Antimicrobial activity of iron-depriving pyoverdines against human opportunistic pathogens
Figures
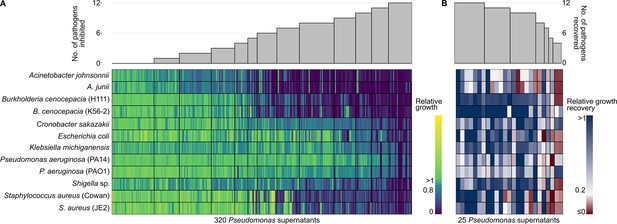
Effect of supernatants from environmental Pseudomonas isolates on the growth of 12 human opportunistic pathogens.
(A) Screen to assess the extent to which pyoverdine-containing supernatants from 320 natural Pseudomonas isolates inhibit the growth of 12 human opportunistic pathogens. The heatmap depicts relative pathogen growth in the supernatant treatment (70% casamino acid medium [CAA] + 30% spent supernatant) relative to the control (70% CAA + 30% sodium chloride solution), with values ranging from stimulation (yellow) to inhibition (blue) based on four independent replicates. Gray bars above the heatmap show the number of pathogens that were at least 20% inhibited in their growth by a given supernatant. The screen returned 25 supernatant candidates that inhibited the growth of all pathogens. (B) Control screen with the 25 top supernatant candidates to check whether pyoverdine causes the observed growth inhibition. The heatmap depicts the level of growth recovery in the 12 pathogens when iron was added to the supernatant, with values ranging from no recovery (red) to full recovery (blue). Growth recovery in iron-rich medium indicates that pyoverdines are involved in growth inhibition in iron-limited medium. Gray bars above the heatmap show the number of pathogens that experienced a relative growth recovery of at least 0.2. The screen returned seven supernatant top candidates for which growth recovery occurred for all pathogens under iron-rich conditions.
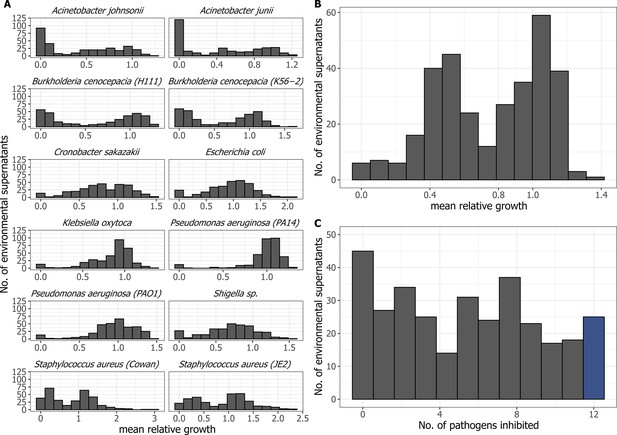
Growth of pathogens in supernatant treatments and the number of pathogens inhibited by a supernatant.
(A) Histograms showing the distribution of relative growth (mean across n = 4 replicates) for each of 12 human opportunistic pathogens when exposed to the supernatants of the 320 natural Pseudomonas spp. (B) Histogram showing the distribution of the mean relative growth across all 12 pathogens when exposed to the 320 supernatants. (C) Histogram showing the number of pathogens that were reduced in their growth by a specific supernatant (at least 20% growth reduction).
