Genetic code expansion, click chemistry, and light-activated PI3K reveal details of membrane protein trafficking downstream of receptor tyrosine kinases
Figures
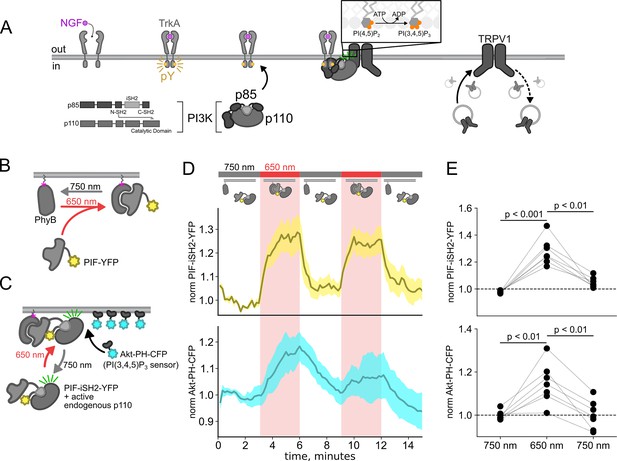
Using the PhyB/PIF system to activate phosphoinositide 3-kinases (PI3K) with light.
The OptoPI3K system reversibly activates PI3K to generate phosphoinositide 3,4,5-trisphosphate (PI(3,4,5)P3) at the plasma membrane (PM). (A) Diagram of PI3K subunits and domains illustrating the regulatory p85 and catalytic p110 subunits. Inter-SH2 (iSH2) domain in p85 subunit interacts with p110. Binding of nerve growth factor (NGF) to TrkA receptor triggers the translocation of PI3K to the PM, phosphorylation of phosphoinositide 4,5-bisphosphate (PI(4,5)P2) to PI(3,4,5)P3, and fusion of TRPV1-containing vesicles with the PM. (B, C) Schematic diagram for OptoPI3K system using PIF-YPF or PIF-iSH2-YFP. PhyB-mCherry is tethered to the PM using CAAX lipidation (magenta star). The iSH2 domain of p85 is fused to PIF so that translocation of PIF-iSH2-YFP, together with endogenous p110, to the PM promotes PI(3,4,5)P3 synthesis upon 650 nm light. (D) Monitoring PIF-iSH2-YFP translocation to and from the PM with 650 and 750 nm light, respectively (top, yellow). Synthesis of PI(3,4,5)P3 follows PIF-iSH2-YFP translocation to the PM, as indicated by the localization of the PI(3,4,5)P3 probe Akt-PH-CFP (bottom, sky blue). F-11 cells transiently expressing PhyB-mCherry-CAAX, PIF-iSH2-YFP, and Akt-PH-CFP were illuminated with 750 or 650 nm light as indicated with the upper bar. Collected traces of PIF-iSH2-YFP and Akt-PH-CFP normalized to the initial baselines during the first episode of 750 nm illumination. The black line indicates the mean of the data and the colored envelope represents the standard error of the mean (n=8). Because of the very low density of PI(3,4,5)P3 present in the PM even in light- or NGF-stimulated cells (Auger et al., 1989), we used total internal reflection fluorescence (TIRF) microscopy to measure PI(3,4,5)P3 density instead of confocal microscopy. TIRF illumination decreases exponentially with distance from the coverslip, selectively illuminating and exciting fluorophores within ~150 nm of the PM (Lakowicz, 2006; Mattheyses and Axelrod, 2006). (E) Scatter plot of PIF-iSH2-YFP and Akt-PH-CFP fluorescence for individual cells. Each point represents the 20 s average for 750 nm (2.66–3 min), 650 nm (5.66–6 min), and 750 nm (8.66–9 min). Translocation of both PIF-iSH2-YFP and Akt-PH-CFP is reversible.
-
Figure 1—source data 1
Excel data for the time courses (Figure 1D and E).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig1-data1-v1.xlsx
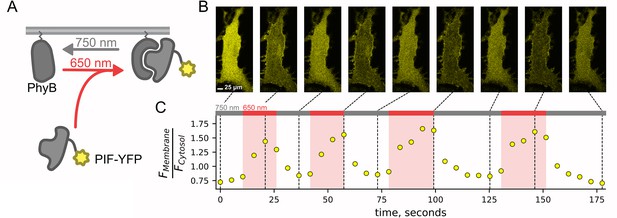
PIF-YFP translocates to plasma membrane (PM) in response to 650 nm light and back to the cytosol in response to 750 nm light.
