Elimination of subtelomeric repeat sequences exerts little effect on telomere essential functions in Saccharomyces cerevisiae
eLife assessment
This important study advances our understanding of the biological significance of the DNA sequence adjacent to telomeres. The data presented convincingly demonstrate that subtelomeric repeats are non-essential and have a minimal, if any, role in maintaining telomere integrity of budding yeast. The work will be of interest to the telomere community specifically and the genome integrity community more broadly.
https://doi.org/10.7554/eLife.91223.4.sa0Important: Findings that have theoretical or practical implications beyond a single subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Convincing: Appropriate and validated methodology in line with current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Telomeres, which are chromosomal end structures, play a crucial role in maintaining genome stability and integrity in eukaryotes. In the baker’s yeast Saccharomyces cerevisiae, the X- and Y’-elements are subtelomeric repetitive sequences found in all 32 and 17 telomeres, respectively. While the Y’-elements serve as a backup for telomere functions in cells lacking telomerase, the function of the X-elements remains unclear. This study utilized the S. cerevisiae strain SY12, which has three chromosomes and six telomeres, to investigate the role of X-elements (as well as Y’-elements) in telomere maintenance. Deletion of Y’-elements (SY12YΔ), X-elements (SY12XYΔ+Y), or both X- and Y’-elements (SY12XYΔ) did not impact the length of the terminal TG1-3 tracks or telomere silencing. However, inactivation of telomerase in SY12YΔ, SY12XYΔ+Y, and SY12XYΔ cells resulted in cellular senescence and the generation of survivors. These survivors either maintained their telomeres through homologous recombination-dependent TG1-3 track elongation or underwent microhomology-mediated intra-chromosomal end-to-end joining. Our findings indicate the non-essential role of subtelomeric X- and Y’-elements in telomere regulation in both telomerase-proficient and telomerase-null cells and suggest that these elements may represent remnants of S. cerevisiae genome evolution. Furthermore, strains with fewer or no subtelomeric elements exhibit more concise telomere structures and offer potential models for future studies in telomere biology.
Introduction
Telomeres, specialized nucleoprotein structures located at the end of linear chromosomes in eukaryotic cells, are crucial for maintaining genomic stability and protecting chromosomal ends from being perceived as DNA breaks (Wellinger and Zakian, 2012). In the budding yeast Saccharomyces cerevisiae, telomeric DNA consists of approximately ~300 ± 75 base pairs of C1-3A/TG1-3 repeats with a 3' G-rich single-stranded overhang (Wellinger and Zakian, 2012). Adjacent to the telomeric TG1-3 repeats, there are subtelomeric repeat elements known as X- and Y’-elements, which vary between telomeres, as well as strains (Chan and Tye, 1983a; Chan et al., 1983b; Louis, 1995). The Y’-elements, immediately internal to the telomeric repeats, are present as a tandem array of 0–4 copies, they fall into two major size classes, 6.7 kb Y’-long (Y’-L) and 5.2 kb Y’-short (Y’-S) (Chan and Tye, 1983a; Chan et al., 1983b). Y’-elements are highly conserved with only ~2% divergence between strains (Louis and Haber, 1992). One entire Y’-element contains two large open-reading frames (ORFs), an ARS consensus sequence (ACS), and a STAR element (subtelomeric anti-silencing regions) consisting of binding sites for Tbf1 and Reb1 (Chan and Tye, 1983a; Chan et al., 1983b; Fourel et al., 1999; Louis and Haber, 1992). The X-element, a much more heterogeneous sequence abutting Y’-elements or telomeric repeats, contains the 473 bp ‘core X’ sequence and the subtelomeric repeats (STRs) A, B, C, and D (Louis and Haber, 1991; Louis et al., 1994). The STRs are found in some chromosome ends, while the ‘core X’ sequence is shared by all chromosomes. Recent long-read sequencing shows that subtelomeric regions display high evolutionary plasticity and are rich in various structure variants such as reciprocal translocations, transpositions, novel insertions, deletions, and duplications (O’Donnell et al., 2023).
Telomeric DNA elongation primarily relies on telomerase, an enzyme comprising a reverse transcriptase, an RNA component, and accessory factors (Palm and de Lange, 2008; Wellinger and Zakian, 2012). In S. cerevisiae, the telomerase holoenzyme consists of the reverse transcriptase Est2, the RNA template TLC1, and accessory factors Est1, Est3, Pop1/Pop6/Pop7 proteins (Lemieux et al., 2016; Lendvay et al., 1996; Lundblad and Szostak, 1989; Singer and Gottschling, 1994). In the absence of telomerase, homologous recombination can take place to replicate telomeres, resulting in telomerase-deficient ‘survivors’ (Lundblad and Blackburn, 1993; Teng and Zakian, 1999). These survivors are broadly categorized into Type I and Type II based on distinct telomere structures (Lundblad and Blackburn, 1993; Teng and Zakian, 1999). Type I survivors possess tandem amplified Y’-elements (both Y’-L and Y’-S) and very short TG1-3 tracts, indicating that Y’-elements serve as substrates for homologous recombination. Type II survivors display long heterogeneous TG1-3 tracts. On solid medium, approximately 90% of the survivors are Type I, while 10% are Type II (Teng et al., 2000). However, in liquid culture, Type II survivors grow faster and eventually dominate the population (Teng and Zakian, 1999). The proteins required for ype I and II survivor formation appear to be different. Type I survivors depend on Rad51, Rad54, Rad55, Rad57, and Pif1 (Chen et al., 2001; Hu et al., 2013; Le et al., 1999). while the formation of Type II survivors requires the Mre11/Rad50/Xrs2 (MRX) complex, KEOPS complex, Rad59, Sgs1, and Rad6, most of which are critical for DNA resection (Chen et al., 2001; He et al., 2019; Hu et al., 2013; Johnson et al., 2001; Le et al., 1999; Huang et al., 2001; Nicolette et al., 2010; Teng et al., 2000; Wellinger and Zakian, 2012; Wu et al., 2017). Although Type I and II pathways are working independently, Kockler et al. found that the proteins involved in each pathway can work together via two sequential steps and contribute to a unified ALT (alternative lengthening of telomeres) process (Kockler et al., 2021).
The amplification of Y’-elements represents a significant feature of telomere recombination in telomerase-null Type I survivors (Lundblad and Blackburn, 1993; Teng and Zakian, 1999), and as a result, extrachromosomal Y’ circular DNAs have been observed in Type I survivors (Larrivée and Wellinger, 2006). Additionally, Y’-element acquisition has been observed in the initiation step of pre-senescence, suggesting a potential role for Y’-elements in Type II survivor formation (Churikov et al., 2014). Furthermore, Y’-elements are mobilized through a transposition-like RNA-mediated process involving Ty1 activity in telomerase-negative survivors (Maxwell et al., 2004). Y’-elements also express potential DNA helicases, Y’-Help, in telomerase-null survivors (Yamada et al., 1998). Thus, Y’-elements play a significant role as donors in homologous recombination-mediated telomere maintenance. The functions of X-elements, on the other hand, are less clear. The ‘core X’ sequence consists of an ACS element and, in most cases, an Abf1 binding site (Louis, 1995), and acts as a protosilencer (Lebrun et al., 2001). In contrast, STRs and Y’-STAR possess anti-silencing properties that limit the spreading of heterochromatin (Fourel et al., 1999). Interestingly, a previous study demonstrated that telomeres with Χ-only ends (containing only X-elements) were more efficiently elongated compared to those with X-Y’ ends (containing both X- and Y’-elements) in tel1Δ rif1Δ strains (Craven and Petes, 1999). Moreover, subtelomeric elements (including X-elements) and associated factors like Reb1 and Tbf1 antagonize telomere anchoring at the nuclear envelope (Hediger et al., 2006). However, considering that X-elements are present in all telomeres while Y’-elements are not, the specific functions of X- and Y’-elements in genome integrity after the evolution of telomerase have long been a subject of questioning (Jäger and Philippsen, 1989; Zakian and Blanton, 1988).
In wild-type yeast strain BY4742, there are 8 Y’-S and 11 Y’-L elements at the 32 telomere loci. Additionally, each telomere locus contains one X-element. The genetic deletion of all X- and Y’-elements to directly investigate the roles of X- and Y’-elements in genome integrity is a challenging and complex task. In this study, we utilized recently reported chromosome-fused budding yeast strains (Shao et al., 2018) to eliminate both X- and Y’-elements completely. This approach allows us to reinvestigate the roles of X- and Y’-elements at telomeres.
Results
Telomere recombination in telomerase-null chromosome-fused yeast strains SY1 to SY12
The functions of Y’-elements have been previously linked to telomere recombination (Churikov et al., 2014; Larrivée and Wellinger, 2006; Lundblad and Blackburn, 1993; Teng and Zakian, 1999). To further investigate the role of Y’-elements in telomere recombination, we utilized a series of chromosome-fused budding yeast strains derived from the wild-type BY4742 strain, including SY1, SY3, SY5, SY7, SY8, SY9, SY10, SY11, SY12, and SY13 (also referred to as SYn for convenience) (Figure 1A; Shao et al., 2018). The remaining subtelomeric elements in SY8 to SY13 strains are listed in Supplementary file 2. We excluded SY14 from these experiments since the presence of circular chromosome was prominent in SY14 tlc1Δ cells (one fused chromosome) (Wu et al., 2020), We generated haploid SYn tlc1Δ TLC1 strains by deleting the chromosomal copy of the TLC1 gene and introducing a plasmid-borne wild-type TLC1 gene (pRS316-TLC1). Clones that lost the pRS316-TLC1 plasmid (containing the URA3 marker) were identified upon counter-selection on 5′-fluoroorotic-acid (5′-FOA) plates and were subsequently re-streaked on YPD plates for at least nine cycles for survivor formation (referred to as the ‘multiple-colony streaking assay’ in ‘Materials and methods’). The telomere patterns of the survivors were then determined through Southern blotting assay (Figure 1B–D).