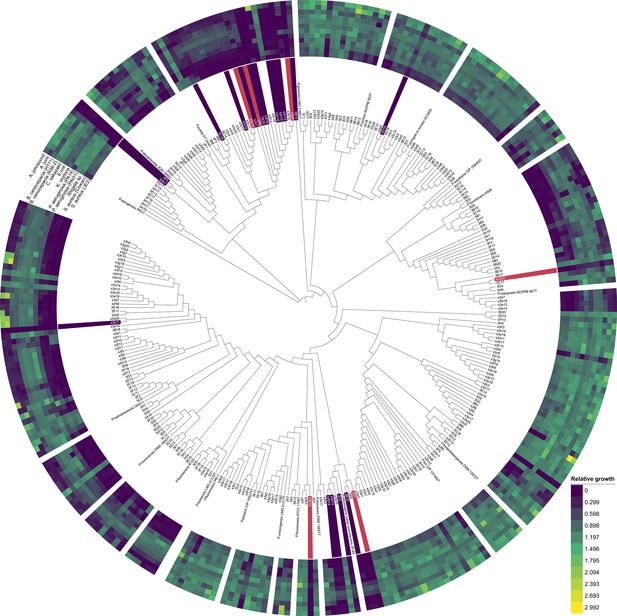
Cladogram of environmental Pseudomonas isolates from soil and pond habitats based on partial rpoD sequences.
The tree includes 297 out of the 320 Pseudomonas isolates for which rpoD sequences with lengths ≥600 bp were available. Twenty-three isolates with shorter sequence lengths had to be excluded. rpoD sequences stem from Butaitė et al., 2018 https://doi.org/10.1111/1462-2920.14355 and are available through the European Nucleotide Archive (ENA, accession number: PRJEB21289). P. aeruginosa PAO1 was used as the outgroup. rpoD sequences from 20 well-characterized fluorescent pseudomonads were taken from the literature and integrated into the cladogram to cover taxonomic affiliations of our strains. Concentrical rings depict the supernatant-mediated growth effects of the environmental isolates on the 12 pathogens as a heatmap (matching the results from Figure 1), ranging from complete growth inhibition (dark purple) to growth promotion (yellow). Isolates whose supernatants strongly inhibited all 12 pathogens are marked in dark purple, while the top candidates, which inhibited those pathogens exclusively under iron-limited conditions, are marked in red (rpoD sequences available for 23 out of these 25 strains).
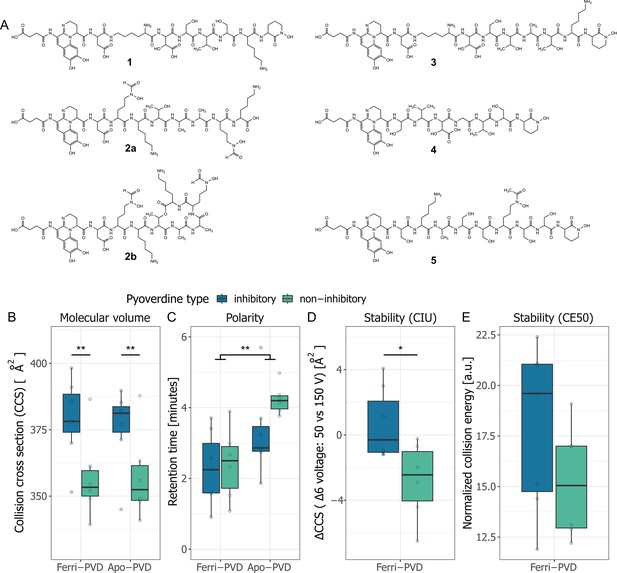
Chemical structure of growth-inhibitory pyoverdines and their properties compared to non-inhibitory pyoverdines.
(A) The chemical structures of the seven top candidate pyoverdines were elucidated using ultra-high-performance liquid chromatography high-resolution tandem mass spectrometry (UHPLC-HR-MS/MS) and revealed five unique pyoverdine structures (1–5) differing in their peptide backbone (see Rehm et al., 2022 for an in-depth chemical analysis). Pyoverdine 1 is a novel structure (from isolate 3A06). Pyoverdine 2 can occur in either a linear 2a or a cyclic 2b form (from isolate 3G07). Pyoverdine 3 was found in three different isolates, originating from the same soil sample (from isolates s3b09, s3b10, and s3b12). Pyoverdine 4 and 5 are from isolate s3c13 and s3e20, respectively. (B) The collision-cross sections (CCS) values of inhibitory pyoverdines are higher than for non-inhibitory pyoverdines. (C) Molecule polarity, measured as the chromatographic retention time, is higher for ferri-pyoverdines (iron loaded) than for apo-pyoverdines (iron free). (D) Iron complex stability, assessed by the collision-induced unfolding (CIU) of ferri-pyoverdines, was significantly higher for inhibitory than non-inhibitory pyoverdines. (E) The normalized collision energy (CE50) necessary to fragment 50% of ferri-pyoverdines was not different between inhibitory and non-inhibitory pyoverdines. Box plots show the median and the first and third quartiles across the seven inhibitory and six non-inhibitory pyoverdines. Whiskers represent the 1.5× interquartile range. Significance levels are based on ANOVAS (∗p < 0.05 and ∗∗p < 0.01).