The PhyB-PIF light-inducible interaction is rapid (a few tens of seconds) and fully reversible (within ~30 s) and can be repeated multiple times. (A) Schematic diagram for optogenetic PhyB-PIF system. PhyB-mCherry loaded with the chromophore phycocyanobilin localizes to the PM due to a CAAX tag to induce lipidation (squiggly line). Illumination with 650 nm light induces a conformational change in PhyB that increases its affinity for PIF-YFP, effectively recruiting PIF-YFP to the PM. The conformational change in PhyB reverses with illumination with 750 nm light, causing PIF-YFP to dissociate and return to the cytoplasm. (B) A representative confocal experiment with an NIH3T3 cell stably expressing PhyB-mCherry-CAAX and PIF-YFP. Images were obtained at times indicated in (C) and are all shown on the same lookup table. During illumination with 650 nm light, PIF-YFP translocated to the PM quickly, with a corresponding decrease in cytoplasmic fluorescence. (C) Ratio of measured PIF fluorescence at region of interest (ROI) placed at the PM (FMembrane) and cytoplasm (Fcytosol) from the cell in (B). These data recapitulate previously published data (Levskaya et al., 2009; Toettcher et al., 2011).
-
Figure 1—figure supplement 1—source data 1
Confocal images (.lsm) generated by Zeiss 710 confocal microscope (Figure 1—figure supplement 1B).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Excel data for the time course (Figure 1—figure supplement 1C).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig1-figsupp1-data2-v1.xlsx
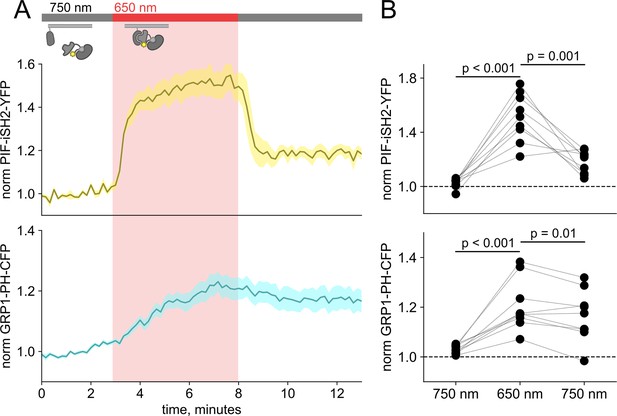
Detection of phosphoinositide 3,4,5-trisphosphate (PI(3,4,5)P3) generated by PIF-iSH2-YFP at the plasma membrane (PM) using GRP1-PH-CFP.
(A) Total internal fluorescence (TIRF) measurements of F-11 cells expressing PhyB-mCherry, PIF-iSH2-YFP, and GRP1-PH-CFP (n=9). Upon 650 nm illumination, PIF PIF-iSH2-YFP translocated to the PM quickly (upper trace) while GRP1-PH-CFP moved slower (lower trace). Normalized to the initial 3 min control period. (B) Scatter plot of PIF-iSH2-YFP and GRP1-PH-CFP fluorescence for individual cells. Each point represents the 30 s average for 750 nm (2.5–3 min), 650 nm (7.5–8 min), and 750 nm (12.5–13 min). Translocation of both PIF-iSH2-YFP and GRP1-PH-CFP is reversible.
-
Figure 1—figure supplement 2—source data 1
Excel data for the time courses (Figure 1—figure supplement 2A and B).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig1-figsupp2-data1-v1.xlsx
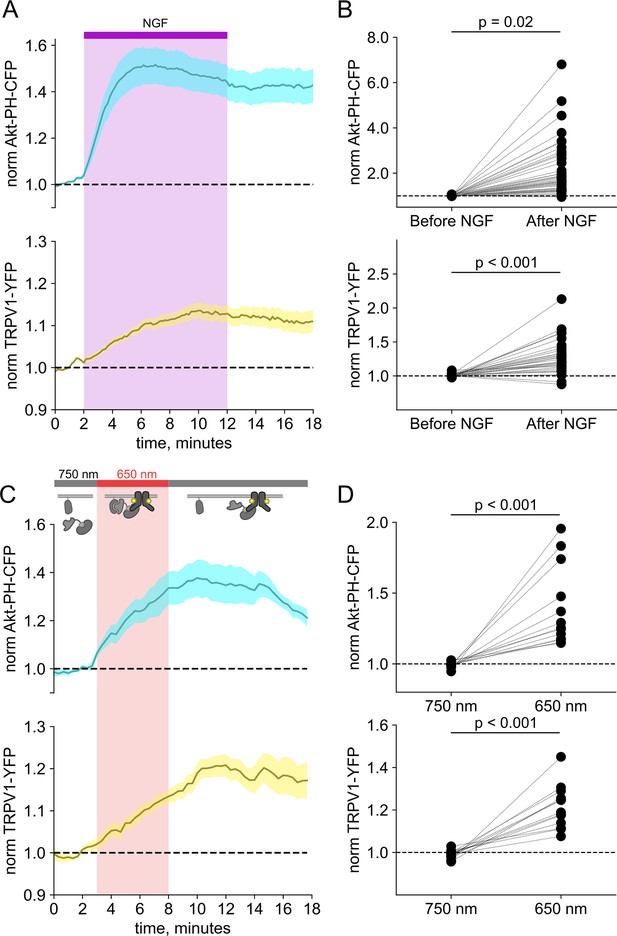
Activation of phosphoinositide 3-kinases (PI3K) with light is sufficient to induce trafficking of TRPV1 to the plasma membrane (PM).