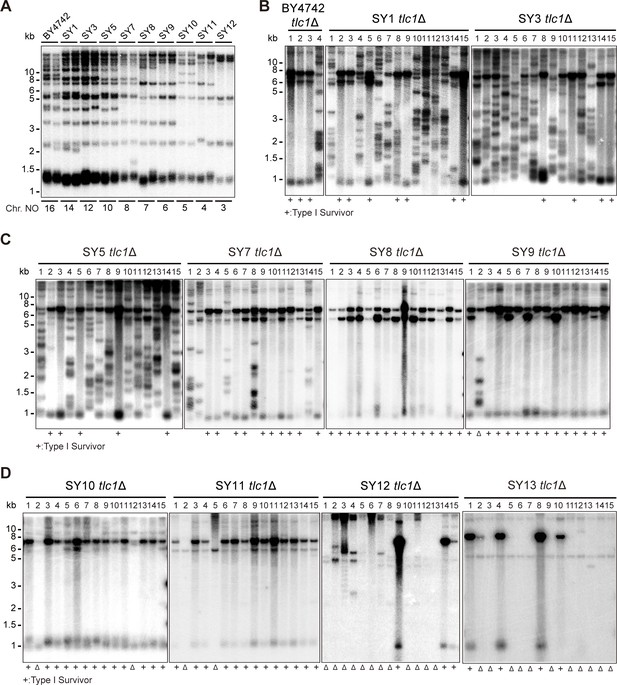
Telomere structures in SYn tlc1Δ survivors.
Telomere Southern blotting assay was performed to examine telomere structure. The genomic DNA extracted from BY4742 (wild type) and SYn strains (labeled on top) was digested with XhoI and subjected to Southern hybridization with a TG1-3 probe. (A) Telomerase-proficient strains (labeled on top), whose chromosome numbers are labeled at the bottom. Two independent clones of each strain were examined. (B–D) SYn tlc1Δ survivors generated on plates. In total, 4 (BY4742 tlc1Δ) and 15 (SYn tlc1Δ) individual survivor clones (labeled on top of each panel) of each strain were examined. ‘+’ at the bottom indicates Type I survivors. ‘Δ’ marks the survivors which are non-canonical Type I or Type II.
-
Figure 1—source data 1
Original file for the Southern blotting analysis in Figure 1A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data1-v1.zip
-
Figure 1—source data 2
File containing Figure 1A and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data2-v1.zip
-
Figure 1—source data 3
Original file for the Southern blotting analysis in Figure 1B for BY4742 tlc1Δ and SY1 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data3-v1.zip
-
Figure 1—source data 4
Original file for the Southern blotting analysis in Figure 1B for SY3 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data4-v1.zip
-
Figure 1—source data 5
File containing Figure 1B and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data5-v1.zip
-
Figure 1—source data 6
Original file for the Southern blotting analysis in Figure 1C for SY5 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data6-v1.zip
-
Figure 1—source data 7
Original file for the Southern blotting analysis in Figure 1C for SY7 tlc1Δ and SY8 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data7-v1.zip
-
Figure 1—source data 8
Original file for the Southern blotting analysis in Figure 1C for SY9 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data8-v1.zip
-
Figure 1—source data 9
File containing Figure 1C and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data9-v1.zip
-
Figure 1—source data 10
Original file for the Southern blotting analysis in Figure 1D for SY10 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data10-v1.zip
-
Figure 1—source data 11
Original file for the Southern blotting analysis in Figure 1D for SY11 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data11-v1.zip
-
Figure 1—source data 12
Original file for the Southern blotting analysis in Figure 1D for SY12 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data12-v1.zip
-
Figure 1—source data 13
Original file for the Southern blotting analysis in Figure 1D for SY13 tlc1Δ.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data13-v1.zip
-
Figure 1—source data 14
File containing Figure 1D and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig1-data14-v1.zip
The canonical telomerase-independent survivors can be broadly categorized into two types: Type I and Type II survivors, based on the restriction fragments generated after XhoI digestion (Lundblad and Blackburn, 1993; Teng and Zakian, 1999). Type I survivors exhibit tandem duplication of Y’-elements and very short TG1-3 tracts, while Type II survivors contain long heterogeneous TG1-3 sequences. Consistent with previous reports, BY4742 tlc1Δ cells generated both Type I (subtelomeric Y’-element recombination) and Type II (TG1-3 recombination) survivors (Figure 1B; Hu et al., 2013). Intriguingly, as the number of chromosomes decreased, the frequency of Type II survivors gradually diminished, while Type I survivors became the predominant type (Figure 1B–D). Furthermore, non-canonical survivors with distinct patterns from Type I or Type II emerged in SY9 tlc1Δ (six chromosomes), SY10 tlc1Δ (five chromosomes), SY11 tlc1Δ (four chromosomes), SY12 tlc1Δ (three chromosomes), and SY13 tlc1Δ (two chromosomes) (Figure 1C and D indicated by triangles at the bottom of the panels). Notably, the Y’-telomere band of ~1.2 kb was not detected in two clones of SY11 tlc1Δ cells (clones 2 and 5), the majority of clones of SY12 tlc1Δ cells (except for clones 9, 14, and 15), and the majority of clones of SY13 tlc1Δ cells (except for clones 1, 4, 8, and 10) (Figure 1D). We speculate that either the Y’-elements have eroded or the chromosomal ends containing Y’-elements have fused with other ends in these non-canonical survivors. These findings suggest that the ratio of survivor types is influenced by the number of chromosomes.
Characterizing the survivor pattern in SY12
To determine the chromosomal end structures of the non-canonical survivors shown in Figure 1, we selected SY12 tlc1Δ survivors for further analysis. In the SY12 strain, there are six telomeres corresponding to the native chromosomes I-L, X-R, XIII-L, XI-R, XVI-L, and XIV-R. We employed Southern blotting after NdeI digestion to validate the telomere and subtelomere structures (Figure 2—figure supplement 1A). The results revealed that, in the SY12 strain used in our study, only the XVI-L telomere contained a single copy of the Y’-element, while all telomeres harbored X-elements (Figure 2—figure supplement 1B). For simplicity, we referred to the chromosomes containing the original I, XIII, and XVI as chromosome 1, 2, and 3, respectively (Figure 2A, left panel).
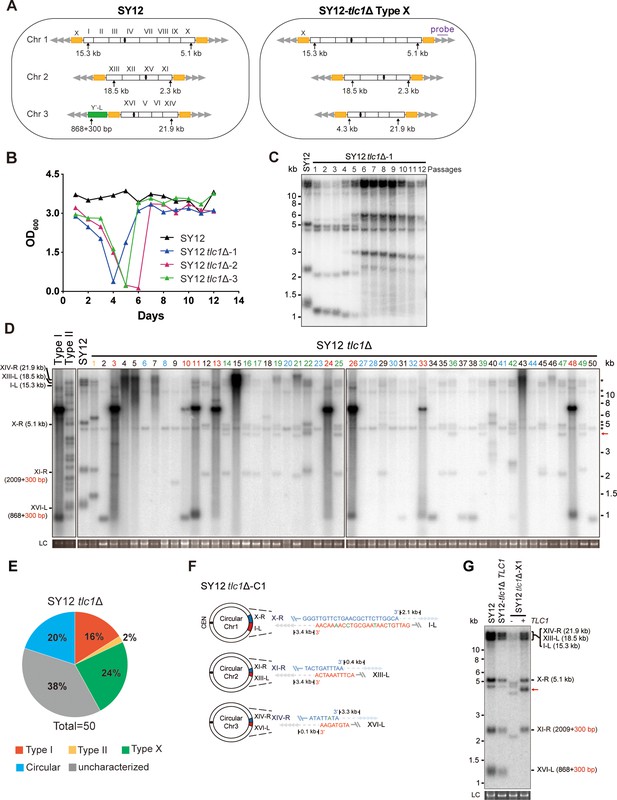
Survivor formation in SY12 tlc1Δ strain.
(A) Schematic representation of chromosome (and telomere) structures (not drawn to scale) in the SY12 strain (left panel) and the Type X survivor (right panel). The Roman numerals, native chromosomes; the Arabic numerals on the left, chromosome numbers of SY12; yellow box, X-element; green box, Y’-element; tandem gray triangles, telomeres; black circles, centromere; vertical arrows and numbers, positions and lengths of the terminal Xhol digestion fragments detected by the telomeric TG1-3 probe. Chromosome numbers are omitted in the Type X survivor (right panel). (B) Cell viability assay in liquid medium. The growth of SY12 (labeled in black) and SY12 tlc1Δ (three clones labeled in blue, purple, and green, respectively) strains were monitored every 24 hr for 12 d. (C) Telomeric Southern blotting assay of SY12 tlc1Δ survivors. Genomic DNAs prepared from SY12 tlc1Δ survivors assayed in (B) were digested with XhoI and subjected to Southern blotting with a TG1-3 probe. (D) Telomere Southern blotting assay of SY12 tlc1Δ survivors obtained on solid medium. Genomic DNA from 50 independent SY12 tlc1Δ clones (labeled on top) was digested with XhoI and hybridized to a telomere-specific TG1–3 probe. Type II survivors: in orange; Type I survivors: in red; circular survivors: in blue; Type X survivors: in green; uncharacterized survivors: in black. Theoretical telomere restriction fragments of the SY12 strain are indicated on the left. The red arrows indicate the new band of about 4.3 kb emerged in Type X survivors. The asterisks indicate the non-specific bands. Genomic DNA stained with Gelred was used as a relative loading control (LC). (E) The ratio of survivor types in SY12 tlc1Δ strain. n = 50; Type I, in red; Type II, in orange; Type X, in green; uncharacterized survivor, in gray; circular survivor, in blue. (F) Schematic of three circular chromosomes and fusion sequences in the SY12 tlc1Δ-C1 survivor. The sequence in blue indicates the sequences of X-R, XI-R, or XIV-R, the sequence in red indicates the sequences of I-L, XIII-L, or XVI-L. Bases in green are mis-paired. The numbers above or below the schematic line (chromosome) indicate the distance to the corresponding telomeres. (G) Telomere Southern blotting analysis of an SY12 tlc1Δ Type X survivor at the 20th re-streak after TLC1 reintroduction. The red arrows indicate the new band of about 4.3 kb emerged in Type X survivors. LC: loading control.