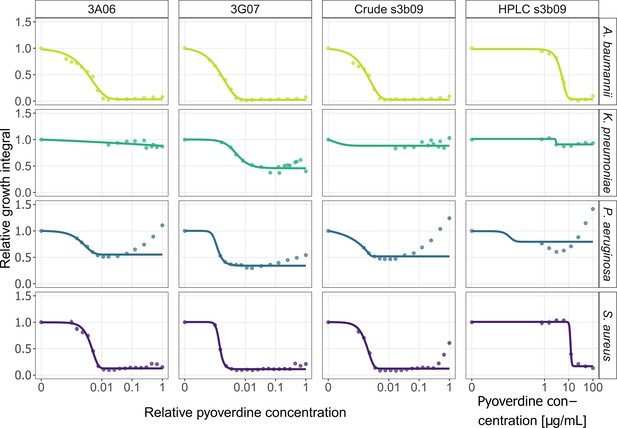
Pyoverdine dose–response curves for A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus.
We exposed the four human opportunistic pathogens to three pyoverdines (3A06, 3G07, s3b09) that were among the most potent ones. We used crude-purified extracts of all three pyoverdines and a high-performance liquid chromatography (HPLC)-purified variant for pyoverdine s3b09. The absolute concentrations of the crude-purified extracts are unknown and therefore expressed relative to the weighed amount of 6 mg. The absolute concentration of the HPLC-purified variant is given in µg/mL. Growth values are scaled relative to the untreated control in casamino acid medium (CAA) medium. Dots and error bars show mean values and standard errors, respectively, across a minimum of three replicates per concentration. Dose–response curves were fitted using four- or five-parameter logistic regressions.
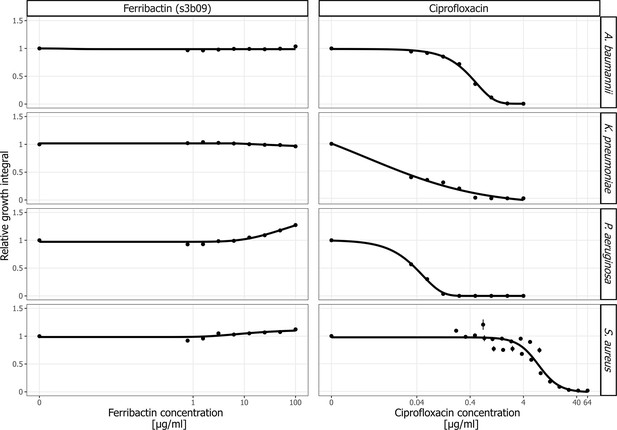
Ferribactin and ciprofloxacin dose-response curves for A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus.
We exposed the four human opportunistic pathogens to a concentration gradient of high-performance liquid chromatography (HPLC)-purified ferribactin s3b09 and ciprofloxacin. Growth values are scaled relative to the untreated control in untreated casamino acid medium (CAA) medium. Dots and error bars show mean values and standard errors, respectively, across three replicates. Dose–response curves were fitted using four- or five-parameter logistic regressions.