Simultaneous total internal fluorescence (TIRF) measurement of phosphoinositide 3,4,5-trisphosphate (PI(3,4,5)P3) (cyan) and TRPV1 (yellow) in the PM in response to either (A) nerve growth factor (NGF) or (C) light. (A) F-11 cells were transfected with TrkA/p75NTR, Akt-PH-CFP, and TRPV1-YFP. NGF (100 ng/mL) was applied during the times indicated by the bar/shading. Plotted are the PM-associated fluorescence in Akt-PH-CFP (top, cyan) and TRPV1-YFP (bottom, yellow) within the cell footprints. Data are reproduced from Stratiievska et al., 2018. (C) F-11 cells transfected with PhyB-mCherry-CAAX, PIF-iSH2 (without a fluorescent tag), Akt-PH-CFP, and TRPV1-YFP were illuminated with 750 or 650 nm light as indicated. Color scheme as in (A), with line indicating the mean and envelope indicating the standard error of the mean (n=13 for Akt-PH-CFP; n=16 for TRPV1-YFP). Note the poor or irreversible increase of PM PI(3,4,5)P3 in the PM. Inset cartoons depict the model for retention of iSH2 at the PM via binding to TRPV1. (B, D) Scatter plots for individual cells. Each point represents the 20 s average for before (0.83–1.17 min) and after NGF (14.8–15.2 min) or for before (1.31–1.63 min) and after 650 nm (10.8–11.2 min).
-
Figure 2—source data 1
These data are reproduced from Stratiievska et al., 2018 (Figure 2A and B).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig2-data1-v1.xlsx
-
Figure 2—source data 2
Excel data for time courses and scatter plots (Figure 2C and D).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig2-data2-v1.xlsx
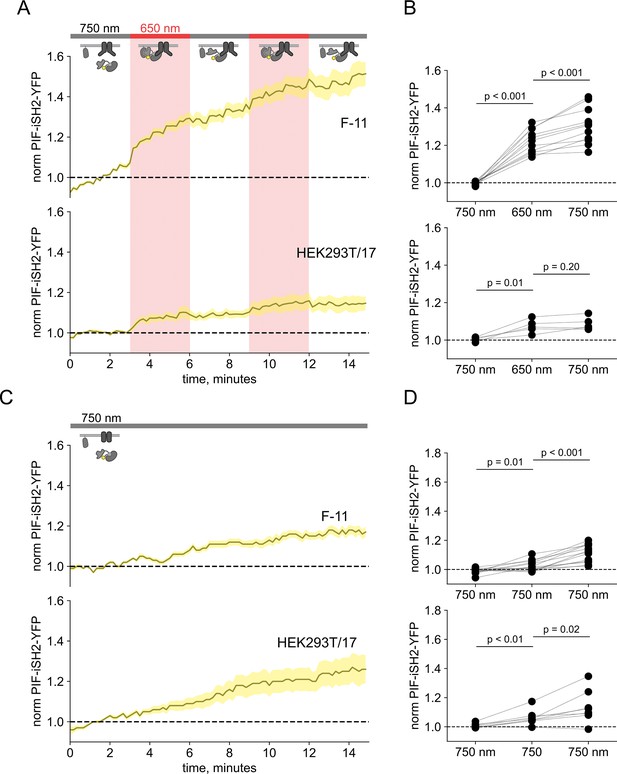
750 nm light fails to cause inter-SH2 (iSH2) dissociation from the plasma membrane (PM) in TRPV1-expressing cells.
(A) Total internal fluorescence (TIRF) measurements of PIF-iSH2-YFP from F-11 and HEK293T/17 cells expressing TRPV1-CFP (n=5–17). The color code and lines/envelopes have the same meaning as in Figure 2. Inset cartoons depict the model for retention of iSH2 at the PM via binding to TRPV1, which contains the PI3K-binding ankyrin repeat domain (ARD). (B) Scatter plot of PIF-iSH2-YFP fluorescence for individual cells. Each point represents the 20 s average for 750 nm (1.33–1.66 min), 650 nm (4.33–4.66 min), and 750 nm (7.33–7.66 min). Translocation of PIF-iSH2-YFP is irreversible. (C, D) The same experiments with continuous illumination at 750 nm (n=5–13).
-
Figure 2—figure supplement 1—source data 1
Excel data for time courses and scatter plots in F-11 and HEK cells.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig2-figsupp1-data1-v1.xlsx
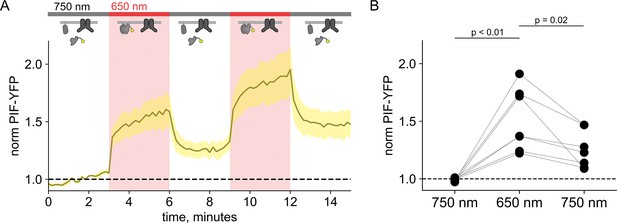
PIF-YFP dissociates from the plasma membrane (PM) in response to 750 nm light even in TRPV1-expressing cells.