-
Figure 2—source data 1
File containing output results of growth analysis of the SY12 tlc1Δ strain in Figure 2B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data1-v1.zip
-
Figure 2—source data 2
Original file for the Southern blotting analysis in Figure 2C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data2-v1.zip
-
Figure 2—source data 3
File containing Figure 2C and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data3-v1.zip
-
Figure 2—source data 4
Original file for the Southern blotting analysis in Figure 2D.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data4-v1.zip
-
Figure 2—source data 5
Original file for the Southern blotting analysis in Figure 2D.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data5-v1.zip
-
Figure 2—source data 6
Original file for the loading control of Southern blotting analysis in Figure 2D.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data6-v1.zip
-
Figure 2—source data 7
Original file for the loading control of Southern blotting analysis in Figure 2D.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data7-v1.zip
-
Figure 2—source data 8
File containing Figure 2D and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data8-v1.zip
-
Figure 2—source data 9
File containing the original scans of the loading control of the Southern blotting analysis in Figure 2D.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data9-v1.zip
-
Figure 2—source data 10
Original file for the Southern blotting analysis in Figure 2G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data10-v1.zip
-
Figure 2—source data 11
Original file for the loading control of Southern blotting analysis in Figure 2G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data11-v1.zip
-
Figure 2—source data 12
File containing Figure 2G and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data12-v1.zip
-
Figure 2—source data 13
File containing the original scans of the loading control of the Southern blotting analysis in Figure 2G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig2-data13-v1.zip
We conducted a re-examination of telomere recombination upon telomerase inactivation in SY12 cells. Deletion of TLC1 in SY12 cells resulted in cell senescence, and different clones recovered at various time points in liquid medium (Figure 2B). Telomere Southern blotting analysis showed progressive shrinking of the telomeric XhoI fragments over time, and TG1-3 recombination occurred to maintain telomeres (Figure 2C). Since the liquid culture contained a mixture of different colonies, we employed a multiple-colony streaking assay and Southern blotting analysis to examine the telomere patterns of 50 independent SY12 tlc1Δ survivors (Figure 2D, Figure 2—figure supplement 2). Among these survivors, eight clones (labeled in red, 16% of the survivors tested) exhibited the typical Type I telomere structure characterized by Y’-element amplification (Figure 2D and E and Supplementary file 5). This was confirmed by Southern blotting analysis using a Y’ probe (Figure 2—figure supplement 2). The emergence of Type I survivor in SY12 strain which only contain one Y’-element indicates that multiple Y’-elements in tandem are not strictly required for Type I formation. Clone 1 (labeled in orange, 2% of the survivors tested) displayed heterogeneous telomeric TG1-3 tracts (Figure 2D and E and Supplementary file 5), indicating it was a Type II survivor. This was further confirmed by restoring the telomere length to the level observed in SY12 cells through the reintroduction of the TLC1 gene into one representative clone (named SY12 tlc1Δ-T1) and subsequent passaging on yeast complete (YC) medium lacking uracil (Ura-) for 20 cycles (Figure 2—figure supplement 3A).
Notably, 10 of the examined clones (labeled in blue, 20% of the survivors tested) displayed no telomere signals associated with canonical Type I or II survivors (Figure 2D and E and Supplementary file 5). Their hybridization patterns were strikingly similar to those of SY14 tlc1Δ survivors (Wu et al., 2020), which survived through intra-chromosomal circularization. To investigate whether the three chromosomes in these SY12 tlc1Δ survivors had undergone intra-chromosomal fusions, we selected a clone, namely SY12 tlc1Δ-C1, and performed PCR-mapping assay to determine the erosion points of each chromosome end, as previously described (Wu et al., 2020). A PCR product of the predicted length would be obtained only if the corresponding chromosome region was intact. The PCR-mapping assay precisely identified the borders of telomere erosion for the three chromosomes in SY12 tlc1Δ-C1 cells. For chromosome 1 (Figure 2A, left panel), the chromosome regions approximately 3.3 kb and 1.9 kb proximal to telomere I-L and X-R, respectively, had been lost (Figure 2—figure supplement 4 and Supplementary file 3). Regarding chromosome 2, the terminal ~3.8 kb of telomere XIII-L and ~2.5 kb of telomere XI-R remained intact (Figure 2—figure supplement 4 and Supplementary file 3). For chromosome 3, the terminal ~0.1 kb of telomere XVI-L was intact, while the terminal ~3.4 kb of telomere XIV-R was preserved (Figure 2—figure supplement 4 and Supplementary file 3). To confirm the chromosome fusion events, we performed PCR-sequencing analysis. If a given pair of primers, oriented to different chromosome ends, produced PCR products, it indicated that the corresponding arms had fused. The results revealed that the three chromosomes in SY12 tlc1Δ-C1 cells had undergone intra-chromosomal fusions through microhomology-mediated end joining (MMEJ) (Wu et al., 2020), resulting in the formation of circular chromosomes (Figure 2F and Supplementary file 3). Notably, the fusion junctions of the three chromosomes in SY12 tlc1Δ-C1 cells differed in nucleotide sequence and length (22 bp, 8 bp, and 5 bp in chromosomes 1, 2, and 3, respectively). Moreover, the sequences involved in the ends-fusion were not perfectly complementary (Figure 2F). For example, the fusion sequence of chromosome 3 was 5 bp long and contained one mismatch. To further verify the chromosome structure in the ‘circular survivors’ SY12 tlc1Δ-C1 (Figure 2F), we performed the pulsed-field gel electrophoresis (PFGE) analysis. Control strains included SY12 (three linear chromosomes) and SY15 (one circular chromosome). The PFGE result confirmed that like the single circular chromosome in SY15 cells, the circular chromosome in the SY12 tlc1Δ-C1 survivors could not enter the gel, while the linear chromosomes in SY12 were separated into distinct bands, as expected (Figure 2—figure supplement 5). Thus, the survivors shown in Figure 2D, which displayed an identical hybridization pattern to the SY12 tlc1Δ-C1 clone, were all likely ‘circular survivors’. Consistently, the telomere signals detected in the SY12 strain were still not observed in the SY12 tlc1Δ-C1 survivor after reintroducing a plasmid-borne wild-type TLC1 gene (Figure 2—figure supplement 3B).
Twelve clones of SY12 tlc1Δ survivors (labeled in green, 24% of the survivors tested) exhibited no Y’-telomere signals compared to SY12 cells but displayed different lengths of TG1-3 tracts (Figure 2D and E and Supplementary file 5). Due to their non-canonical telomere structures, characterized by the absence of both Y’- amplification and superlong TG1-3 sequences, we designated these SY12 tlc1Δ survivors (labeled in green, Figure 2D) as Type X. In Type X survivors, the DNA bands with sizes of approximately 2.3 kb, 5.1 kb, 15.3 kb, 18.5 kb, and 21.9 kb were roughly comparable to the telomeres of XI-R, X-R, I-L, XIII-L, and XIV-R in SY12 cells (indicated on the left in the panel). The newly emerged band at approximately 4.3 kb likely originated from the XVI-L telomere (indicated by the red arrow on the right in the panel) (Figure 2D), where the Y’-elements had been eroded, leaving only the TG1-3 tracts at the very ends (Figure 2A, right panel). It remains unclear whether Y’-element erosion is common in telomerase-null BY4742 Type II survivors. However, in SY12 tlc1Δ cells, the remaining single copy of the Y’-element could not find homology sequences to repair telomeres, whereas the multicopy X-element could easily find homology sequences to repair telomeres and form the Type X survivors. To verify this notion, we reintroduced the TLC1 gene into one representative clone (named SY12 tlc1Δ-X1) and examined the telomere length. As expected, the telomeres of X-R and XI-R were restored to the lengths observed in wild-type SY12 cells, and accordingly, the newly emerged 4.3 kb band was also elongated (Figure 2G). Given that the restriction fragments of telomeres I-L (15.3 kb), XIII-L (18.5 kb), and XIV-R (21.9 kb) were quite long, detecting minor changes in telomere length was challenging under the assay conditions of Southern blotting. To determine the chromosomal end structure of the Type X survivor, we randomly selected a typical Type X survivor, and performed PCR-sequencing analysis. The results revealed the intact chromosome ends for I-L, X-R, XIII-L, XI-R, and XIV-R, albeit with some mismatches compared with the S. cerevisiae S288C genome (http://www.yeastgenome.org/), which possibly arising from recombination events that occurred during survivor formation. Notably, the sequence of the Y’-element in XVI-L could not be detected, while the X-element remained intact (Figure 2—figure supplement 6). These data indicated that Type X survivors possess linear chromosomes with telomeres terminating in TG1-3 repeats, while the Y’-element has been eroded (Figure 2A, right panel). Consistently, no Y’ signals were detected in these 12 Type X survivors (labeled in green, Figure 2—figure supplement 2), suggesting that the Y’-element has not been translocated to other telomeres and is not essential for yeast cell viability.
In addition to the aforementioned Type I, Type II, circular, and Type X survivors, there were some clones (labeled in black, 38% of the survivors tested) which exhibited non-uniform telomere patterns and were not characterized (Figure 2D and E and Supplementary file 5). We speculated that combinations of diverse mechanisms were occurring within each ‘uncharacterized survivor’. For instance, in the case of two survivors (clones 9 and 18, Figure 2D) in which only one hybridization signal could be detected, pointing to the possibility that two chromosomes underwent intra-chromosomal fusions while one retained its ends through TG1-3 recombination. However, the sizes of the two telomere restriction fragments on the linear chromosome were too close to be distinguished and separated, resulting in only one hybridization signal. Alternatively, it is also plausible that three chromosomes experienced intra-chromosomal fusions, with one fusion point containing TG1-3 repeats. For the uncharacterized clones 4, 5, 7, 15, and 43, they exhibited significant amplification of TG1-3 sequences, and the telomeres of these survivors did not resolve into distinct bands (Figure 2D). We hypothesize that the observed telomere patterns in these survivors could be attributed to extensive TG1-3 recombination. However, we cannot exclude the possibility of coexisting diverse mechanisms within a survivor, such as telomere elongation through TG1-3 amplification, as well as intra- and inter-chromosomal fusions. Since we could not figure out the telomere structures in these survivors, we classified them as ‘uncharacterized survivors’.
To further determine the genetic requirements for survivors in SY12, we constructed the SY12 tlc1Δ rad52Δ pRS316-TLC1 strain. The plasmid-borne wild-type TLC1 gene (pRS316-TLC1) was counter-selected on 5′-FOA plates. SY12 tlc1Δ rad52Δ cells were measured by the cell viability assay (see ‘Materials and methods’). The results showed double deletion of TLC1 and RAD52 in SY12 strain could slightly accelerate senescence, and SY12 tlc1Δ rad52Δ survivors could be generated but took much longer to recover than the SY12 tlc1Δ survivors (Figure 2—figure supplement 7A), suggesting that Rad52 is not strictly required for survivor generation in the SY12 strain in liquid. We also passaged SY12 tlc1Δ rad52Δ cells on solid medium until survivor emerged. Southern blotting of 25 clones revealed that neither Type I nor II survivors were found, and instead circular survivors except clone 20 were obtained (labeled in blue, Figure 2—figure supplement 7B). We conclude that the formation of circular survivors in the SY12 tlc1Δ rad52Δ strain is mediated by MMEJ as observed in the SY14 tlc1Δ rad52Δ strain (Wu et al., 2020), but not RAD52 mediate pathways. Since no Type X survivor was detected in SY12 tlc1Δ rad52Δ strain, we constructed the SY12 tlc1Δ rad51Δ pRS316-TLC1 and SY12 tlc1Δ rad50Δ pRS316-TLC1 strain to investigate on which pathway the formation of the Type X survivor relied. After being counter-selected on 5′-FOA plates, cells were passaged on solid medium until survivor arose. Southern blotting assay indicated the emergence of Type X survivors even in the absence of Rad51 (labeled in green, clones 2, 5, 11, and 18, Figure 2—figure supplement 8A). In contrast, no Type X survivor was detected in the SY12 tlc1Δ rad50Δ strain (Figure 2—figure supplement 8B). These data suggest that the formation of the Type X survivor depends on Rad50-mediated Type II pathway.