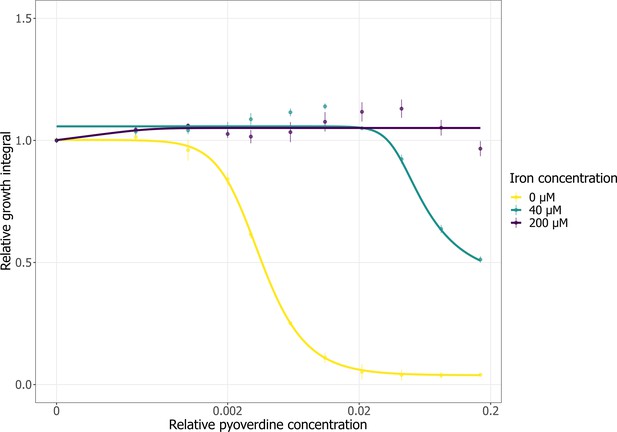
The effect of iron-saturated pyoverdines on the growth of A. baumannii.
We exposed A. baumannii to increasing crude pyoverdine s3b09 concentrations (relative concentrations 0.16–0.0004) in casamino acid medium (CAA) medium (yellow line, n = 5), or in CAA medium supplemented with either 40 µM (green line, n = 3) or 200 µM FeCl3 (purple line, n = 3). Growth values are scaled relative to the pyoverdine untreated control, independently for each of the three media (CAA with either 0, 40, or 200 µM FeCl3). Dots and error bars show mean values and standard errors, respectively. Dose–response curves were fitted using four- or five-parameter logistic regressions.
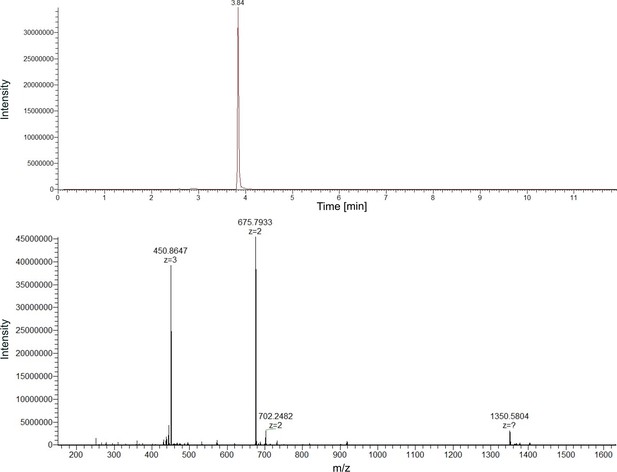
High-resolution mass spectrum (HRMS) of purified pyoverdine s3b09.
Mass chromatogram (upper panel) and spectrum (lower panel) of the high-performance liquid chromatography (HPLC)-purified pyoverdine s3b09. The compound elutes at a retention time of 3.84 min. It is the compound with the highest abundance in the measured sample. Based on these data, we define the measured sample as highly purified pyoverdine.
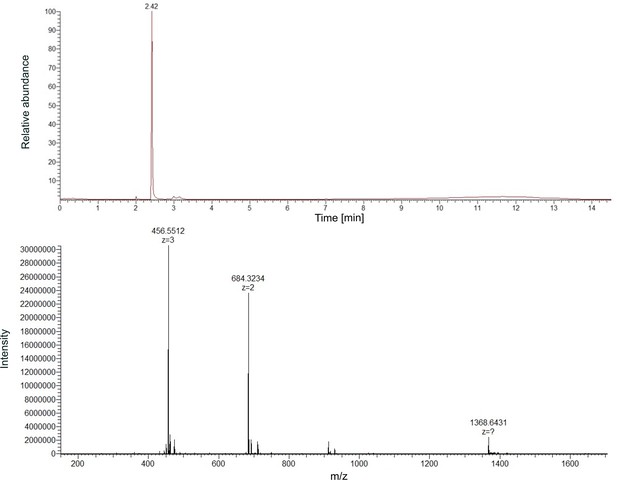
High-resolution mass spectrum (HRMS) of purified ferribactin (precursor molecule of pyoverdine s3b09).
Mass chromatogram (upper panel) and spectrum (lower panel) of ferribactin. The compound elutes at a retention time of 2.42 min and has the highest intensity in the measured sample.