TRPV1 does not retain PIF lacking inter-SH2 (iSH2) at the PM of F-11 cells. (A) Normalized total internal fluorescence (TIRF) fluorescence was recorded in F-11 cells transfected with PhyB-mCherry-CAAX, PIF-YFP (no iSH2 domain), and either TRPV1-CFP or TRPV1 (no fluorescent tag). Reversible translocation of PIF-YFP upon 650 nm illumination demonstrates that PIF lacking iSH2 domain does not interact with TRPV1 channels on the PM (n=8). (B) Scatter plot of PIF-iSH2-YFP fluorescence for individual cells. Each point represents the 20 s average for 750 nm (1.33–1.66 min), 650 nm (4.33–4.66 min), and 750 nm (7.33–7.66 min).
-
Figure 2—figure supplement 2—source data 1
Excel data for time course and scatter plot.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig2-figsupp2-data1-v1.xlsx
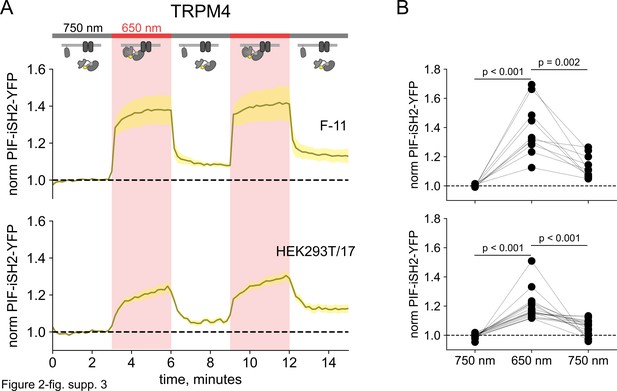
750 nm light succeeds in causing inter-SH2 (iSH2) dissociation from the plasma membrane (PM) in TRPM4-expressing cells.
(A) Total internal fluorescence (TIRF) measurements of PIF-iSH2-YFP from F-11 and HEK293T/17 cells expressing TRPM4-CFP (n=7–17). Inset cartoons depict the model for no retention of iSH2 at the PM via binding to TRPM4, which has no ankyrin repeats. (B) Scatter plot of PIF-iSH2-YFP fluorescence for individual cells. Each point represents the 20 s average for 750 nm (1.33–1.66 min), 650 nm (4.33–4.66 min), and 750 nm (7.33–7.66 min).
-
Figure 2—figure supplement 3—source data 1
Excel data for time courses and scatter plots in F-11 and HEK cells.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig2-figsupp3-data1-v1.xlsx
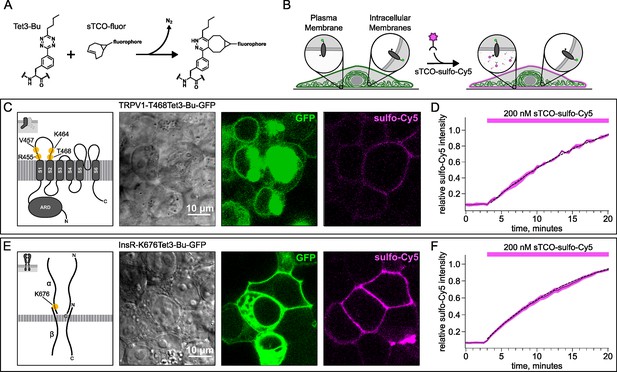
Labeling the TRPV1 and InsR with membrane-impermeant sTCO-Cy5.
Confocal imaging illustrates the labeling of membrane proteins incorporating the noncanonical amino acid (ncAA) Tet3-Bu with sTCO-sulfo-Cy5 in HEK293T/17 cells. The membrane-impermeable dye labeled only the proteins on the plasma membrane (PM). (A) Schematic of the reaction between Tet3-Bu and sTCO-conjugated dyes. (B) Cartoon representing the selective labeling of membrane proteins incorporating Tet3-Bu at an extracellular site with membrane-impermeant sTCO-sulfo-Cy5. (C, E) Confocal images of HEK293T/17 cells expressing (C) TRPV1-468Tet3-Bu-GFP or (E) InsR-676Tet3-Bu-GFP. GFP fluorescence reflects expression of the proteins in the confocal volume across the field of view. Initially the cells did not show any detectable Cy5 fluorescence but after incubation of several minutes of 200 nM sTCO-sulfo-Cy5 showed Cy5 fluorescence at the PM. The Cy5 images shown for (C) TRPV1-Tet3-Bu and (E) InsR-Tet3-Bu were obtained at the end of the experiment (20 min). (D, F) The graphs summarize the Cy5 fluorescence at the PM in (D) TRPV1-Tet3-Bu-GFP or (F) InsR-Tet3-Bu-GFP-expressing cells. Solid traces represent the mean and envelopes the standard error of the mean (n=3 for TRPV1 and n=11 for InsR). Dashed traces represent a fit to the mean with a single exponential (tau = 17.8 min for TRPV1; tau = 13.8 min for InsR). Fits to the individual time courses for all the cells gave a mean of 18.2 min for TRPV1 (±2.2 min) and 19.3 min for InsR (±4.0 min).
-
Figure 3—source data 1
Original confocal microscopic images for TRPV1 labeling (Figure 3C).