Taken together, our results indicate that telomerase inactivation in SY12 cells leads to cell senescence and the emergence of survivors with diverse telomere patterns, including Y’-amplification (Type I), elongated TG1-3 tracts (Type II), intra-chromosomal end-to-end joining (circular), Y’- loss (Type X), and uncharacterized.
Deletion of all of the X- and Y’-elements in the SY12 strain
We aimed to determine whether the subtelomeric X-elements are dispensable or not. In the SY12 strain, there are six X-elements distributed among six telomeres (Figure 2A, left panel). To precisely delete all X- and Y’-elements in SY12 strains, we employed a method that combines the efficient CRISPR-Cas9 cleavage system with the robust homologous recombination activity of yeast, as previously described (Shao et al., 2018; Shao et al., 2019). Briefly, the Cas9 nuclease cleaved the unique DNA sequences adjacent to the subtelomeric region (site S1) with the guidance of gRNA1. The resulting chromosome break was repaired through homologous recombination (HR) using the provided chromosome ends excluding the X- and Y’-elements. Subsequently, the URA3 marker and the guide RNA expression plasmid (pgRNA) were eliminated by inducing gRNA2 expression on pCas9 using galactose (Figure 3—figure supplement 1). This approach allowed us to initially delete the Y’-element and X-element in XVI-L, generating the SY12YΔ strain (Figure 3A, Supplementary file 4, and Supplementary file 6). Subsequently, through five successive rounds of deletions, we removed all remaining X-elements, resulting in the SY12XYΔ strain (Figure 3A, Supplementary file 4, and Supplementary file 6). To confirm the series of deletions, we performed PCR analysis using a primer located within the deletion region and another primer annealing upstream of the region (indicated by purple arrows in Figure 3—figure supplement 1, primers are shown in Supplementary file 1). This analysis verified the complete deletion of the subtelomeric X- and Y’-elements (Figure 3B, rows 3–7). Additionally, we conducted a separate PCR analysis using primers specific to either X- or Y’-elements, which confirmed the absence of both X- and Y’-elements in the SY12XYΔ strain (Figure 3B, row 8). Subsequently, we inserted a Y’-long element (cloned from the native XVI-L sequence, which does not contain the centromere-proximal short telomere sequence) into the left arm of chromosome 3 in the SY12XYΔ strain, resulting in the SY12XYΔ+Y strain containing a single Y’-element but no X-element (Figure 3A and Supplementary file 4). The successful insertion was confirmed by PCR analysis (Figure 3B, lane 9).
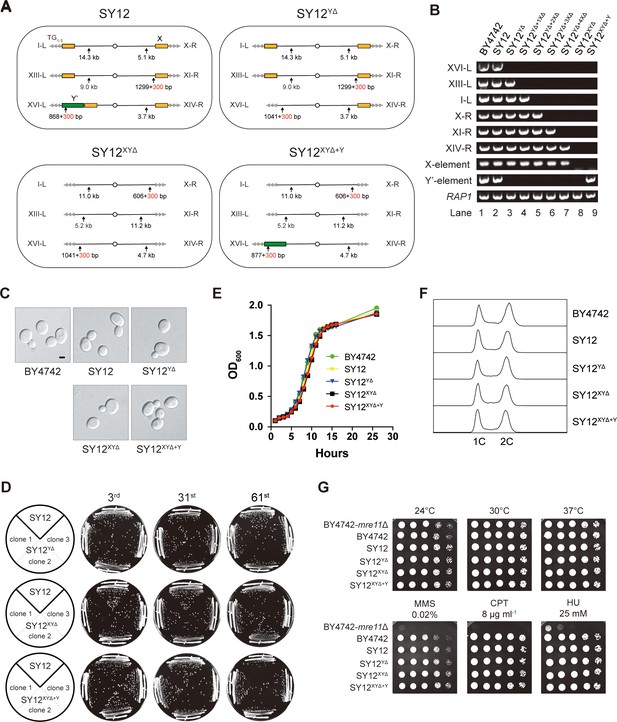
Characterization of SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ strains.
(A) Schematic of chromosome structures in the SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ strains. Yellow box, X-element; green box, Y’-element; tandem gray triangles, telomeres. Vertical arrows and numbers indicate the positions and sizes of the sites and length of Xhol and PaeI-digested terminal fragments. (B) PCR analyses of the engineered sites of the individual telomeres (labeled on the left) in BY4742, SY12, SY12YΔ, SY12YΔ+1XΔ, SY12YΔ+2XΔ, SY12YΔ+3XΔ, SY12YΔ+4XΔ, SY12XYΔ, and SY12XYΔ+Υ strains (labeled on top). Primer sequences for the PCR analyses are listed in Supplementary file 1. RAP1 was an internal control. (C) Morphology of BY4742, SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ cells in the exponential growth phase (30°C in YPD). Shown are DIC images. Scale bar, 2 μm. (D) Growth analysis of the SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ strains. Several clones of the SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ strains were re-streaked on YPD plates 61 times at intervals of 2 d. Shown were the 3rd, 31st, and 61st re-streaks. (E) Growth analysis of BY4742, SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ cells in liquid culture. Error bars represent standard deviation (s.d.), n = 3. (F) Fluorescence-activated cell sorting (FACS) analysis of DNA content of BY4742, SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ cells. (G) Dotting assays on YPD plates at low (24°C) and high (37°C) temperatures, or on YPD plates containing methyl methane sulfonate (MMS), camptothecin (CPT), or hydroxyurea (HU) at the indicated concentrations. The BY4742 mre11Δ haploid strain serves as a negative control because Mre11 is involved in the repair of double-stranded breaks (Lewis et al., 2004).
-
Figure 3—source data 1
PCR identify of SY12 subtelomeric deletion strains in Figure 3B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data1-v1.zip
-
Figure 3—source data 2
File containing Figure 3B and original scans of PCR identify of SY12 subtelomeric deletion strains.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data2-v1.zip
-
Figure 3—source data 3
Original file for the morphology analysis in Figure 3C for BY4742 strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data3-v1.zip
-
Figure 3—source data 4
Original file for the morphology analysis in Figure 3C for SY12 strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data4-v1.zip
-
Figure 3—source data 5
Original file for the morphology analysis in Figure 3C for SY12YΔ strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data5-v1.zip
-
Figure 3—source data 6
Original file for the morphology analysis in Figure 3C for SY12XYΔ strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data6-v1.zip
-
Figure 3—source data 7
Original file for the morphology analysis in Figure 3C for SY12XYΔ+Y strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data7-v1.zip
-
Figure 3—source data 8
File containing Figure 3C and original photos of morphology analysis of SY12 subtelomeric deletion strains.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data8-v1.zip
-
Figure 3—source data 9
Original file for the growth analysis in Figure 3D for SY12YΔ strain at the third streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data9-v1.zip
-
Figure 3—source data 10
Original file for the growth analysis in Figure 3D for SY12YΔ strain at the 31st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data10-v1.zip
-
Figure 3—source data 11
Original file for the growth analysis in Figure 3D for SY12YΔ strain at the 61st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data11-v1.zip
-
Figure 3—source data 12
Original file for the growth analysis in Figure 3D for SY12XYΔ strain at the third streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data12-v1.zip
-
Figure 3—source data 13
Original file for the growth analysis in Figure 3D for SY12XYΔ strain at the 31st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data13-v1.zip
-
Figure 3—source data 14
Original file for the growth analysis in Figure 3D for SY12XYΔ strain at the 61st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data14-v1.zip
-
Figure 3—source data 15
Original file for the growth analysis in Figure 3D for SY12XYΔ+Y strain at the third streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data15-v1.zip
-
Figure 3—source data 16
Original file for the growth analysis in Figure 3D for SY12XYΔ+Y strain at the 31st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data16-v1.zip
-
Figure 3—source data 17
Original file for the growth analysis in Figure 3D for SY12XYΔ+Y strain at the 61st streaks.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data17-v1.zip
-
Figure 3—source data 18
File containing Figure 3D and original photos of growth analysis of SY12 subtelomeric deletion strains.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data18-v1.zip
-
Figure 3—source data 19
File containing output results of growth analysis of the SY12 subtelomeric deletion strains in Figure 3E.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data19-v1.zip
-
Figure 3—source data 20
Original FACS analysis results of Figure 3F.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data20-v1.zip
-
Figure 3—source data 21
Original file for the dotting assay on YPD plate at 24°C in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data21-v1.zip
-
Figure 3—source data 22
Original file for the dotting assay on YPD plate at 30°C in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data22-v1.zip
-
Figure 3—source data 23
Original file for the dotting assay on YPD plate at 37°C in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data23-v1.zip
-
Figure 3—source data 24
Original file for the dotting assay on YPD plate containing MMS in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data24-v1.zip
-
Figure 3—source data 25
Original file for the dotting assay on YPD plate containing CPT in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data25-v1.zip
-
Figure 3—source data 26
Original file for the dotting assay on YPD plate containing HU in Figure 3G.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data26-v1.zip
-
Figure 3—source data 27
File containing Figure 3G and original photos of dotting assays of SY12 subtelomeric deletion strains.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig3-data27-v1.zip
Subtelomeric X- and Y’-elements are dispensable for cell proliferation, various stress responses, telomere length control, and telomere silencing
The SY12YΔ, SY12XYΔ, and SY12XYΔ+Y cells, cultured in YPD medium at 30°C, exhibited the same cell morphology as the parental strains SY12 and BY4742 (Figure 3C). To assess the stability of their genomes, we restreaked several clones of SY12YΔ, SY12XYΔ, and SY12XYΔ+Y strains on YPD plates for a total of 61 times at 2-day intervals (Figure 3D). Similar to the SY12 strain, the progeny colonies of SY12YΔ, SY12XYΔ, and SY12XYΔ+Y grew robustly on solid medium (Figure 3D). Moreover, SY12YΔ, SY12XYΔ, and SY12XYΔ+Y cells exhibited growth rates comparable to those of SY12 and BY4742 cells in liquid medium (Figure 3E). Fluorescence-activated cell sorting (FACS) analysis revealed that SY12YΔ, SY12XYΔ, and SY12XYΔ+Y had the same 1C and 2C DNA content as wild-type cells (Figure 3F), indicating that the X- and Y’-elements are not necessary for cell proliferation under normal conditions. Additionally, the growth of SY12YΔ, SY12XYΔ, and SY12XYΔ+Y cells at different temperatures (24 and 37°C) (Figure 3G, upper panel) closely resembled that of SY12 and BY4742 cells. Furthermore, SY12YΔ, SY12XYΔ, SY12XYΔ+Y, SY12, and BY4742 cells exhibited similar sensitivities to various genotoxic agents, including hydroxyurea (HU), camptothecin (CPT), and methyl methanesulfonate (MMS) (Figure 3G, lower panel). These results indicate that the X- and Y’-elements are dispensable for cellular responses to cold or heat treatment and DNA damage challenges, consistent with a recent study of ‘synthetic yeast genome project’, namely Sc2.0, showing that thousands of genome-wide edits, including the deletion of subtelomeric repetitive sequences, deletion of introns, and relocation of tRNAs genes, yielded a strain that displays comparable growth with wild-type strain (Richardson et al., 2017; Zhao et al., 2023).