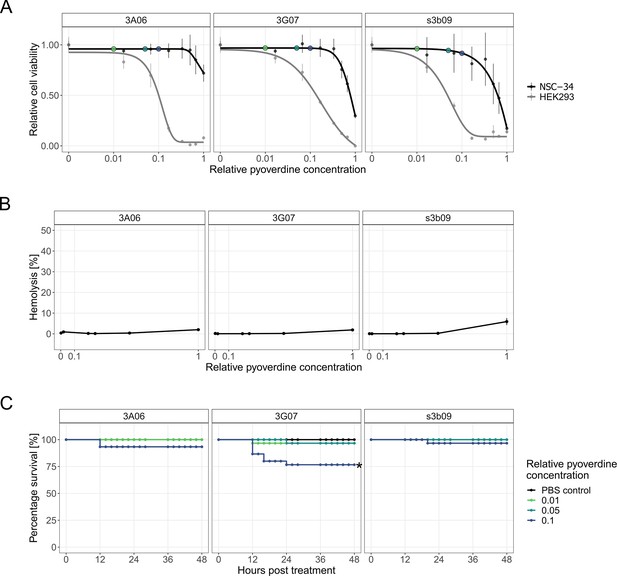
Toxicity assays for pyoverdines from environmental Pseudomonas spp. against human cell lines, sheep erythrocytes, and the host larvae of G. mellonella.
(A) We exposed mouse neuroblastoma-spinal cord (NSC-34) and human embryonic kidney 293 (HEK-293) cells to three crude-purified pyoverdines (3A06, 3G07, s3b09) that were among the most potent ones to inhibit bacterial growth. An MTT assay was used to assess the metabolic activity of cells as an indicator of cell viability and proliferation. Cell viability data are scaled relative to the pyoverdine-free treatment, whereby dots and error bars show means and standard error across three replicates, respectively. The absolute concentrations of the crude-purified pyoverdines are unknown and concentrations are therefore expressed relative to the highest one used. Colored dots indicate relative pyoverdine dosages used for the in vivo experiments. Dose–response curves were fitted using five-parameter logistic regressions. (B) We evaluated the hemolytic activity of the pyoverdines by adding them to sheep erythrocytes along a concentration gradient (range 0.002–1). Triton X-100 and PBS served as positive and negative control, respectively. Hemolytic activity is scaled relative to the positive control, dots and error bars show means and standard error across six replicates from two independent experiments, respectively. (C) To assess the toxic effects of pyoverdine on the host, we injected pyoverdines (three relative concentrations, 0.01, 0.05, 0.1) into larvae 4 hr after a mock infection with PBS. The percentage of larval survival was tracked over 48 hr post-treatment. Data stem from three independent experiments with each 10 larvae per infection and treatment. Significance based on the log-rank test (adjusted for multiple comparisons using the Holm method).
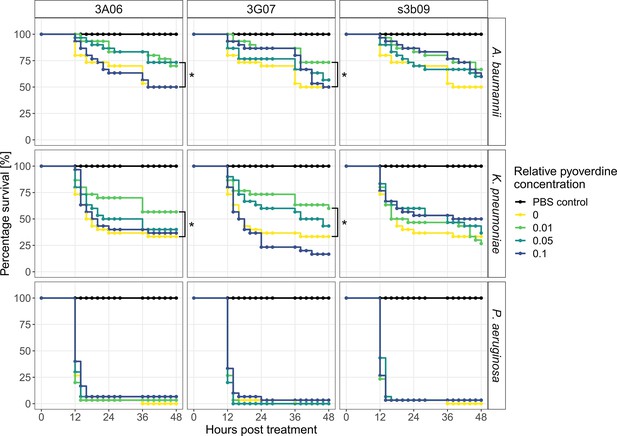
Pyoverdine treatments significantly increase survival of the host G. mellonella when infected with A. baumannii and K. pneumoniae.