Figure 3—source data 1_BF_TRPV1.lsm: the images contain GFP (green) and bright field (black-white). Figure 3—source data 1_GFP_Cy5_ TRPV1.lsm: the images contain GFP (green) and Cy5 (red). The last images were shown.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-data1-v1.zip
-
Figure 3—source data 2
Original confocal microscopic images for InsR labeling (Figure 3E).
Figure 3—source data 1_BF_InsR.lsm: the images contain GFP (blue) and bright field (black-white). Figure 3—source data 1_GFP_Cy5_InsR.lsm: the images contain GFP (blue) and Cy5 (red). The last images were shown.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-data2-v1.zip
-
Figure 3—source data 3
Excel data for scatter plots for TRPV1 and InsR labeling (Figure 3D and F).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-data3-v1.xlsx
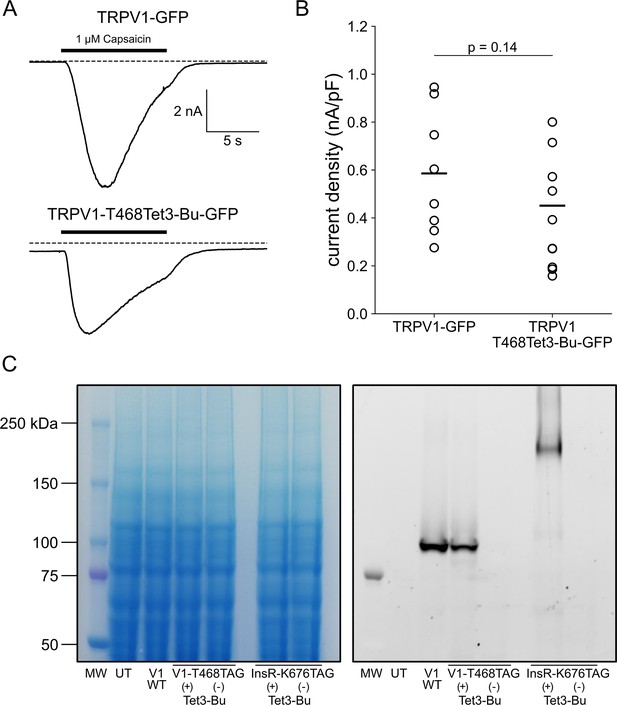
Incorporation of Tet3-Bu into TRPV1 and InsR.
(A) Whole-cell patch clamp recording demonstrates that TRPV1 incorporating Tet3-Bu remains functional. Application of 1 μM capsaicin to HEK-293T/17 cells transfected with either TRPV1-GFP (top) or TRPV1-468Tet3-Bu (bottom) induced inward currents at a holding potential of –60 mV. (B) Collected whole-cell electrophysiology data from all cells with peak capsaicin-activated currents normalized to the area of cell membrane (n=8 and 9 for TRPV1-GFP and TRPV1-468Tet3-Bu, respectively). (C) In-gel fluorescence screening demonstrates that expression of full-length TRPV1-468Tet3-Bu or InsR-676Tet3-Bu requires the presence of Tet3-Bu (‘Tet3-Bu (+)’). Wild type TRPV1-GFP is shown for reference. Detergent-extracted cell lysates were run on SDS/PAGE. GFP fluorescence (left) in the gels was measured and then gels were stained with Coomassie blue dye (right).
-
Figure 3—figure supplement 1—source data 1
Representative whole-cell patch clamp recordings.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp1-data1-v1.xlsx
-
Figure 3—figure supplement 1—source data 2
Whole-cell electrophysiology data from all cells with peak capsaicin-activated currents.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp1-data2-v1.xlsx
-
Figure 3—figure supplement 1—source data 3
Original gel image with Coomassie blue staining, original fluorescent gel image, and labeled gel images.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp1-data3-v1.zip
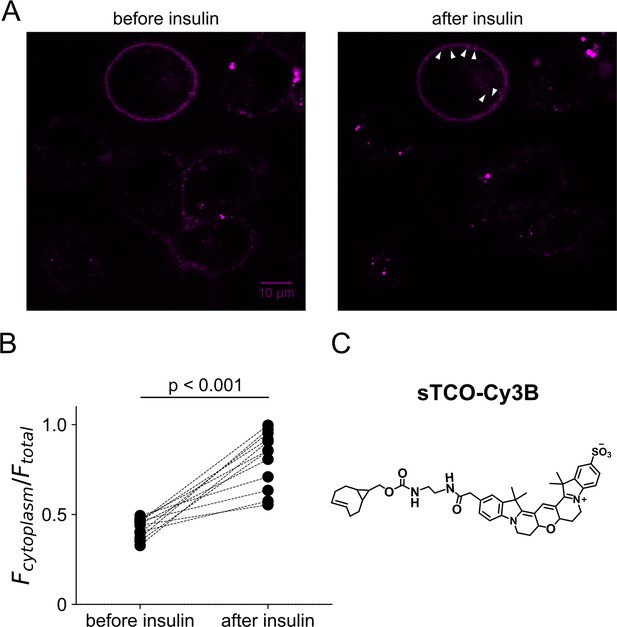
Activity of InsR-K676TAG measure as its recycling upon insulin treatment.
