Recognition and cleavage of human tRNA methyltransferase TRMT1 by the SARS-CoV-2 main protease
Figures
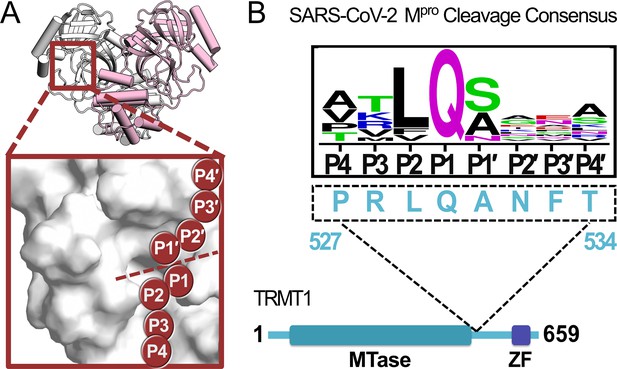
A peptide sequence found in human TRMT1 fits the cleavage consensus for the SARS-CoV-2 main protease (Mpro).
(A) Overview of the structure of the SARS-CoV-2 Mpro homodimer (PDB 7BB2) with substrate peptide residues (P4-P3-P2-P1-P1′-P2′-P3′-P4′) illustrated in the Mpro active site (inset); proteolytic cleavage takes place between substrate residues P1 and P1′ (dotted line). (B) The TRMT1(527–534) sequence found in a linker region between the TRMT1 SAM methyltransferase (MTase) and zinc finger (ZF) domains is consistent with the SARS-CoV-2 Mpro cleavage consensus sequence.
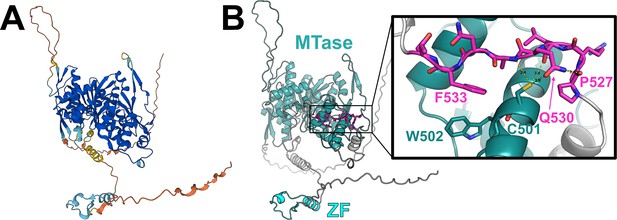
AlphaFold-predicted31,32 structure of human TRMT1.
(A) TRMT1 structural model colored by AlphaFold prediction confidence (dark blue = very high confidence, cyan = confident, yellow = low confidence, orange = very low confidence). (B) TRMT1 structural model colored by domain (SAM-dependent methyltransferase, MTase = teal; zinc finger, ZF = cyan; linker and unstructured regions = gray) with the TRMT1 (527–534) cleavage sequence highlighted in magenta. Inset shows closeup of the surface-exposed TRMT1 (527–534) cleavage sequence (magenta) and the AlphaFold-predicted contacts between the residues in the cleavage sequence and the surface of the MTase domain.
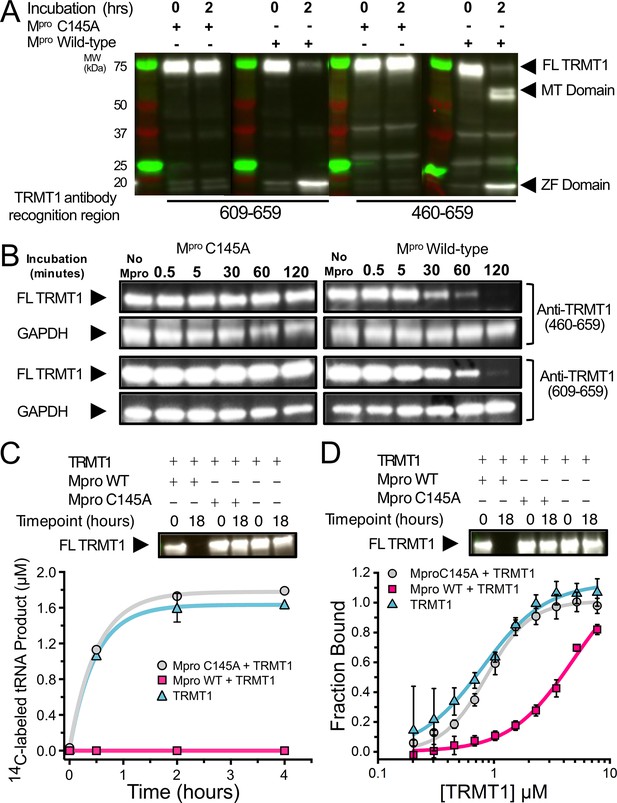
SARS-CoV-2 Mpro cleaves full-length (FL) human TRMT1 which impacts methyltransferase activity and tRNA binding.