Next, we examined the effects of X- and Y’-element elimination on telomeres. Southern blotting assay revealed that SY12YΔ, SY12XYΔ, and SY12XYΔ+Y cells maintained stable telomeres at a length of approximately 300 bp, comparable to that in SY12 cells (Figure 4A), indicating that the X- and Y’-elements are not required for telomere length regulation. To determine whether the deletion of X- and Y’-elements abolishes telomere silencing, we constructed haploid strains of SY12YΔ sir2Δ, SY12XYΔ sir2Δ, SY12XYΔ+Y sir2Δ, SY12 sir2Δ, and BY4742 sir2Δ. We then performed real-time RT-PCR to quantify the expression of the MPH3 and HSP32 genes, located near the subtelomeric region of X-R (X-only end) and XVI-L (X-Y’ end), respectively (Figure 4B), and found that the increase of the MPH3 or HSP32 expression upon SIR2 deletion in SY12YΔ, SY12XYΔ, and SY12XYΔ+Y strains was more significant than that in the BY4742 or the SY12 strain, indicating that telomere silencing remains effective in the absence of X-and Y’-elements (Figure 4B). These findings align with previous studies showing that telomeres without an X- or Y’-element exert a position effect on the transcription of neighboring genes (Aparicio et al., 1991), and that X- and Y’-elements function as modulators of TPE (Fourel et al., 1999; Lebrun et al., 2001; Ottaviani et al., 2008).
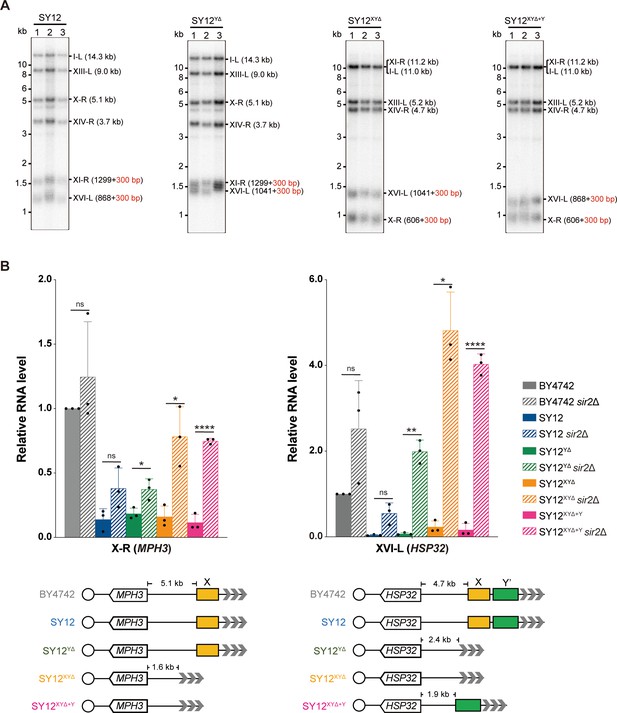
Telomere length and telomere silencing analyses of SY12YΔ, SY12ΧΥΔ, and SY12XYΔ+Υ strains.
(A) Southern blotting analysis of telomere length in SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ (labeled on top) cells. Genomic DNA prepared from three independent clones of SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ strains were digested with XhoI and PaeI, and then subjected to Southern blotting with a TG1-3 probe. The numbers in brackets indicate the telomere length of the corresponding chromosomes. (B) Expressions of MPH3 and HSP32 in ΒΥ4742, SY12, SY12YΔ, SY12XYΔ, and SY12XYΔ+Υ cells were detected by qRT-PCR. The numbers above the schematic line (lower panels) indicate the distance to the corresponding subtelomeric elements or telomeres. The RNA levels of MPH3 and HSP32 were normalized by ACT1. The wild-type value is arbitrarily set to 1. Error bars represent standard deviation (s.d.), n = 3. ‘ns’, p>0.5 (Student’s t-test); *p<0.05 (Student’s t-test); **p<0.01 (Student’s t-test); ****p<0.0001 (Student’s t-test).
-
Figure 4—source data 1
Original file for the Southern blotting analysis in Figure 4A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig4-data1-v1.zip
-
Figure 4—source data 2
File containing Figure 4A and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig4-data2-v1.zip
-
Figure 4—source data 3
File containing output results of qPCR.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig4-data3-v1.zip
In conclusion, the SY12YΔ, SY12XYΔ, and SY12XYΔ+Y strains behave similarly to the wild-type SY12 strain under all tested conditions (Figures 3 and 4). Their simplified telomere structure makes them potentially useful tools for telomere studies.
Y’-elements are not strictly required for the formation of Type II survivors
The BY4742 strain harbors 19 Y’-elements distributed among 17 telomere loci. Numerous studies have emphasized the significance of Y’-elements in telomere recombination. For instance, Type I survivors exhibit significant amplification of Y’-elements (Lundblad and Blackburn, 1993; Teng and Zakian, 1999) and survivors show a marked induction of the potential DNA helicase Y’-Help1 encoded by Y’-elements (Yamada et al., 1998). Additionally, the acquisition of Y’-elements by short telomeres delays the onset of senescence (Churikov et al., 2014).
To investigate the requirement of Y’-elements in survivor formation, we deleted TLC1 in SY12YΔ cells and conducted a cell viability assay. The results demonstrated that three individual colonies underwent senescence and subsequently recovered at different passages in liquid media (Figure 5A). Further analysis through Southern blotting revealed that the telomeres of SY12YΔ tlc1Δ cells underwent progressive shortening with each passage until reaching critically short lengths. Subsequently, TG1-3 recombination occurred, leading to abrupt telomere elongation (Figure 5B).
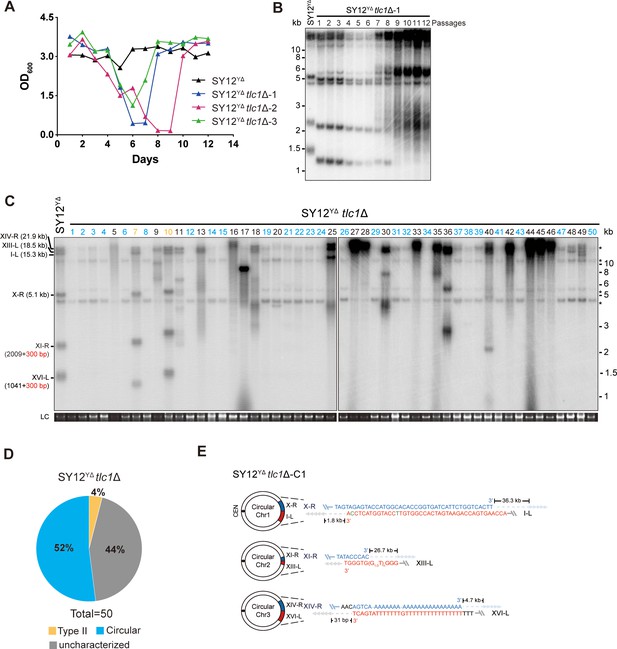
Survivor analysis of SY12YΔtlc1Δ strain.
(A) Cell viability assay in liquid medium. The growth of SY12YΔ (labeled in black) and SY12YΔtlc1Δ (three clones labeled in blue, purple, and green, respectively) strains were monitored every 24 hr for 12 d. (B) Telomeric Southern blotting assay of SY12YΔtlc1Δ survivors. Genomic DNAs prepared from SY12YΔtlc1Δ survivors assayed in (A) were digested with XhoI and subjected to Southern blotting with a TG1-3 probe. (C) Telomere Southern blotting analysis of SY12YΔtlc1Δ survivors obtained on solid medium. Genomic DNAs of 50 independent survivors (labeled 1–50 on top) were digested with XhoI and hybridized by a TG1-3 probe. Type II survivors: in orange; circular survivors: in blue; uncharacterized survivors: in black. Theoretical telomere restriction fragments of the SY12YΔ strain are indicated on the left. LC: loading control. (D) The ratio of survivor types in SY12 YΔtlc1Δ strain. n = 50; Type II, in orange; uncharacterized survivor, in gray; circular survivor, in blue. (E) Schematic of three circular chromosomes and fusion sequences in the SY12YΔtlc1Δ-C1 survivor. The sequence in blue indicates the sequences of X-R, XI-R, or XIV-R, the sequence in red indicates the sequences of I-L, XIII-L, or XVI-L. Bases in green are mis-paired, dashes are deleted. The numbers above or below the schematic line (chromosome) indicate the distance to the corresponding telomeres.
