Identification of CFAP52 as a novel diagnostic target of male infertility with defects of sperm head-tail connection and flagella development
Figures
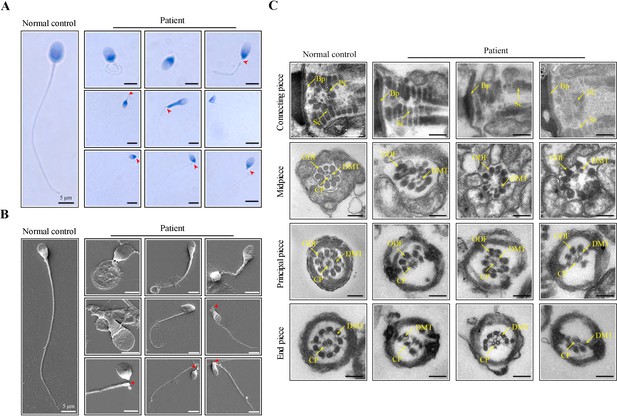
Morphological and ultrastructural defects in spermatozoa from the infertile patient.
(A, B) Papanicolaou staining and scanning electron microscopy (SEM) results showed aberrant spermatozoa morphologies in the infertile patient. Spermatozoa from the patient displayed defective sperm flagella, including absent, short, coiled, and bent flagella. Notably, sperm decapitation was also observed. The red arrowheads indicate the sperm-head connecting piece. Scale bars, 5 μm. (C) Transmission electron microscopy (TEM) analyses of the spermatozoa from a healthy control and the infertile patient. Deformed sperm heads, defects of the connecting piece (missing or defective PC and Sc), and abnormalities of the flagella (disorganized arrangements and/or absence of CPs, DMTs, and ODFs) were observed in the patient’s spermatozoa. PC, proximal centriole; Sc, segmented column; Bp, basal plate; CP, central-pair microtubule; ODF, outer dense fibre; DMT, doublet microtubules. Scale bars, 100 nm. All experiments were repeated three times with similar results.
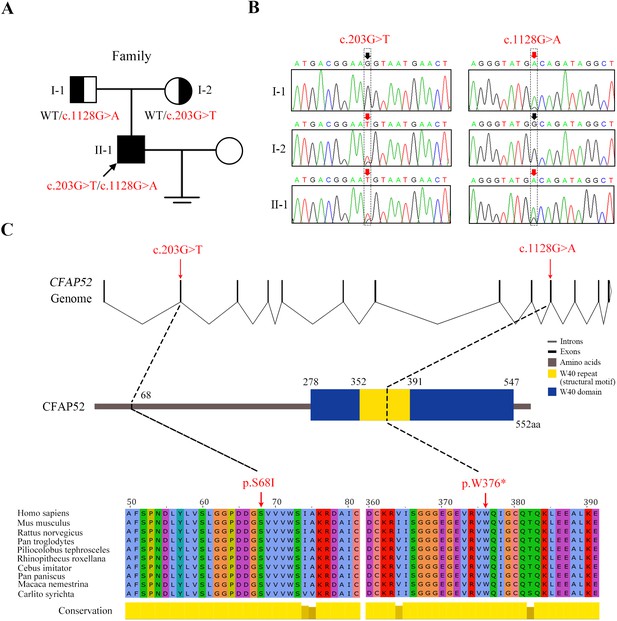
Novel mutations in CFAP52 identified in the infertile family.
(A) Pedigree of the infertile family; the black arrow indicates the proband (II-1). (B) Sanger sequencing confirmed that the proband carried biallelic mutations (c.203G>T/c.1128G>A) in CFAP52 and that the parents harboured the heterozygous mutation. The red arrows indicate the positions of variants. (C) The localization of CFAP52 variants in the genome, CFAP52 protein structure, and conservation of mutant amino acids in various species. The NCBI reference sequence number of CFAP52 is NM_001080556.2.
-
Figure 2—source data 1
All candidate variants identified in the patients by WES.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig2-data1-v1.zip
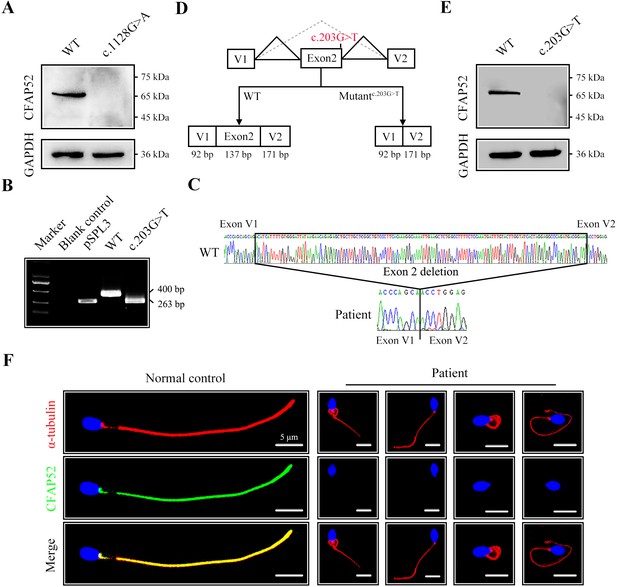
The impact of mutations on CFAP52 expression.