(A) Western blots of recombinantly purified FL TRMT1 incubated with 10 µM catalytically inactive (Cys145Ala) or active (wild-type [WT]) SARS-CoV-2 Mpro at 37°C. Incubation with WT Mpro results in proteolysis of FL TRMT1 and the appearance of cleavage products corresponding the zinc finger (ZF) domain (observed with both anti-TRMT1 (609–659) and anti-TRMT1 (460–659) antibodies) and the methyltransferase (MTase) domain (observed with only anti-TRMT1 (460–659) antibody). (B) Western blots of endogenous human TRMT1 in HEK293T cell lysate incubated with 10 µM of either catalytically inactive (Cys145Ala) or active (WT) Mpro at 37°C. Endogenous FL TRMT1 is stable in human cell lysate over the course of a 2 hr incubation with C145A Mpro (left) and is rapidly proteolyzed upon incubation with WT Mpro (right). GAPDH was stained in conjunction with TRMT1 antibodies and used as a loading control. (C) Recombinant, FL TRMT1 cleaved for 18 hr with WT Mpro (complete cleavage confirmed by western blot using anti-TRMT1 (460–659), top panel), has no observable methyltransferase activity on a human FL tRNAphe substrate (bottom panel, pink squares); in contrast, robust tRNA methylation activity is still seen with TRMT1 incubated for 18 hr with Mpro Cys145Ala or no protease (bottom panel, gray circles and blue triangles, respectively). TRMT1 tRNA methyltransferase activity was measured by monitoring radiolabel incorporation into tRNA substrate in reactions with cofactor 14C-SAM. (D) Recombinant, FL TRMT1 cleaved for 18 hr with WT Mpro (complete cleavage confirmed by western blot using anti-TRMT1 (460–659), top panel), has reduced binding affinity (~6-fold change) for human tRNAPhe (bottom panel, pink squares) compared to uncleaved TRMT1 that had been incubated for 18 hr with either Mpro Cys145Ala or no protease (bottom panel, gray circles and blue triangles, respectively). TRMT1-tRNA binding was measured by electrophoretic mobility shift assay (EMSA), where bound and unbound tRNA species at different TRMT1 concentrations were separated, visualized by SYBR Gold staining, and quantified using ImageJ to obtain fraction bound values. Methyltransferase and binding assays in (C, D) were carried out in triplicate and errors are shown as SEM; fitted kinetic and binding parameters are shown in Figure 2—source data 1.
-
Figure 2—source data 1
Table of methyltransferase activity (kobs) and tRNA binding affinity (KD) parameters for Figure 2C and D.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-data1-v1.docx
-
Figure 2—source data 2
Raw, uncropped immunoblots for gel images in Figure 2.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-data2-v1.zip
-
Figure 2—source data 3
Annotated, uncropped immunoblots for gel images in Figure 2.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-data3-v1.zip
-
Figure 2—source data 4
Primary kinetic and binding data for Figure 2C and D.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-data4-v1.xlsx
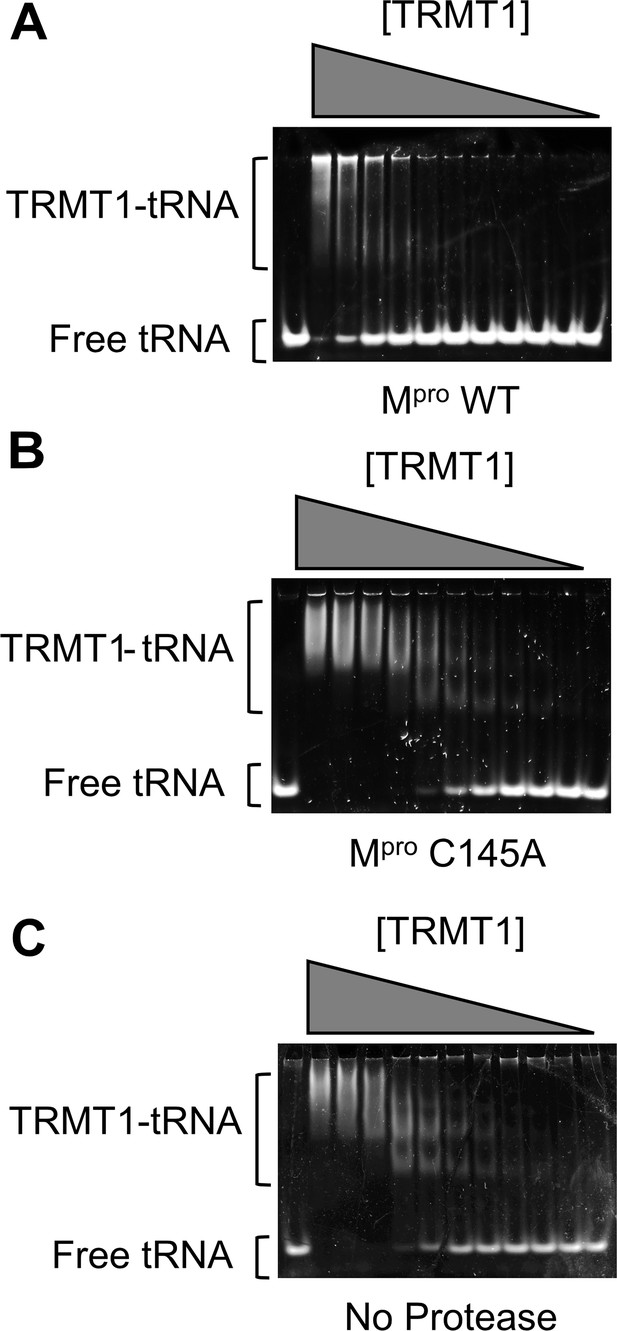
TRMT1 binding to tRNAphe substrate was measured using an electrophoretic mobility shift assay (EMSA).