-
Figure 5—source data 1
File containing output results of growth analysis of the SY12YΔ tlc1Δ strain.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data1-v1.zip
-
Figure 5—source data 2
Original file for the Southern blotting analysis in Figure 5B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data2-v1.zip
-
Figure 5—source data 3
File containing Figure 5B and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data3-v1.zip
-
Figure 5—source data 4
Original file for the Southern blotting analysis in Figure 5C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data4-v1.zip
-
Figure 5—source data 5
Original file for the Southern blotting analysis in Figure 5C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data5-v1.zip
-
Figure 5—source data 6
Original file for the loading control of Southern blotting analysis in Figure 5C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data6-v1.zip
-
Figure 5—source data 7
Original file for the loading control of Southern blotting analysis in Figure 5C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data7-v1.zip
-
Figure 5—source data 8
File containing Figure 5C and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data8-v1.zip
-
Figure 5—source data 9
File containing the original scans of the loading control of the Southern blotting analysis in Figure 5C.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig5-data9-v1.zip
Next, we examined the telomere patterns of 50 independent SY12YΔ tlc1Δ survivors using a multiple-colony streaking assay and Southern blotting analysis. Out of the 50 clones analyzed, no Type I survivors were detected due to the deletion of Y’-elements in SY12YΔ strain (Figure 5C). Two clones (labeled in orange, 4% of the survivors tested) displayed heterogeneous telomere tracts (Figure 5C and D and Supplementary file 5). Reintroduction of TLC1 into a representative clone (named SY12YΔ tlc1Δ-T1) resulted in telomere length restoration similar to SY12YΔ cells (Figure 5—figure supplement 1A), indicating their classification as Type II survivors. Twenty-six clones (labeled in blue, 52% of the survivors tested) exhibited patterns identical to that of the SY12 tlc1Δ circular survivors (Figures 5C and D and 2D and Supplementary file 5). Further mapping of erosion borders and sequencing of fusion junctions (Figure 5E, Figure 5—figure supplement 2, and Supplementary file 3) confirmed that three chromosomes from a randomly selected clone (named SY12YΔ tlc1Δ-C1) underwent intra-chromosomal fusions mediated by microhomology sequences. The erosion sites and fusion sequences differed from those observed in SY12 tlc1Δ-C1 cells (Figure 2F), suggesting the stochastic nature of intra-chromosome end fusion by MMEJ. As expected, the telomere Southern blotting pattern (XhoI digestion) of the SY12YΔ tlc1Δ-C1 survivor remained unchanged following telomerase reintroduction (Figure 5—figure supplement 1B). Further PFGE analysis confirmed that the chromosomes in SY12YΔ tlc1Δ-C1 were circulated (Figure 2—figure supplement 5). Notably, a significant proportion of the survivors displayed telomere signals that were different from those of either the Type II or circular survivors (labeled in black, 44% of the survivors tested, Figure 5C and D and Supplementary file 5), and they were uncharacterized survivors. Further deletion of RAD52 in the SY12YΔ tlc1Δ cells affected, but did not eliminate, survivor generation (Figure 5—figure supplement 3A). Southern blotting assay confirmed that most of the recovered clones were circular survivors, and two were uncharacterized survivors (clones 9 and 16, labeled in black, Figure 5—figure supplement 3B), suggesting that survivor formation in SY12YΔ tlc1Δ rad52Δ cells does not strictly rely on the homologous recombination. Overall, these findings indicate that Y’-elements are not strictly required for Type II survivor formation (Churikov et al., 2014).
X-elements are not strictly necessary for survivor generation
To investigate the contribution of X-elements to telomere recombination, we employed the SY12XYΔ+Y strain, which contains only one Y’-element in the subtelomeric region, and the SY12XYΔ tlc1Δ strain, which lacks both the X- and Y’-elements. Subsequently, we deleted TLC1 in the SY12XYΔ+Y and SY12XYΔ strains and conducted a cell viability assay. Consistently, the deletion of TLC1 in SY12XYΔ+Y and SY12XYΔ resulted in telomere shortening, senescence, and the formation of Type II survivors (Figure 6—figure supplement 1). Then, 50 independent clones of SY12XYΔ+Y tlc1Δ or SY12XYΔ tlc1Δ survivors were examined using Southern blotting (Figure 6A and B).
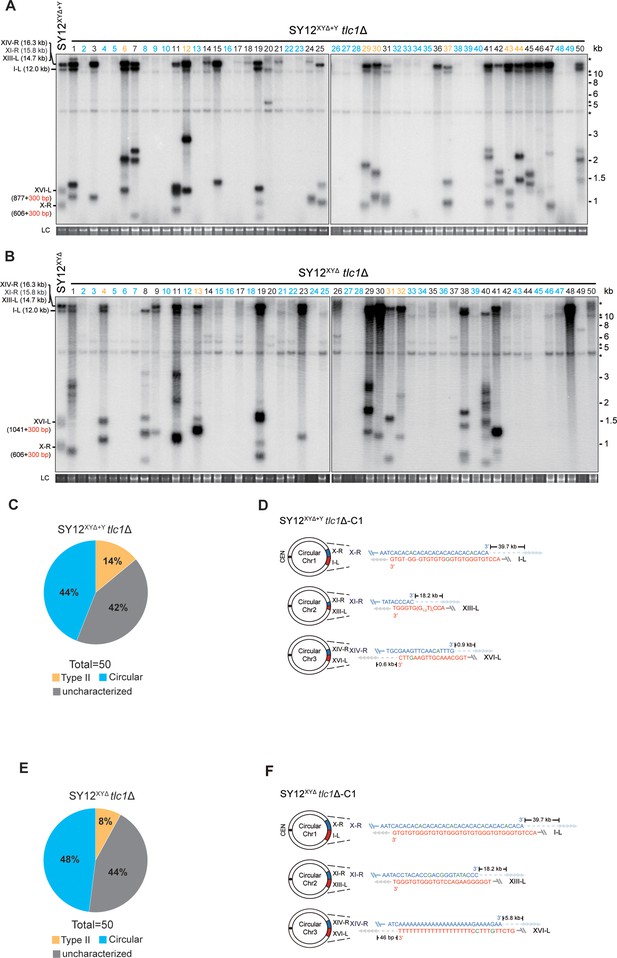
Survivor analysis of SY12XYΔtlc1Δ and SY12XYΔ+Ytlc1Δ strains.
(A, B) Telomere Southern blotting analysis of SY12XYΔ+Ytlc1Δ (A) and SY12XYΔ tlc1Δ (B) survivors obtained on solid medium. 50 independent survivors (labeled 1–50 on top) were randomly picked, and their genomic DNAs were digested with XhoI and subjected to the Southern blotting assay with a TG1-3 probe. Type II survivors: in orange; circular survivors: in blue; uncharacterized survivors: in black. The sizes of individual telomere restriction fragments of the SY12XYΔ+Y and SY12XYΔ strain are indicated on the left. LC: loading control. (C, E) The percentage of survivor types in SY12 XYΔ+Ytlc1Δ (C) and SY12 XYΔtlc1Δ (E) strains. n = 50; Type II, in orange; uncharacterized survivor, in gray; circular survivor, in blue. (D, F) Schematic of three circular chromosomes and fusion sequences in the SY12XYΔ+Ytlc1Δ-C1 (D) and SY12XYΔ tlc1Δ-C1 (F) survivors, respectively. The sequence in blue indicates the sequences of X-R, XI-R, or XIV-R, the sequence in red indicates the sequences of I-L, XIII-L, or XVI-L. Bases in green are mis-paired, dashes are deleted. The numbers above or below the schematic line (chromosome) indicate the distance to the corresponding telomeres.
-
Figure 6—source data 1
Original file for the Southern blotting analysis in Figure 6A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data1-v1.zip
-
Figure 6—source data 2
Original file for the Southern blotting analysis in Figure 6A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data2-v1.zip
-
Figure 6—source data 3
Original file for the loading control of Southern blotting analysis in Figure 6A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data3-v1.zip
-
Figure 6—source data 4
Original file for the loading control of Southern blotting analysis in Figure 6A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data4-v1.zip
-
Figure 6—source data 5
File containing Figure 6A and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data5-v1.zip
-
Figure 6—source data 6
File containing the original scans of the loading control of the Southern blotting analysis in Figure 6A.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data6-v1.zip
-
Figure 6—source data 7
Original file for the Southern blotting analysis in Figure 6B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data7-v1.zip
-
Figure 6—source data 8
Original file for the Southern blotting analysis in Figure 6B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data8-v1.zip
-
Figure 6—source data 9
Original file for the loading control of Southern blotting analysis in Figure 6B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data9-v1.zip
-
Figure 6—source data 10
Original file for the loading control of Southern blotting analysis in Figure 6B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data10-v1.zip
-
Figure 6—source data 11
File containing Figure 6B and original scans of the relevant Southern blotting analysis.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data11-v1.zip
-
Figure 6—source data 12
File containing the original scans of the loading control of the Southern blotting analysis in Figure 6B.
- https://cdn.elifesciences.org/articles/91223/elife-91223-fig6-data12-v1.zip
Among the SY12XYΔ+Y survivors analyzed, 22 clones underwent chromosomal circularization (labeled in blue, 44% of the survivors tested, Figure 6A and C and Supplementary file 5). We randomly selected a clone named SY12XYΔ+Y tlc1Δ-C1, and the results of erosion-border mapping and fusion junction sequencing showed that it had undergone intra-chromosomal fusions mediated by microhomology sequences (Figure 6D, Figure 6—figure supplement 2, and Supplementary file 3). Subsequently, Southern blotting revealed that the chromosome structure of SY12XYΔ+Y tlc1Δ-C1 remained unchanged after TLC1 reintroduction (Figure 6—figure supplement 3), and PFGE analysis confirmed the circular chromosome structure in SY12XYΔ+Y tlc1Δ-C1 (Figure 2—figure supplement 5). Additionally, seven clones utilized the Type II recombination pathway and exhibited heterogeneous telomeric TG1-3 tracts (labeled in orange, 14% of the survivors tested, Figure 6A and C and Supplementary file 5). Reintroduction of TLC1 into a representative clone (named SY12XYΔ+Y tlc1Δ-T1) restored the telomere length to normal (Figure 6—figure supplement 3). These findings indicate that the majority of cells underwent intra-chromosomal circularization or TG1-3 recombination. While even though there is a Y’-element, no Type I survivors were generated in SY12XYΔ+Y tlc1Δ survivors (Figure 6A). We speculated that the short TG1-3 repeats located centromere-proximal to the Y’-elements play a crucial role in strand invasion and subsequent Y’-recombination. This speculation is consistent with a previous report stating that Type I events are virtually absent in the yeast strain Y55, which lacks TG1-3 repeats centromere-proximal to the Y’-element (Huang et al., 2001). We also observed some clones displayed non-canonical telomere signals like SY12 tlc1Δ ‘uncharacterized’ survivors (labeled in black, 42% of the survivors tested, Figure 6A and C and Supplementary file 5). Overall, these data suggest that X-elements are not strictly necessary for survivor formation.