F-11 cells were transfected with the genetically modified receptor and inspected with a confocal microscope. (A) After labeling with membrane-impermeable sTCO-Cy3B dyes, the receptor was visible in the plasma membrane (PM). Application of 100 nM insulin triggered the generation of endocytic granules (arrow heads). In some cells the receptor recycling was so vigorous that PM receptors nearly disappeared. (B) Scatter plot of the ratio of fluorescence between the cytoplasm and total cell area in individual cells (n=12). (C) Structure of sTCO-Cy3B.
-
Figure 3—figure supplement 2—source data 1
Original confocal microscopic images for InsR endocytosis before (9th image) and after insulin (22nd image) treatment.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp2-data1-v1.zip
-
Figure 3—figure supplement 2—source data 2
Excel data for scatter plot for InsR endocytosis (Figure 3—figure supplement 2B).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp2-data2-v1.xlsx
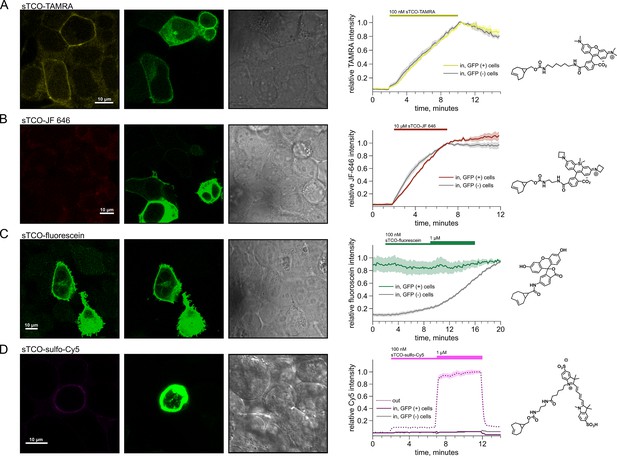
Labeling of cells with sTCO conjugates of TAMRA, JF 646, fluorescein, and sulfo-Cy5.
HEK293T/17 cells expressing InsR-Tet3-Bu-GFP were labeled with sTCO dyes. For all dyes, shown are (left) confocal images of a representative field of HEK293T/17 cells incubated with the indicated concentration of sTCO-conjugated dye, (center) plots of the kinetics of cell labeling, and (right) diagrams of the fluorescent dyes. The confocal images were obtained ~30 s after washing off of extracellular dyes. GFP and bright-field images are presented to demonstrate GFP-expressing (GFP(+)) and non-expressing (GFP(-)) cells. (A) 100 nM sTCO-TAMRA; (B) 10 µM sTCO-JF 646; (C) 100 nM and 1 µM sTCO-fluorescein; and (D) 100 nM and 1 µM sTCO-sulfo-Cy5. For the plots, the intracellular fluorescence was normalized to the peak value at the end of the dye applications (A–C) or to the fluorescence from 1 µM extracellular sTCO-sulfo-Cy5 (D). The thick lines represent the mean of 6–9 cells for each dye, and the envelopes represent the standard errors of the mean.
-
Figure 3—figure supplement 3—source data 1
Original confocal microscopic images for TAMRA (90th image, plus GFP and bright field), JF 646 (72nd image, plus GFP and bright field), fluorescein (126th image, plus GFP and bright field), and Cy5 (115th image, plus GFP and bright field).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp3-data1-v1.zip
-
Figure 3—figure supplement 3—source data 2
Excel data for labeling kinetics of sTCO dyes (Figure 3—figure supplement 3A–D).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig3-figsupp3-data2-v1.xlsx
Activity of InsR-K676TAG measure as its recycling upon insulin treatment.
The movie reflects the same measurements as Figure 3—figure supplement 2A. Images were recorded every 10 s. The time stamp is indicated in the upper right corner and the time of insulin application is indicated in the upper left corner.
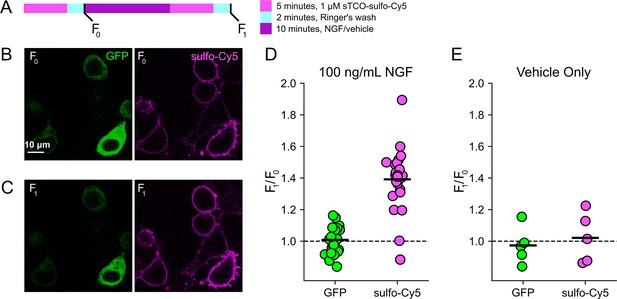
Click chemistry labeling of TRPV1-468Tet3-Bu-GFP with sTCO-Cy5 to measure nerve growth factor (NGF)-induced trafficking of TRPV1 to the plasma membrane (PM).