Representative EMSA gels are shown for tRNA binding experiments where TRMT1 was preincubated overnight with Mpro wild-type (WT) (A), Mpro C145A (B), or no protease (C); after overnight treatment with protease (or no protease), binding was measured by EMSA to 300 nM tRNAphe, with a TRMT1 dilution series from 7.8 → 0.1 μM. For an unbound tRNA migration reference, the first lane of each gel contained tRNAphe without TRMT1. The cleaved TRMT1 generated by Mpro WT (A) had diminished affinity for tRNA, whereas TRMT1 incubated with Mpro C145A (B) had comparable tRNA binding affinity to TRMT1 incubated with no protease (C). KDs for the TRMT1-tRNA interactions under each of these conditions are listed in Figure 2—source data 1 and were determined by quantifying fraction bound from the free tRNA and TRMT1-tRNA bands shown above, plotting this against [TRMT1], and fitting these plots (shown in Figure 2D) to a standard single-site ligand binding equation. We note that the cleaved TRMT1-tRNA complexes in (A) migrate at significantly higher apparent molecular weights, and are retained in the wells, as compared to uncleaved TRMT1-tRNA complexes. Based on our complementary kinetic experiments shown in Figure 2C, although cleaved TRMT1 apparently retains some binding affinity for tRNA, these are non-functional complexes or oligomers.
-
Figure 2—figure supplement 1—source data 1
Raw, uncropped EMSA gel images for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Annotated, uncropped EMSA gel images for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig2-figsupp1-data2-v1.zip
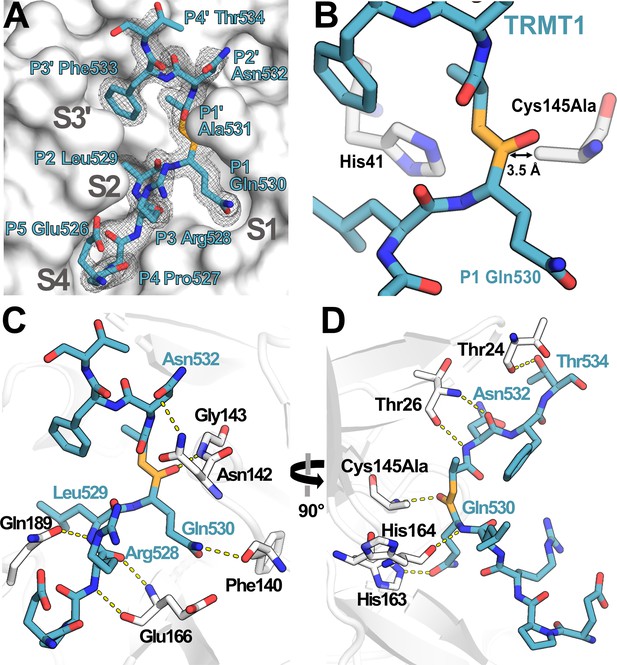
Structure of human TRMT1 (526–536) peptide bound to SARS-CoV-2 Mpro.
(A) TRMT1 peptide (blue) bound in Mpro active site (gray) showing substrate binding pockets S1, S2, S4, and S3′. Fo-Fc omit electron density map of TRMT1 peptide bound to Mpro is shown contoured at 1σ. TRMT1 Gln P1, an ultra-conserved residue in the Mpro cleavage consensus which is critical for Mpro-mediated proteolysis, is nestled in the S1 pocket of the Mpro active site. The scissile P1 – P1′ amide linkage of TRMT1 is colored orange. (B) The TRMT1 P1 Gln amide is positioned for cleavage near Mpro catalytic dyad residues His41 and Cys145Ala in the protease active site. (C, D) Direct hydrogen bond contacts formed between Mpro residues (white) and the bound TRMT1 peptide (light blue) are illustrated as yellow dashed lines; (C and D) are views rotated by 90°, highlighting different TRMT1-Mpro hydrogen bonding interactions. Mpro Phe140 and His163 recognize the TRMT1 P1 Gln530 sidechain; TRMT1 Asn532 and Mpro Asn142 engage in sidechain-sidechain interaction; additional backbone hydrogen bond contacts include Mpro Thr24-TRMT1 Thr534, Mpro Thr26-TRMT1 Asn532, Mpro Asn142-TRMT1 Asn532, Mpro Glu166-TRMT1 Arg528, and Mpro Gln189-TRMT1 Leu529; many of these interactions are consistent with canonical Mpro-peptide substrate contacts in the active site.