Larvae of the greater wax moth were first infected with either A. baumannii, K. pneumoniae, or P. aeruginosa and then treated with one of three pyoverdines (3A06, 3G07, or s3b09) at four relative pyoverdine concentrations (0, 0.01, 0.05, 0.1). All panels show the larvae survival over 48 hr post-treatment. Data stem from three independent experiments with each 10 larvae per infection and treatment. Asterisks indicate significant differences in larval survival between treated and untreated infections based on Cox proportional hazard regressions (p<0.05).
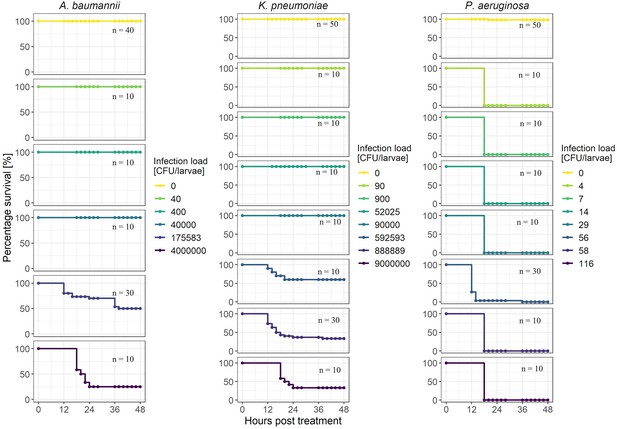
Survival curves of G. mellonella infected with A. baumannii, K. pneumoniae, or P. aeruginosa at different infection loads.
To determine the infection load at which approximately 50% of larvae die (LD50) after 48 hr, we infected larvae with different infection loads with one of three human opportunistic pathogens, A. baumannii, K. pneumoniae, or P. aeruginosa. All panels show the percentage of survival of wax moth larvae over 48 hr post infection. Based on the resulting killing curves, we selected 1.8 * 105 CFU/larvae for A. baumannii and 8.9 * 105 CFU/larvae for K. pneumoniae for the main infection and treatment experiments. These values correspond closely to LD50. In contrast, no LD50 value could be determined for P. aeruginosa as 100% of the larvae died, regardless of the infection dose. For the main experiments, we took 56 CFU/larvae.
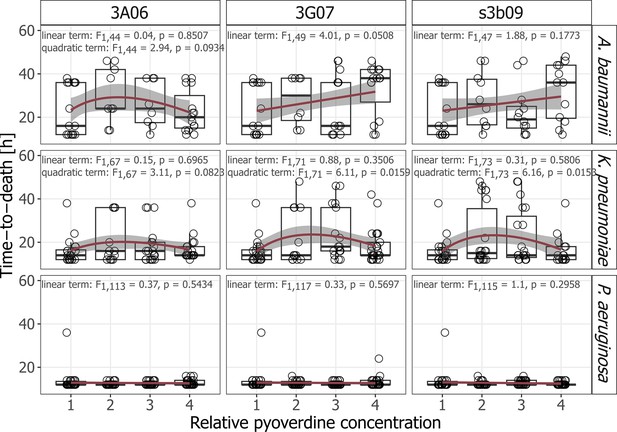
Variation in the time-to-death of G. mellonella larvae in response to pyoverdine treatment.
Larvae were infected with either A. baumannii, K. pneumoniae, or P. aeruginosa and treated with either pyoverdine 3A06, 3G07, or s3b09 at relative pyoverdine concentrations 0, 0.01, 0.05, or 0.1. Data stem from three independent experiments, each with 10 larvae per infection and treatment. For this analysis, only larvae that died during the experiment could be included. Box plots show the median and the first and third quartiles, and whiskers represent the 1.5× interquartile range. Red lines and shaded areas show the linear or quadratic regression lines (based on square-root transformed data) and 95% confidence intervals, respectively.