Among the SY12XYΔ survivors, 24 displayed a ‘circular survivor’ pattern (labeled in blue, 48% of the survivors tested, Figure 6B and E and Supplementary file 5). Additional PCR-sequencing assays and PFGE analysis of the SY12XYΔ tlc1Δ-C1 cells confirmed the occurrence of intra-chromosomal fusions mediated by microhomology sequences (Figure 6F, Figure 6—figure supplement 4, Supplementary file 3, and Figure 2—figure supplement 4). Reintroduction of TLC1 into a representative clone named SY12XYΔtlc1Δ-C1 could restore its telomere length to WT level (Figure 6—figure supplement 5A). Also, 4 of 50 survivors harbored Type II telomere structure (labeled in orange, 8% of the survivors tested, Figure 6B and E and Supplementary file 5). Reintroduction of TLC1 into a representative clone named SY12XYΔtlc1Δ-T1 could restore its telomere length to WT level (Figure 6—figure supplement 5B). Some of the survivors (labeled in black, 44% of the survivors tested, Figure 6B and E and Supplementary file 5) were not characterized. Like in SY12 tlc1Δ cells, Rad52 is not strictly required for the formation of circular survivors in SY12XYΔ tlc1Δ rad52Δ and SY12XYΔ+Y tlc1Δ rad52Δ strains (Figure 6—figure supplement 6A and B). To investigate whether Type I-specific mechanisms are still utilized in the survivor formation in Y’-less strain, we deleted RAD51 in SY12XYΔ tlc1Δ, and found that SY12XYΔ tlc1Δ rad51Δ strain was able to generate three types of survivors, including Type II survivor, circular survivor, and uncharacterized survivor (Figure 6—figure supplement 7A), similar to the observations in SY12XYΔ tlc1Δ strain (Figure 6B). Notably, the proportions of circular and uncharacterized survivors in the SY12XYΔ tlc1Δ rad51Δ strain were 36% (9/25) and 32% (8/25) (Figure 6—figure supplement 7B and Supplementary file 5), respectively, lower than 48% and 44% in the SY12XYΔ tlc1Δ strain (Figure 6E and Supplementary file 5). Accordingly, the ratio of Type II survivor in SY12XYΔ tlc1Δ rad51Δ was (32% of the survivors tested, Figure 6—figure supplement 7B and Supplementary file 5) was higher than SY12XYΔ tlc1Δ strain (8% of the survivors tested, Figure 6E and Supplementary file 5), suggesting that Type I-specific mechanisms still contribute to the survivor formation even in the Y’-less strain SY12XYΔ. Collectively, the aforementioned data suggest that X-elements, as well as Y’-elements, are not essential for the generation of Type II survivors.
Discussion
The wild-type yeast strain BY4742, commonly used in laboratories, possesses 19 Y’-elements at 17 telomere loci and 32 X-elements at 32 telomere loci. This abundance of Y’-elements and X-elements poses challenges for loss-of-function studies, highlighting the need for a strain lacking all Y’-elements and X-elements. Fortunately, we have previously constructed the single-chromosome yeast strain SY14, which contains only one copy of Y’-element and two copies of X-element (Shao et al., 2018), and could have been an ideal tool. However, the telomerase-null survivors of SY14 mainly bypassed senescence through chromosomal circularization, providing limited insights into the roles of Y’- and X-elements in telomere maintenance (Wu et al., 2020). Therefore, in this study, we employed the SY12 strain, which has three chromosomes, to investigate the functions of Y’- and X-elements at telomeres (Figure 2A, left panel).
We constructed the SY12YΔ, SY12XYΔ+Y, and SY12XYΔ strains, which lack the Y’-element, X-elements, and both X- and Y’-elements, respectively (Figure 3A). Surprisingly, the SY12YΔ, SY12XYΔ, and SY12XYΔ+Y strains exhibited minimal defects in cell proliferation, genotoxic sensitivity, and telomere homeostasis (Figures 3 and 4). These results demonstrate, for the first time, that both X- and Y’-elements are dispensable for cellular functions. Thus, the SY12YΔ, SY12XYΔ, and SY12XYΔ+Y strains established in this study, with their simplified telomere structures, are valuable resources for telomere biology research.
Subtelomeric regions are known to be highly variable and often contain species-specific homologous DNA sequences. In the case of fission yeast, subtelomeric regions consist of subtelomeric homologous (SH) and telomere-distal sequences. Previous studies have shown that SH sequences in fission yeast do not significantly impact telomere length, mitotic cell growth, or stress responses. However, they do play a role in buffering against the spreading of silencing signals from the telomere (Tashiro et al., 2017). Though the ‘core X’ sequence acts as a protosilencer (Lebrun et al., 2001), the X-STRs and Y’-STAR possess anti-silencing properties that limit the spreading of heterochromatin in budding yeast (Fourel et al., 1999), the telomere position effect remains effective in the strains that lack both X- and Y’-elements (Figure 4B). Given the remarkable differences in both sequence and size between the subtelomeric regions of budding yeast and fission yeast, it is difficult to compare the extent to which subtelomeric elements affect telomere silencing.
Amplification of Y’-element(s) is a characteristic feature of canonical Type I survivors. Type I survivors emerged in SY12 strain, indicating that multiple Y’-elements in tandem are not strictly required for Type I recombination (Figure 2D). Interestingly, the telomerase-null SY12YΔ and SY12XYΔ cells, lacking Y’-elements, failed to generate Type I survivors but could generate Type II survivors, indicating that the acquisition of Y’-elements is not a prerequisite for Type II survivor formation (Figures 5C and 6B). These observations support the notion that Type I and Type II survivors form independently, although both may utilize a common alternative telomere-lengthening pathway (Kockler et al., 2021). Moreover, a subset of SY12 tlc1Δ, SY12YΔ tlc1Δ, SY12XYΔ+Y tlc1Δ, and SY12XYΔ tlc1Δ cells could escape senescence and become survivors through microhomology-mediated intra-chromosomal end-to-end fusion (chromosome circularization) (Figures 2D, 5C, and 6A and B, labeled in blue). Notably, the survivors with all circular chromosomes were readily recovered from the telomerase-null SY11 to SY14, but not SY1 to SY10 cells (Figure 1). Several reasons could account for this. First, a smaller number of telomeres provides fewer recombination donors and acceptors, resulting in less efficient inter-chromosomal homologous recombination (e.g., TG1-3 tracts recombination or Y’-element acquisition). Second, the continuously shortened telomeres of linear chromosomes may trigger another round of senescence, while survivors with circular chromosomes do not encounter end-replication problems and therefore exhibit greater stability. Third, the presence of homologous sequences at both chromosome ends appears to be a minimum requirement for microhomology-mediated intra-chromosomal end-to-end fusion. With fewer homologous sequences, the probability of chromosome circularization decreases, and with more chromosomes, the likelihood of circularizing each chromosome within a cell diminishes. Fourth, in cells with fewer telomeres, intra-chromosomal telomere fusions are more likely to occur, while lethal inter-chromosomal fusions are competed out. However, we can speculate that in telomerase-null cells with eroded chromosome ends, stochastic repair mechanisms such as homologous recombination, microhomology-mediated end joining, and inter- and intra-chromosomal fusions operate simultaneously. Only those survivors that maintain a relatively stable genome and robust growth can be experimentally recovered.
S. cerevisiae (budding yeast) and Schizosaccharomyces pombe (fission yeast) are the most commonly used laboratory systems, separated by approximately 1 Gya (billion years ago) according to molecular-clock analyses (Hedges, 2002). Despite both species having genomes are both over 12 megabases in length, haploid S. cerevisiae contains 16 chromosomes, while S. pombe has only 3 chromosomes (Forsburg, 2005). The telomerase-independent mechanisms for maintaining chromosome ends differ between these two yeasts. In budding yeast, homologous recombination is the primary mode of survival in telomerase-deficient cells, resulting in the generation of Type I or Type II survivors (McEachern and Haber, 2006). Telomerase- and recombination-deficient cells occasionally escape senescence through the formation of palindromes at chromosome ends in the absence of EXO1 (Maringele and Lydall, 2004). Fission yeast cells lacking telomerase can also maintain their chromosome termini by recombining persistent telomere sequences, and survivors with all intra-circular chromosomes (Nakamura et al., 1998) or intermolecular fusions (Tashiro et al., 2017; Wang and Baumann, 2008) have been observed. In our research, some SY12 tlc1Δ cells, which have three chromosomes, also bypassed senescence by circularizing their chromosomes (Figure 2D), suggesting that a lower chromosome number increases the likelihood of recovering survivors containing circular chromosomes.
While most eukaryotes employ telomerase for telomere replication, some eukaryotes lack telomerase and utilize recombination as an alternative means to maintain telomeres (Biessmann and Mason, 1997). In Drosophila, telomeres are replicated through a retrotransposon mechanism (Levis et al., 1993; Louis, 2002). The structure and distribution of Y’-elements in S. cerevisiae suggest their origin from a mobile element (Jäger and Philippsen, 1989; Louis and Haber, 1992), and Y’-elements can be mobilized through a transposition-like RNA-mediated process (Maxwell et al., 2004). In telomerase-deficient yeast cells, homologous recombination can acts as a backup mechanism for telomere replication (Lundblad and Blackburn, 1993), and the reintroduction of telomerase efficiently inhibits telomere recombination and dominates telomere replication (Chen et al., 2009; Peng et al., 2015; Teng and Zakian, 1999), These findings suggest that subtelomeric region amplification mediated by recombination and/or transposition may represent ancient telomere maintenance mechanisms predating the evolution of telomerase (de Lange, 2004). Therefore, subtelomeric X- and Y’-elements might be considered as evolutionary ‘fossils’ in the S. cerevisiae genome, and their elimination has little impact on telomere essential functions and genome stability.
Materials and methods
Yeast strains and plasmids
Request a detailed protocolYeast strains used in this study are listed in Supplementary file 6. The plasmids for gene deletion and endogenous expression of TLC1 were constructed based on the pRS series as described previously (Sikorski and Hieter, 1989). We use PCR to amplify the upstream and downstream sequence adjacent to the target gene, and then the PCR fragments were digested with different restriction enzymes and inserted into pRS plasmids. Plasmids were introduced into budding yeast by standard procedures, and transformants were selected on auxotrophic medium (Orr-Weaver et al., 1981).
Multiple-colony streaking assay
Request a detailed protocolSingle clones of indicated yeast strains were randomly picked and streaked on extract-peptone-dextrose (YPD) plates. Thereafter, several clones of their descendants were passaged by successive re-streaks at 30°C. This procedure was repeated dozens of times every 2 d.