-
Figure 3—source data 1
Table of data and refinement statistics for Mpro-TRMT1 crystal structure.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig3-data1-v1.docx
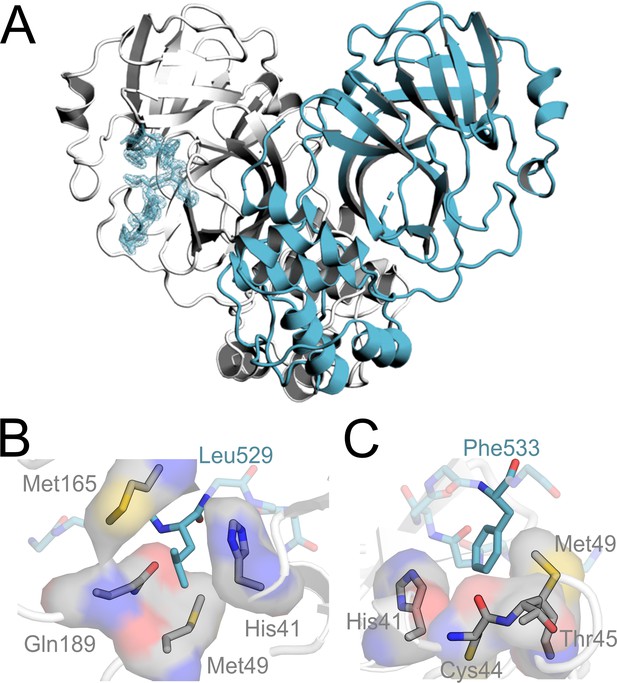
The Mpro-TRMT1 (526–536) co-crystal structure uncovers how TRMT1 peptide residues occupy the Mpro active site substrate binding pockets.
(A) Structure of human TRMT1 (526–536) peptide in complex with the SARS-CoV-2 Mpro dimer. Only one protomer of the Mpro dimer has TRMT1 bound in the active site. Fo-Fc omit electron density map of TRMT1 peptide bound to Mpro contoured at 1σ. (B) TRMT1 (526–536) P2 residue Leu529 packs against Mpro residues His41, Met49, Gln189, and Met165 in the Mpro S2 substrate binding pocket. (C) TRMT1 (526–536) P3′ residue Phe533 sits in the S3′ pocket, composed of Mpro residues His41, Cys44, Thr45 (backbone), and Met49.
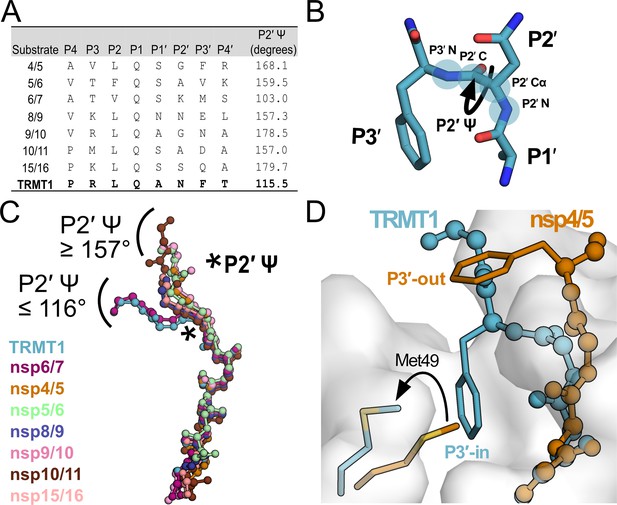
Mpro-peptide structures illustrate two distinct substrate binding modes, P3′-in and P3′-out.
(A) Comparison of known Mpro substrate cleavage sequences and the P2′ Ψ backbone dihedral angles measured in the corresponding C145A Mpro-peptide structures for each substrate. We included all known C145A Mpro-viral peptide structures in this analysis, except those that were missing the P3′ residue or had poorly defined electron density for the C-terminal portion of the peptide; structures used in this analysis are PDB IDs: 7MGS, 7T8M, 7DVW, 7T9Y, 7TA4, 7TA7, 7TC4, and 9DW6. Additionally, since a C145A Mpro-nsp6/7 structure was not available, we used an H41A Mpro-nsp6/7 structure (PDB 7VDX) for this analysis. (B) Section of an Mpro-bound peptide substrate showing residues P1′, P2′, and P3′, with the key P2′ Ψ dihedral angle illustrated with a curved arrow; the four backbone atoms that define the P2′ Ψ dihedral angle are labeled and highlighted with blue circles (P2′N–P2′Cα–P2′C–P3′N). (C) Alignment of peptide substrate backbones in the Mpro active site reveals two distinct binding modes at the C-terminal end of the bound peptides characterized by P2′ Ψ dihedral angles ≥157° (nsp4/5, nsp5/6, nsp8/9, nsp9/10, nsp10/11, nsp15/16) or ≤116° (TRMT1, nsp6/7). Peptide overlays were generated by aligning SARS-CoV-2 Mpro-peptide substrate structures in PyMOL. The location of the P2′ Ψ dihedral angle in the substrate peptide backbone is denoted with a star. (D) Alignment of nsp4/5- and TRMT1-bound Mpro structures showing divergent C-terminal peptide substrate binding modes in the Mpro active site. The backbone geometry of nsp4/5 (P2′ Ψ=168°) positions the P3′ Phe sidechain away from the Mpro surface (‘P3′-out’ conformation), while the TRMT1 backbone geometry (P2′ Ψ=115°) positions the P3′ Phe sidechain toward the Mpro active (‘P3′-in’ conformation) site where it displaces Mpro Met49 to open and occupy the S3′ pocket.