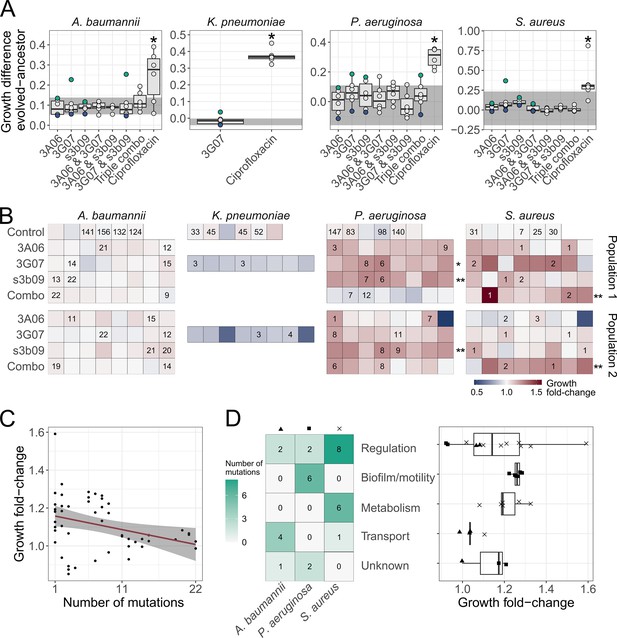
Phenotypic and genotypic analysis of experimentally evolved pathogens reveal weak levels of resistance evolution against pyoverdine treatment.
(A) We exposed evolved and ancestral pathogen populations to the treatment in which they evolved in and quantified their growth (area under the curve). Growth values were scaled relative to the ancestor in untreated medium, and the panels show the scaled growth differences between evolved and ancestral populations. The shaded areas show the scaled growth difference between the ancestor and the populations evolved in untreated medium and is representative of medium adaptation. The blue and green dots represent the pathogen populations with the lowest (population 1) and highest (population 2) scaled growth difference, respectively, which were subsequently used to pick clones. The dots show mean values across two independent replicates and asterisks show significant growth increases relative to the medium-adapted control. Box plots show the median and the first and third quartiles across the six independently evolved populations. Whiskers represent the 1.5× interquartile range. Significance levels are based on two-sided t-tests, Welch’s t-tests, or Wilcoxon tests (∗p < 0.05) (B) We repeated the above growth assays with 208 individual clones evolved under the pyoverdine treatments (picked from population 1 and population 2) and 24 populations evolved in growth medium alone (control). Each square represents a clone or a control population, and the number indicates the number of mutations identified based on whole-genome sequencing. The heatmap shows the fold-change in growth relative to the evolved control populations. Asterisks depict significant fold-increases in growth compared to control populations (based on one- and two-way ANOVAs, where ∗p < 0.05 and ∗∗p < 0.01). (C) The relationship between the number of mutations and fold-change in growth across all sequenced clones (n = 52) The red line and shaded area are the regression line and 95% confidence interval, respectively. (D) Heatmap showing the number of mutations per pathogen and per functional gene categories across clones together with the respective fold-change in growth (n = 32). For this analysis, we excluded intergenic mutations and deletions larger than 600 bp. Triangle, square, and cross depict growth fold-change of A. baumannii, P. aeruginosa, and S. aureus, respectively.
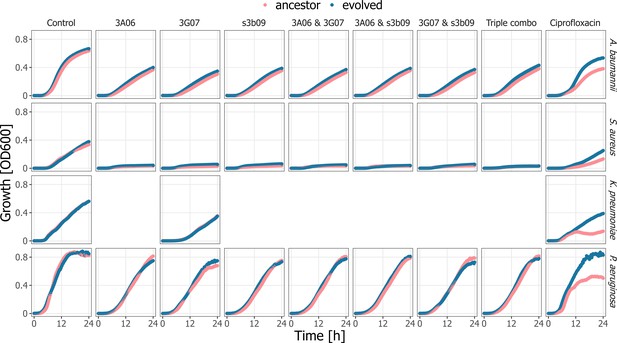
Population growth kinetics of the pathogens A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus before and after experimental evolution.
