the bottom left are NHE1 inhibited (gold). The actual Fluorescence eXclusion Microscopy (FxM) signal is visible within the cell footprints. The video is 40 min long with chemoattractant uncaging after the first 10 min. The scale bars are 50 µm. The right panel shows the corresponding median volume of the population in cyan and gold for control and iNHE1, respectively. The corresponding 95% confidence interval is in light cyan or gold. The volume is relative to the average over the 2 min window prior to uncaging. Data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
NHE1-inhibition leads to impaired motility versus control, which is rescued by mild hypoosmotic shock.
Each panel is a representative video of control, NHE1-inhibited, and hypoosmotically shocked NHE1-inhibited cells, respectively. Each video is 40 min long and chemoattractant uncaging occurs after 10 min. Scale bars are 50 µm. Data available via Dryad; see https://doi.org/10.7272/Q6NS0S5N.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/90551/elife-90551-mdarchecklist1-v1.docx
-
Supplementary file 1
Extended data tables for CRISPR screen and volunteer demographic information.
(A) Chemoattractant-induced swelling genome-wide CRISPR KO screen hits. Rankings determined using MAGeCK Li et al., 2014. Fold change is the median fold enrichment in the dense bin versus the other two bins of the functional guides. FDR is the false discovery rate of each gene given the distribution of negative control guides in the library. See Methods section for details. (B) Volunteer Demographic Information. Demographic information collected for the volunteer donors according to the Institutional Review Board-approved study protocol at the University of California - San Francisco (Study #21-35147). (C) Guides used to make single gene knockouts in HL-60s. The two highest performing guides from the genome-wide screen were chosen to make single gene knockouts in the HL-60 cell line. See Methods for details.
- https://cdn.elifesciences.org/articles/90551/elife-90551-supp1-v1.docx