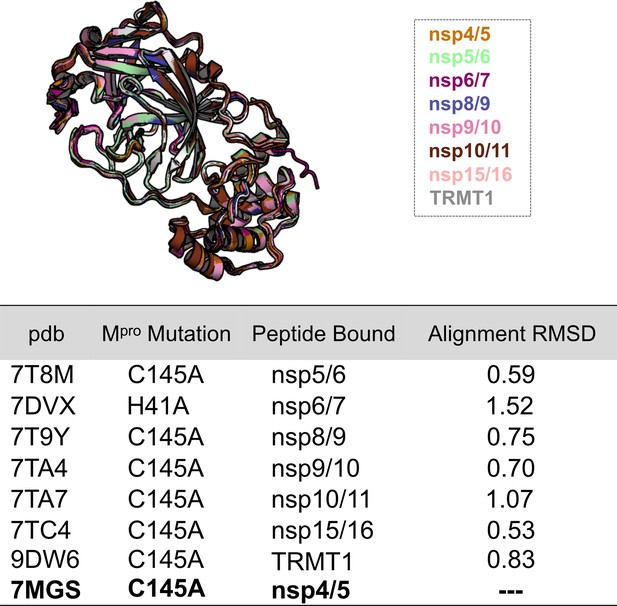
An alignment of Mpro structures for each Mpro-peptide complex used in the analysis shown in Figure 4A and C (top).
Calculated all-atom RMSDs are derived from each structure alignment to Mpro Cys145Ala bound to nsp4/5 (7MGS) (bottom). The overall structure of the Mpro backbone is highly similar regardless of the bound peptide substrate.
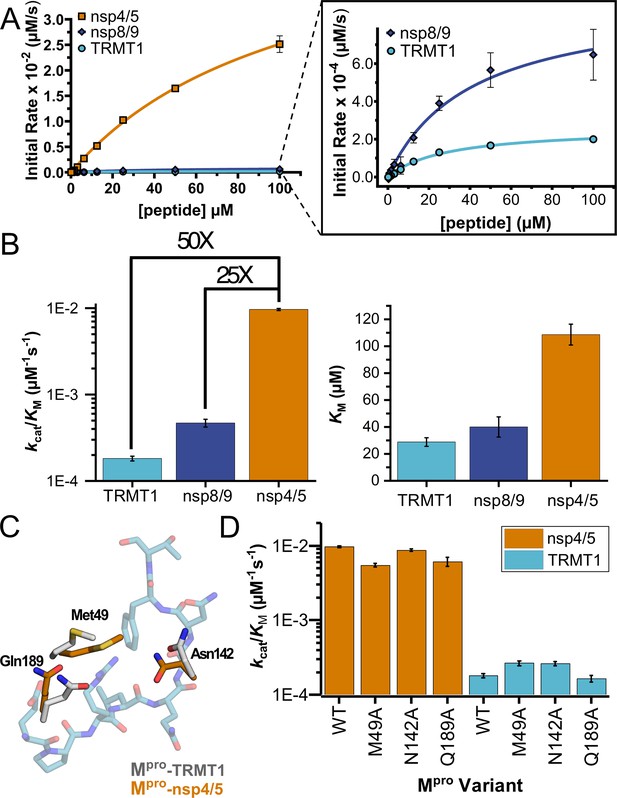
Human TRMT1 peptides are cleaved with similar catalytic efficiencies to known Mpro substrates.
