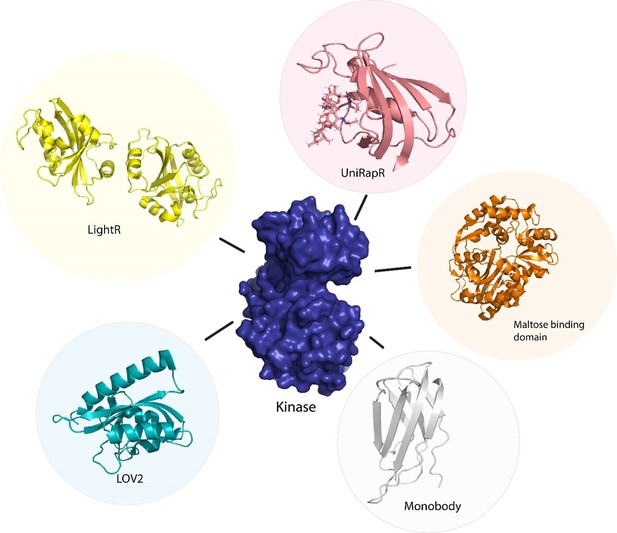
Allosteric regulation of kinase activity in living cells
Figures
Figure 2
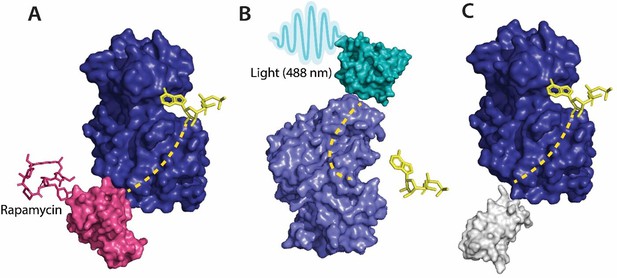
Various tools used as allosteric regulators.
(A) Allosteric activation of kinase (ERK2 kinase, PDB ID: 4GT3) by uniRapR (PDB ID: 7F2J) domain that binds to small molecule (rapamycin) causing activation of the protein. (B) Allosteric inhibition of kinase (ERK2 kinase, PDB ID: 4GT3) by insertion of an optogenetic control protein, LOV2 (PDB ID: 2V0W), causing conformational change upon irradiation by blue light. (C) Activation of kinase using monobodies (PDB ID: 3RZW).
-
Figure 2—source data 1
Source data for Figure 2.
- https://cdn.elifesciences.org/articles/90574/elife-90574-fig2-data1-v1.docx
Additional files
Download links
A two-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Allosteric regulation of kinase activity in living cells
eLife 12:RP90574.
https://doi.org/10.7554/eLife.90574.4