HEK293T/17 cells expressing TRPV1-Tet3-Bu-GFP and NGF receptor were labeled with extracellular sTCO-sulfo-Cy5 and inspected with confocal microscopy. (A) Experimental protocol. Cells were incubated with 1 µM sTCO-sulfo-Cy5 for 5 min and free dye removed from the bath by washing for 2 min with dye-free Ringer’s solution (‘pulse-chase’ labeling, F0). Then the cells were treated with 100 ng/mL NGF for 10 min before the second sulfo-Cy5 labeling (F1). Confocal images after initial sTCO-sulfo-Cy5 labeling (B) and after the 10 min treatment with NGF and subsequent sTCO-sulfo-Cy5 labeling (C). (D) Summary scatter plot from multiple measurements, with individual experiments shown as dots and the mean of the experiments as black bars. The effect of NGF on GFP and sulfo-Cy5 signals is presented as a ratio of F1/F0 (n=24). After NGF treatment, the ratio increased for sulfo-Cy5 significantly (p<0.001) but not for GFP (p=0.64). (E) The same experiment without NGF treatment (‘vehicle only’, n=5). Vehicle treatment did not change both GFP (p=0.63) and sulfo-Cy5 (p=0.78).
-
Figure 4—source data 1
Original confocal microscopic images for TRPV1 trafficking.
F0: 15th image, F1: 32nd image, GFP (green) and Cy5 (red) (Figure 4B and C).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig4-data1-v1.zip
-
Figure 4—source data 2
Excel data for scatter plot for TRPV1 trafficking (Figure 4D and E).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig4-data2-v1.xlsx
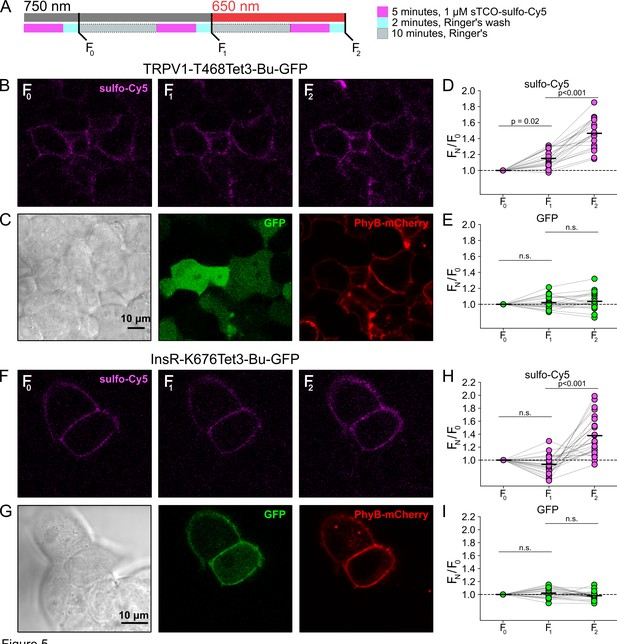
Measuring light-activated phosphoinositide 3-kinases (PI3K)-induced TRPV1 and InsR trafficking to the plasma membrane (PM) using click chemistry.
(A) Illustration of the experimental protocol. HEK293T/17 cells expressing TRPV1-468Tet3-Bu-GFP and InsR-676Tet3-Bu-GFP. (B) Confocal images of sulfo-Cy5 obtained at different stages as depicted in (A). (C) Bright-field (left) and confocal (middle and right) images obtained at the end of experiment. Comparison of bright field (left) and GFP (middle) distinguishes TRPV1-Tet3-Bu-GFP-expressing cells from untransfected cells. PhyB-mCherry images (red) indicate that most TRPV1-positive cells expressed significant levels of the PhyB/PIF machinery for activating PI3K. (D, E) Summary scatter plots from multiple experiments, with individual cells shown as dots and the mean shows as black lines (n=20). The effects of 750 and 650 nm illumination on sulfo-Cy5 (D) and GFP (E) are presented as ratios of fluorescence intensity after illumination with the indicated wavelength of illumination to the initial fluorescence. (F–I) The same experiment for InsR-676Tet3-Bu-GFP trafficking (n=24).
-
Figure 5—source data 1
Original confocal microscopic images for TRPV1 trafficking (Figure 5B and C).
Figure 5—source data 1_TRPV1_Cy5_750_1.lsm, Figure 5—source data 1_TRPV1_Cy5_750_2.lsm, Figure 5—source data 1_TRPV1_Cy5_650.lsm: the mages contain GFP (green) and Cy5 (red). Figure 5—source data 1_TRPV1_ BF_PhyB.lsm: the mages contain bright field and PhyB (magenta).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-data1-v1.zip
-
Figure 5—source data 2
Scatter plot for TRPV1 trafficking (Figure 5D and E).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-data2-v1.xlsx
-
Figure 5—source data 3
Original confocal microscopic images for InsR trafficking (Figure 5F and G).