(A) Kinetics of nsp4/5, nsp8/9, and TRMT1 peptide cleavage by Mpro. To initiate the reaction, 50 nM enzyme was added to 100–0.097 µM peptide. Each fluorogenic peptide was conjugated with a quenching moiety, and upon peptide cleavage, the fluorescence of the cleavage product was measured to determine initial rates of the reaction. Nsp4/5 cleavage rates were faster than those observed for the nsp8/9 or TRMT1 peptides, but nsp8/9 and TRMT1 sequences exhibit similar Mpro-mediated cleavage rates. (B) The catalytic efficiency (kcat/KM) of TRMT1 peptide cleavage by Mpro is similar to that for nsp8/9 peptide cleavage; both of these substrates are cleaved significantly slower than the nsp4/5 sequence. This suggests that TRMT1 is a feasible substrate for Mpro. (C) Illustration of changes in Mpro Met49, Asn142, and Gln189 residue positioning in TRMT1-bound (white) vs nsp4/5-bound (orange) structures. The TRMT1 peptide is shown in blue; nsp4/5 peptide is not shown. (D) No major changes in catalytic efficiency are observed for nsp4/5 and TRMT1 peptide cleavage upon mutagenesis of key Mpro residues involved in TRMT1 binding and recognition. All kinetic assays were carried out in triplicate and errors are shown as SEM.
-
Figure 5—source data 1
Table of kinetic parameters associated with Figure 5.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig5-data1-v1.docx
-
Figure 5—source data 2
Primary fluorescence data and calibration and correction calculations used to determine kinetic parameters in Figure 5.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig5-data2-v1.xlsx
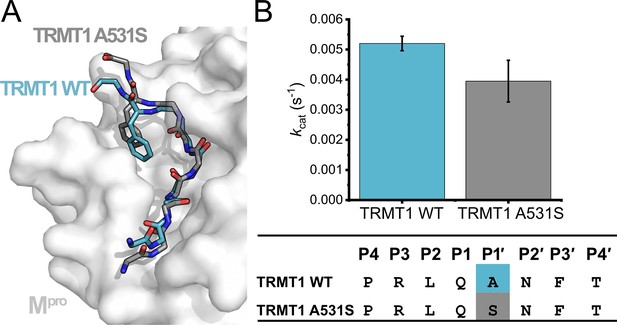
Analysis of a point mutation to P1′ Ala residue of TRMT1 (526–536).
TRMT1 (A531S) contains a Ser at position P1′, which conforms to the Mpro cleavage consensus (Figure 1B) but is predicted to disfavor the P3′-in binding conformation observed for TRMT1 in the Mpro active site. (A) Molecular dynamics simulated structure of Mpro in complex with TRMT1 A531S (gray peptide) predicts this peptide remains in the P3′-in conformation, as observed with TRMT1 wild-type (WT) (blue peptide). (B) No major difference is measured for cleavage of the WT vs A531S TRMT1 peptide by Mpro kinetic data comparing kcat for cleavage of the TRMT1 (526–536) peptide with either the WT TRMT1 sequence or an Ala531 to Ser mutation.
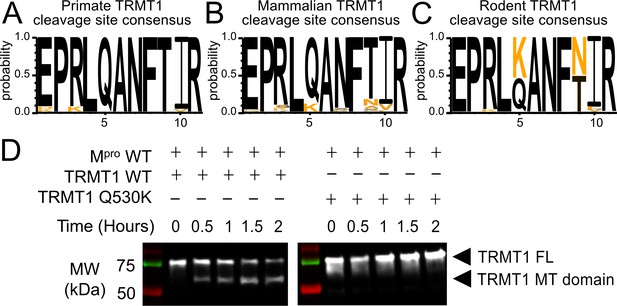
The Mpro-targeted cleavage sequence is conserved in most mammalian TRMT1 proteins, except rodents where TRMT1 is resistant to cleavage.
(A–C) The Mpro-targeted TRMT1 cleavage site sequence (human TRMT1 residues 526–536) is highly conserved in primates (A) and most mammals (B), with the notable exception of rodents (C), where the glutamine Q530 residue most critical for Mpro-directed cleavage is substituted to a lysine in Muroidea. Sequence logo plots of the cleavage site in TRMT1 (526–536) were produced with WebLogo3. The human reference sequence is in black and orange residues show the differences. (D) Wild-type (WT) human TRMT1 is cleaved over the course of a 2 hr incubation with Mpro (left western blot panel), whereas human TRMT1(Q530K), which has the Q to K mutation found in Muroidea, is entirely resistant to cleavage during a 2 hr incubation with Mpro (right western blot panel).
-
Figure 6—source data 1
Data used for sequence analyses in Figure 6A–C.