Telomere Southern blotting
Request a detailed protocolSouthern blotting was performed as previously described (Hu et al., 2013). Yeast genomic DNA was extracted by a phenol chloroform method. Restriction fragments were separated by electrophoresis in 1% agarose gel, transferred to Amersham Hybond-N+ membrane (GE Healthcare), and hybridized with α-32P dCTP-labeled probe.
Cell viability assay
Request a detailed protocolCell viability assay was performed as previously described with a few modifications (Le et al., 1999). Three independent single colonies of indicated strains were grown to saturation at 30°C. Then the cell density was measured every 24 hr by spectrometry (OD600), and the cultures were diluted to the density at OD600 = 0.01. This procedure was repeated several times to allow the appearance of survivors. The genomic DNA samples at indicated time points were harvested for telomere length analysis.
Molecular analysis of circular chromosomes
Request a detailed protocolFusion events were determined by PCR amplification and DNA sequencing. Genomic DNA was extracted by phenol chloroform. First, we use primers pairs located at different sites of each chromosome arm at an interval of 1 kb (listed in Supplementary file 1) to determine the erosion site of each chromosome; PCR was performed as standard procedures in 10 μl reactions by TaKaRa Ex Taq. To amplify the sequence of fusion junction, we use pairs of primers oriented to different arm of each chromosome; PCR was performed as standard procedures in 50 μl reactions by TaKaRa LA Taq. The fragments were purified by kit (QIAGEN), then they were sequenced directly or cloned into the pMD18-T Vector (TaKaRa) for sequencing.
CRISPR-Cas9-mediated X- and Y’-elements deletion
Request a detailed protocolX- and Y’-elements were deleted as described (Shao et al., 2018; Shao et al., 2019). Briefly, pgRNA and a DNA targeting cassette, containing a selection marker, a homology arm (DR1), a direct repeat (DR2), and telomeric repeats, were co-introduced into indicated cells harboring pCas9. pCas9 nuclease was directed to a specific DNA sequence centromere-proximal to the subtelomeric region with the guidance of gRNA1, where it induces a double-stranded break. Homologous recombination between the broken chromosome and the provided DNA targeting cassette caused the deletion of X- and Y’-elements. The positive transformants identified by PCR were transferred into the galactose-containing liquid medium, which induces the expression of the gRNA2 on pCas9 to cut at the target site near the URA3 gene and on the backbone of pgRNA. Then the culture was plated on the medium containing 5′-FOA to select for eviction of the URA3 marker.
Cell growth assay
Request a detailed protocolThree individual colonies of the indicated strains were inoculated into 5 ml liquid medium and incubated at 30°C. The cell cultures were then diluted in 30 ml of fresh YPD medium to the density at OD600 = 0.1. Then the density of cells was measured by spectrometry (OD600) hourly.
FACS assay
Request a detailed protocolThe FACS analysis was performed as previously described (He et al., 2019). Yeast cells were cultured at 30°C until the log phase, and then 1 ml of the cells was harvested. The cells were washed with cold sterile ddH2O and fixed with 70% ethanol overnight at 4°C. The following day, the cells were washed with 50 mM sodium citrate buffer (pH 7.2) and then digested with 0.25 mg/ml RNase A at 37°C for 2–3 hr, followed by 0.2 mg/ml Protease K at 50°C for 1 hr. Both RNase A and Protease K were diluted in sodium citrate buffer. The cells were resuspended in 500 μl sodium citrate buffer and then sonicated for 45 s at 100% power. The DNA of the cells was stained with 20 μg/ml propidium iodide (PI) at 4°C overnight or at room temperature for 1 hr. FACS analysis was performed on a BD LSRII instrument.
Serial dilution assay
Request a detailed protocolA single colony per strain was inoculated into 3 ml liquid medium and incubated at 30°C. The cell cultures were then adjusted to a concentration of OD600 ~ 0.5. Fivefold serially diluted cells were spotted on the indicated plates. The plates were incubated at 30°C for the appropriate time prior to photography.
RNA extraction and RT-qPCR
Request a detailed protocolThree independent single colonies of indicated strains were grown to log phase at 30°C. Yeast pellets from a 1 ml cell culture were digested with Zymolyase 20T (MP Biomedicals, LLC) to obtain spheroplasts. RNA was extracted with RNeasy mini kit (QIAGEN) followed by reverse transcription using the Fastquant RT kit (Tiangen). Real-time PCR was carried out using SYBR Premix Ex Taq II (Takara) on the Applied Biosystems StepOne Real-Time PCR System. Primer pairs used in RT-qPCR are listed in Supplementary file 1. The gene expression levels were normalized to that of ACT1 and the wild-type value is arbitrarily set to 1.
PFGE analysis
Request a detailed protocolDNA plugs for PFGE were prepared according to the manufacturer’s instructions (Bio-Rad) and Ishii et al., 2008. Fresh yeast cells were inoculated in 50 ml YPD and incubated at 30°C until the OD600 reached approximately 1.0. The cells were subsequently harvested, washed twice with cold EDTA buffer (50 mM, pH 8.0), and resuspended in 300 μl of CSB buffer (10 mM pH 7.2 Tris–Cl, 20 mM NaCl, 100 mM pH 8.0 EDTA, 4 mg/ml lyticase) and blended with 300 μl of 2% low-melt agarose (Bio-Rad). Then, 100 μl of resuspended cells were added to each plug and incubated at 4°C for 30 min until the agarose plugs were solidified. The solidified agarose plugs were incubated in lyticase buffer (10 mM pH 7.2 Tris–Cl, 100 mM pH 8.0 EDTA, 1 mg/ml lyticase) at 37°C for 3 hr, followed by incubation in Proteinase K Reaction Buffer (100 mM pH 8.0 EDTA, 0.2% sodium deoxycholate, 1% sodium lauryl sarcosine) containing 1 mg/ml Proteinase K at 50°C for 12 hr. The plugs were washed four times in 25 ml of wash buffer (20 mM Tris, pH 8.0, 50 mM EDTA) for 1 hr each time at room temperature with gentle agitation. The plugs were then fixed into a pulsed field agarose gel (Bio-Rad), and the CHEF-DR II Pulsed Field Electrophoresis System (Bio-Rad) was used for gel electrophoresis. The electrophoresis conditions for separation were as follows: 0.8% agarose gel, 1× TBE buffer, 14°C temperature, first run: initial switch time 1200 s; final switch time 1200 s; run time 24 hr; voltage gradient 2 V/cm; angle 96°; second run: initial switch time 1500 s; final switch time 1500 s; run time 24 hr; voltage gradient 2 V/cm; angle 100°; third run: initial switch time 1800 s; final switch time 1800 s; run time 24 hr; voltage gradient 2 V/cm; angle 106°. The gel was stained with GelstainRed nucleic acid dye (US Everbright), and PFGE Gels were imaged by Tanon 2500.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files; source data files have been provided for all figures.
References
-
Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination eventsMolecular and Cellular Biology 21:1819–1827.https://doi.org/10.1128/MCB.21.5.1819-1827.2001
-
T-loops and the origin of telomeresNature Reviews. Molecular Cell Biology 5:323–329.https://doi.org/10.1038/nrm1359
-
The yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe: models for cell biology researchGravitational and Space Biology Bulletin 18:3–9.
-
KEOPS complex promotes homologous recombination via DNA resectionNucleic Acids Research 47:5684–5697.https://doi.org/10.1093/nar/gkz228
-
The origin and evolution of model organismsNature Reviews. Genetics 3:838–849.https://doi.org/10.1038/nrg929
-
Many yeast chromosomes lack the telomere-specific Y’ sequenceMolecular and Cellular Biology 9:5754–5757.https://doi.org/10.1128/mcb.9.12.5754-5757.1989
-
Telomerase- and capping-independent yeast survivors with alternate telomere statesNature Cell Biology 8:741–747.https://doi.org/10.1038/ncb1429
-
Are Drosophila telomeres an exception or the rule?Genome Biology 3:REVIEWS0007.https://doi.org/10.1186/gb-2002-3-10-reviews0007
-
Telomerase- and recombination-independent immortalization of budding yeastGenes & Development 18:2663–2675.https://doi.org/10.1101/gad.316504
-
Ty1 mobilizes subtelomeric Y’ elements in telomerase-negative Saccharomyces cerevisiae survivorsMolecular and Cellular Biology 24:9887–9898.https://doi.org/10.1128/MCB.24.22.9887-9898.2004
-
Break-induced replication and recombinational telomere elongation in yeastAnnual Review of Biochemistry 75:111–135.https://doi.org/10.1146/annurev.biochem.74.082803.133234
-
Mre11-Rad50-Xrs2 and Sae2 promote 5’ strand resection of DNA double-strand breaksNature Structural & Molecular Biology 17:1478–1485.https://doi.org/10.1038/nsmb.1957
-
How shelterin protects mammalian telomeresAnnual Review of Genetics 42:301–334.https://doi.org/10.1146/annurev.genet.41.110306.130350
-
Creating functional chromosome fusions in yeast with CRISPR-Cas9Nature Protocols 14:2521–2545.https://doi.org/10.1038/s41596-019-0192-0
-
Subtelomeres constitute a safeguard for gene expression and chromosome homeostasisNucleic Acids Research 45:10333–10349.https://doi.org/10.1093/nar/gkx780
-
Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiaeMolecular and Cellular Biology 19:8083–8093.https://doi.org/10.1128/MCB.19.12.8083
-
Rad6-Bre1-mediated H2B ubiquitination regulates telomere replication by promoting telomere-end resectionNucleic Acids Research 45:3308–3322.https://doi.org/10.1093/nar/gkx101
-
Y’-Help1, a DNA helicase encoded by the yeast subtelomeric Y’ element, is induced in survivors defective for telomeraseThe Journal of Biological Chemistry 273:33360–33366.https://doi.org/10.1074/jbc.273.50.33360
-
Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiaeMolecular and Cellular Biology 8:2257–2260.https://doi.org/10.1128/mcb.8.5.2257-2260.1988
Article and author information
Author details
Funding
National Key Research and Development Program of China (2023YFA0913400)
- Jin-Qiu Zhou
The National Natural Science Foundation of China (32150004)
- Jin-Qiu Zhou
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank the members of Zhou lab for discussions and suggestions for this project.
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Reviewed Preprint version 3:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.91223. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Hu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,491
- views
-
- 137
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 2
- citations for umbrella DOI https://doi.org/10.7554/eLife.91223