Growth kinetics (measured at OD600 nm) of ancestral (pink) and evolved (blue) populations of the four pathogens subjected to a no-treatment control, single pyoverdine 3A06, 3G07 or s3b09 treatments, their double and triple combination treatments, and a ciprofloxacin antibiotic treatment.
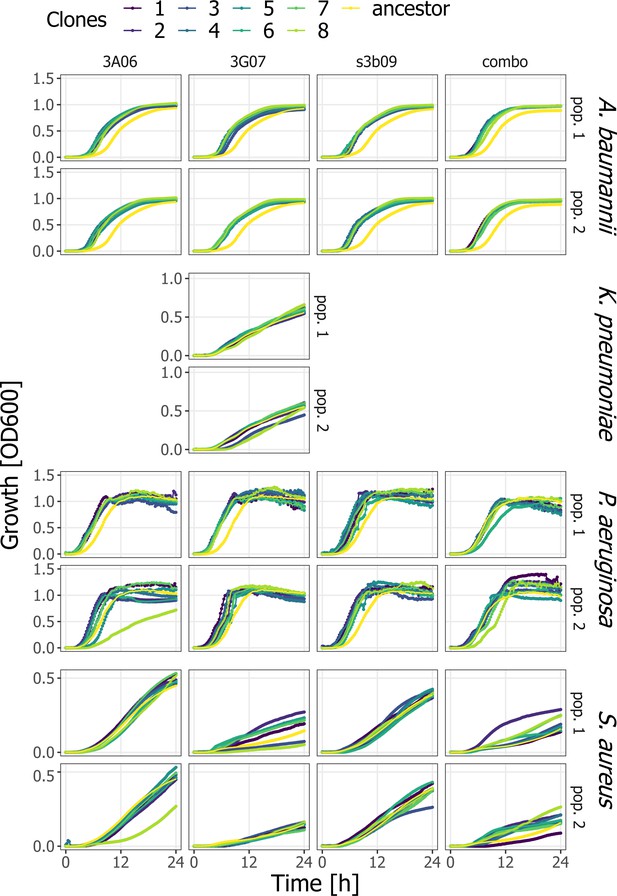
Growth kinetics of evolved clones of the pathogens A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus in comparison to the ancestor.
We exposed the ancestor (yellow) and eight evolved clones per treatment (pyoverdines: 3A06, 3G07, s3b09, combo) for two lineages (pop.1 and pop.2; 208 individual clones in total) to the treatment they evolved in and measured their growth (OD at 600 nm) over time.
Tables
Species and strains of human opportunistic pathogens used for the pyoverdine growth inhibition assay.
| Species and strain name | Class | Gram staining | Frequency of inhibiting supernatants |
|---|---|---|---|
| Acinetobacter johnsonii | Gammaproteobacteria | Negative | 75.0 |
| Acinetobacter junii | Gammaproteobacteria | Negative | 73.1 |
| Burkholderia cenocepacia H111 | Betaproteobacteria | Negative | 52.2 |
| Burkholderia cenocepacia K56-2 | Betaproteobacteria | Negative | 54.7 |
| Cronobacter sakazakii | Gammaproteobacteria | Negative | 48.8 |
| Escherichia coli K12 | Gammaproteobacteria | Negative | 31.6 |
| Klebsiella michiganensis | Gammaproteobacteria | Negative | 30.9 |
| Pseudomonas aeruginosa PA14 | Gammaproteobacteria | Negative | 9.7 |
| Pseudomonas aeruginosa PAO1 | Gammaproteobacteria | Negative | 20.9 |
| Shigella sp. | Gammaproteobacteria | Negative | 57.8 |
| Staphylococcus aureus Cowan | Bacilli | Positive | 47.8 |
| Staphylococcus aureus JE2 | Bacilli | Positive | 41.6 |
Additional files
-
Supplementary file 1
Supplementary tables a-e.
- https://cdn.elifesciences.org/articles/92493/elife-92493-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/92493/elife-92493-mdarchecklist1-v1.docx