Additional data used in sequence analysis can be found at https://doi.org/10.6084/m9.figshare.22004474 and https://doi.org/10.6084/m9.figshare.22004492.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig6-data1-v1.xlsx
-
Figure 6—source data 2
Raw, uncropped immunoblots for Figure 6D.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig6-data2-v1.zip
-
Figure 6—source data 3
Annotated, uncropped immunoblots for Figure 6D.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig6-data3-v1.zip
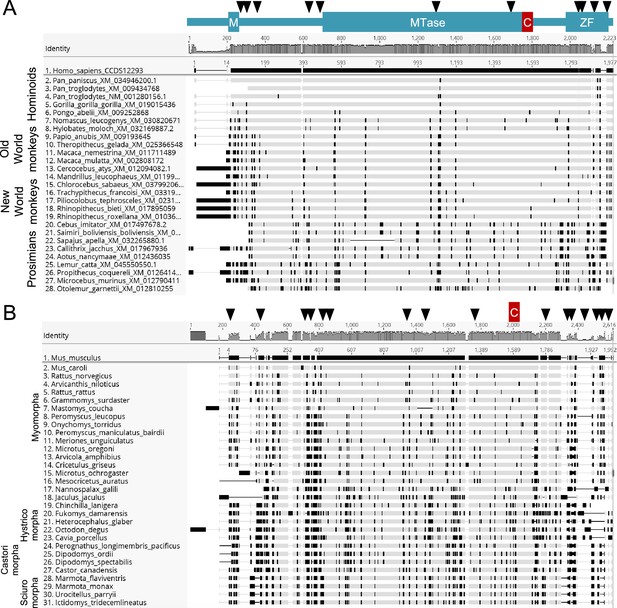
Evolution of mammalian TRMT1.
(A) The Mpro binding/cleavage site in TRMT1 is highly conserved in primates, while there has been rapid evolution at N- and C-termini. Codon alignment of the primate TRMT1 sequences with the human as a reference. The numbering is according to the nucleotide position in the alignment (top) and in the reference sequence (human). Non-synonymous differences to the reference are highlighted in black, from Geneious R9. The sites under positive selection identified by MEME or FUBAR are shown above the alignment with black triangles. Major domains and the cleavage sites are also represented: M for mitochondrial signal, MTase for methyltransferase, C for cleavage by Mpro, ZF for zinc finger. (B) Same as (A) for rodents, with mouse as the reference.
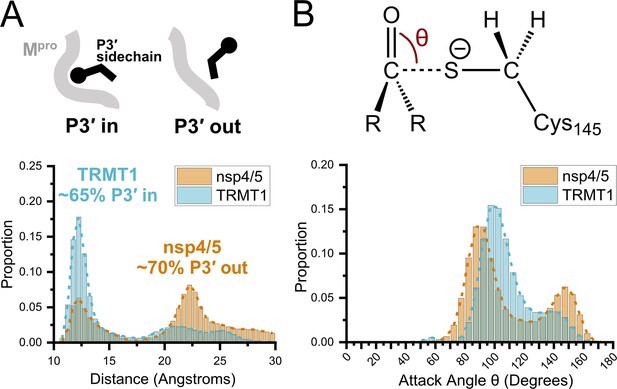
Molecular dynamics (MD) simulations confirm dominant peptide binding conformations and suggest discrimination in cleavage kinetics result from catalytic steps that follow initial binding and nucleophilic attack.
(A) Distribution of the sum of the minimum distance for P3′ Phe residue in nsp4/5 or TRMT1 from three residues (Thr25, Met49, Cys44) which form the S3′ subsite; P3′-in and P3′-out conformations are illustrated above the distribution plot. The much larger proportion of TRMT1 at smaller distances reflects the peptide’s preference for binding in the P3′-in conformation where TRMT1 P3′ Phe occupies the S3′ pocket during the majority of the MD simulation. (B) Distribution of the attack angle of the nucleophilic Mpro Cys145 sulfur atom and the substrate carbonyl carbon atom in the to-be-cleaved amide bond (S–C=O angle θ, top illustration) during the course of the MD simulation. Although nsp4/5 has a higher proportion of attack angles observed closer to the optimal 90° compared to TRMT1, consistent with faster nsp4/5 cleavage kinetics, this small preference is insufficient to explain the 200-fold faster cleavage kinetics of nsp4/5 observed in experimental proteolysis assays.
-
Figure 7—source data 1
Data from molecular dynamics (MD) simulations used to construct distribution plots in Figure 7.
- https://cdn.elifesciences.org/articles/91168/elife-91168-fig7-data1-v1.xlsx
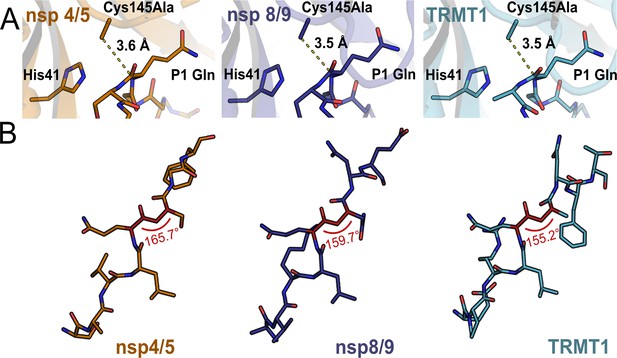
Substrate positioning at the Mpro catalytic site does not readily explain observed differences in cleavage kinetics.