Figure 5—source data 1_InsR_750_1.lsm, Figure 5—source data 1_InsR_750_2.lsm, Figure 5—source data 1_InsR_650.lsm: the mages contain GFP (green) and Cy5 (red). Figure 5—source data 1_BF_PhyB.lsm: the images contain bright field and PhyB (magenta).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-data3-v1.zip
-
Figure 5—source data 4
Excel data for scatter plot for InsR trafficking (Figure 5H and I).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-data4-v1.xlsx
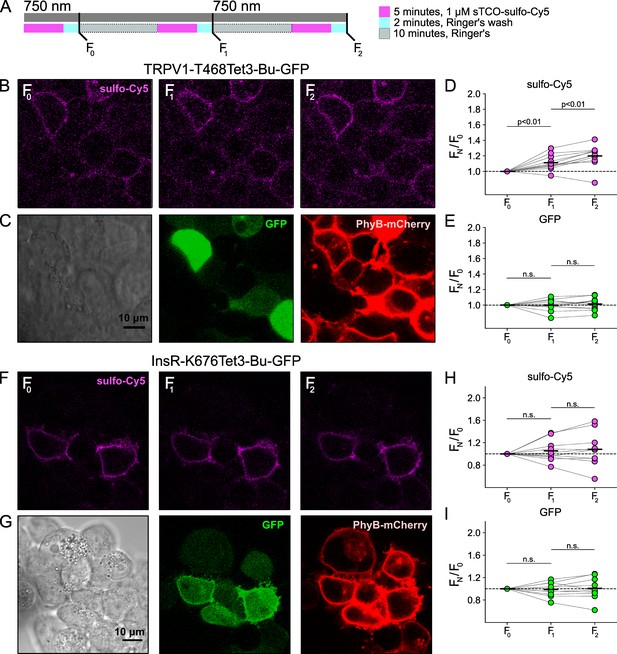
Control experiment for light-activated phosphoinositide 3-kinases (PI3K)-induced TRPV1 and InsR trafficking to the plasma membrane (PM).
The same experiment as Figure 5 without 650 nm illumination. The cells were exposed to 750 nm throughout the recording (n=11 and 10 for TRPV1-Tet3-Bu-GFP and InsR-676Tet3-Bu-GFP, respectively).
-
Figure 5—figure supplement 1—source data 1
Original confocal microscopic images for TRPV1 trafficking (Figure 5—figure supplement 1B and C).
Figure 5—figure supplement 1—source data 1_TRPV1_750_1.lsm, Figure 5—figure supplement 1—source data 1_TRPV1_750_2.lsm, Figure 5—figure supplement 1—source data 1_TRPV1_750_3.lsm: the images contain GFP (green) and Cy5 (red). Figure 5—figure supplement 1—source data 1_TRPV1_BF_PhyB.lsm: the images contain bright field and PhyB (magenta).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-figsupp1-data1-v1.zip
-
Figure 5—figure supplement 1—source data 2
Excel data for scatter plot for TRPV1 trafficking (Figure 5—figure supplement 1D and E).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-figsupp1-data2-v1.xlsx
-
Figure 5—figure supplement 1—source data 3
Original confocal microscopic images for InsR trafficking (Figure 5—figure supplement 1F and G).
Figure 5—figure supplement 1—source data 1_InsR_750_1.lsm, Figure 5—figure supplement 1—source data 1_InsR_750_2.lsm, Figure 5—figure supplement 1—source data 1_InsR_750_3.lsm: the images contain GFP (green) and Cy5 (red). Figure 5—figure supplement 1—source data 1_InsR_BF.lsm: the image contains bright field. Figure 5—figure supplement 1—source data 1_InsR_PhyB.lsm: the image contains PhyB (magenta).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-figsupp1-data3-v1.zip
-
Figure 5—figure supplement 1—source data 4
Excel data for scatter plot for InsR trafficking (Figure 5—figure supplement 1H and I).
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig5-figsupp1-data4-v1.xlsx
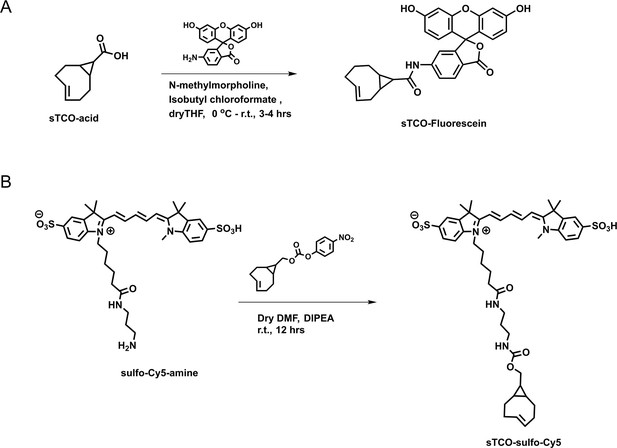
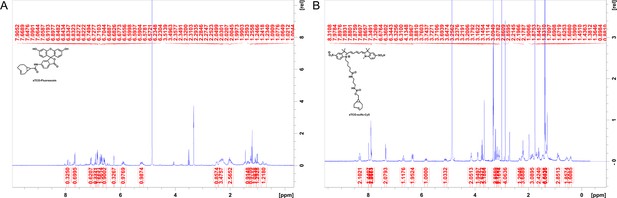
Synthesis of (A) sTCO-fluorescein and (B) sTCO-sulfo-Cy5.
Schemes illustrating the synthesis pathways for the indicated dyes.
-
Figure 6—source data 1
Two NMR data files processed with TopSpin 4.1.4 software.
- https://cdn.elifesciences.org/articles/91012/elife-91012-fig6-data1-v1.zip