(A) One possible explanation for faster cleavage kinetics of nsp4/5 relative to nsp8/9 or TRMT1 (data in Figure 4) could be better positioning of the scissile peptide bond and electrophilic P1 amide carbonyl closer to the nucleophilic Mpro Cys145 residue. However, the measured C145A-P1(CO) distances are nearly identical for nsp4/5-, nsp8/9-, and TRMT1-bound Mpro crystal structures, suggesting this is not the case. (B) Another possible explanation for faster cleavage kinetics of nsp4/5 are deviations in the dihedral angle of the scissile amide (P1(CA)-P1(C)-P1′(N)-P1′(CA)) bond away from 180°, which could indicate ground state destabilization that would result in accelerated peptide bond cleavage. However, the most rapidly cleaved substrate, nsp4/5, has a scissile amide bond dihedral angle closest to 180°, indicating that amide bond planarity of the bound substrate does not play an important role in determining peptide cleavage rates.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Recombinant DNA reagent (plasmid) | pLVX-EF1alpha-SARS-CoV-2-nsp5-2xStrep-IRES-Puro | Addgene | RRID:Addgene_141370 | |
| Recombinant DNA reagent (plasmid) | pLVX-EF1alpha-SARS-CoV-2-nsp5-C145A-2xStrep-IRES-Puro | Addgene | RRID:Addgene_141371 | |
| Recombinant DNA reagent (plasmid) | pETARA-Mpro-WT | This paper | Mugridge Lab plasmid, E. coli expression vector for wild-type Mpro with a GST tag | |
| Recombinant DNA reagent (plasmid) | pETARA-Mpro-C145A | This paper | Mugridge Lab plasmid, E. coli expression vector for Mpro C145A variant with a GST tag | |
| Recombinant DNA reagent (plasmid) | pETARA-Mpro-M49A | This paper | Mugridge Lab plasmid, E. coli expression vector for Mpro M49A variant with a GST tag | |
| Recombinant DNA reagent (plasmid) | pETARA-Mpro-N142A | This paper | Mugridge Lab plasmid, E. coli expression vector for Mpro N142A variant with a GST tag | |
| Recombinant DNA reagent (plasmid) | pETARA-Mpro-Q189A | This paper | Mugridge Lab plasmid, E. coli expression vector for Mpro Q189A variant with a GST tag | |
| Recombinant DNA reagent (plasmid) | TRMT1-pET22b(+)–18del-WT | GenScript | E. coli expression plasmid to generate recombinant human TRMT1 WT | |
| Peptide | TRMT1(526–536) unlabeled Peptide | Peptide 2.0 | EPRLQANFTIR | |
| Peptide | TRMT1(526–536) labeled Peptide | Peptide 2.0 | MCA-EPRLQANFTIR-K(Dnp)K | |
| Peptide | nsp4/5 labeled Peptide | Peptide 2.0 | MCA-SAVLQSGFRKM-K(Dnp)K | |
| Peptide | nsp8/9 labeled Peptide | Peptide 2.0 | MCA-AVKLQNNELSP- K(Dnp)K | |
| Software, algorithm | Origin software | OriginLab Corporation | RRID:SCR_014212; Versions 2021 and 2023b | |
| Software, algorithm | GraphPad Prism software | GraphPad | RRID:SCR_002798; Version 10.0.03 | |
| Cell line (Homo sapiens) | 293T | ATCC | ATCC: CRL-3216 | |
| Antibody | Anti-TRMT1 460–659 | Invitrogen | PA5-96585 | Western blot primary (1:2000) |
| Antibody | Anti-TRMT1 609–659 (Rabbit polyclonal) | Bethyl Laboratories | A304-205A | Western blot primary (1:2000) |
| Antibody | Anti-GAPDH (Rabbit polyclonal) | Invitrogen | PA-1987 | Western blot primary (1:100,000) |
| Antibody | Goat anti-Rabbit IgG (H+L) Secondary Antibody, HRP | Invitrogen | A16096 | Western blot secondary (1:10,000) |
| Commercial assay or kit | Clarity Western ECL Substrate | Bio-Rad Laboratories | 1705060 | HRP substrate for western blot |
| Recombinant DNA reagent | tRNA PheGAA, sequence with T7 promoter (Homo sapiens) | IDT | TAATACGACTCACTATAGCCGAAATAGCTCAGTTGGGAGAGCGTTAGACTGAAGATCTAAAGGTCCCTGGTTCGATCCCGGGTTTCGGCA | |
| Commercial assay or kit | RNA Clean & Concentrator kits | Zymo Research | R1016 | For purification of tRNA product |
| Software, algorithm | ImageJ software | Schneider et al., 2012 | Version 1.54d | For image analysis of EMSA gels |
| Other | Spark microplate reader | Tecan | For plate-based activity assay | |
| Other | FluorChem R imager | Protein Simple | For imaging western blots | |
| Other | Quantulus Scintillation Counter | Revvity | For measuring 14C CPM | |
| Software, algorithm | Coot software | Emsley et al., 2010 | Version 0.8.9.1 | Structure building |
| Software, algorithm | PHENIX software | Liebschner et al., 2019 | Version v.1.17.1–3600 | Structure refinement |