(A) Western blotting analysis showed that CFAP52 expression was not detected in HEK293T cells transfected with the mutant-CFAP52c.1128G>A plasmid. (B) Electrophoresis showed a decrease in the molecular weight of the PCR products generated from mutant CFAP52c.203G>T (263 bp) compared with WT CFAP52 (400 bp) in the minigene experiment. (C) Sanger sequencing of cDNA of the splicing mutation showed the deletion of exon 2 in the c.203G>T variant clone. (D) The pattern diagram depicts the adverse effects caused by the CFAP52 splicing mutation c.203G>T. (E) Western blotting analysis showed that HEK293T cells transfected with the mutant CFAP52c.203G>T plasmid did not express CFAP52. (F) Immunofluorescence staining showed that CFAP52 expression in the patient’s spermatozoa was absent compared with that of a healthy control (blue, DAPI; green, CFAP52; red, α-tubulin). Scale bars, 5 μm. All experiments were repeated three times with similar results.
-
Figure 3—source data 1
Primers for Sanger sequencing and Minigene.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig3-data1-v1.zip
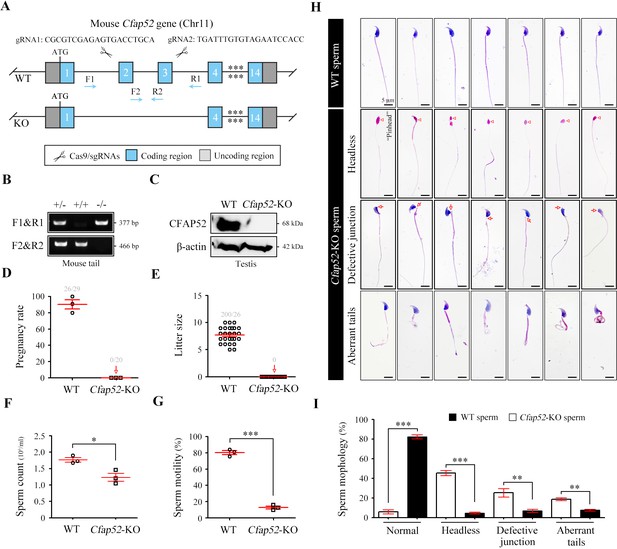
Cfap52 deficiency in mice leads to male sterility, with spermatozoa showing defective head-tail connections and flagella.
(A) Schematic illustration of the targeting strategy for generating Cfap52-KO mice by using CRISPR/Cas9 technology. A detailed procedure is described in the Materials and methods. (B) Representative results of PCR-based genotyping using mouse tail DNA. (C) Immunoblotting of CFAP52 was performed in the testis protein lysates of WT mice and Cfap52-KO mice. β-actin served as a loading control. Western blotting experiments were repeated three times with similar results. (D, E) Fertility assessment experiments were performed on three adult Cfap52-KO male mice and three WT male littermates for 2 months. Of the 20 female mice mated with Cfap52-KO male mice, no pregnancy was observed. (F, G) Sperm counts were counted with a fertility counting chamber under a light microscope, and total motility was assessed by a computer-assisted sperm analysis (CASA) system. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, *p<0.05; ***p<0.001. (H) Morphological analyses of spermatozoa in WT mice and Cfap52-KO mice by Papanicolaou staining. First row, normal morphology of spermatozoa from WT mice; second row, headless spermatozoa in Cfap52-KO mice; third row, spermatozoa with defective head-tail connection in Cfap52-KO mice; fourth row, short-tailed spermatozoa in Cfap52-KO mice. The arrowheads indicate the ‘pinhead’, and the arrows indicate the defective head-tail connection. Scale bars, 5 μm. (I) Percentage of spermatozoa with normal morphology and each type of defect in WT mice and Cfap52-KO mice. At least 100 spermatozoa were counted for each mouse. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, **p<0.01; ***p<0.001.
-
Figure 4—source data 1
Primers for Cfap52-KO mouse genotyping.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig4-data1-v1.zip
-
Figure 4—source data 2
Original blots of Figure 4C.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig4-data2-v1.zip
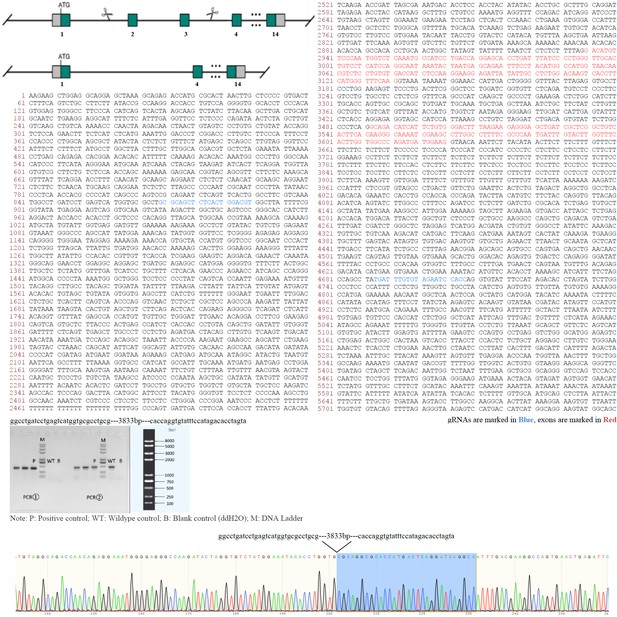
Animal report of generation of Cfap52-KO mice.
Genomic region of mouse Cfap52 locus. The mouse Cfap52-201 transcript (ENSMUST00000021287.11) is located on mouse chromosome 11. Fourteen exons have been identified and exons 2~3 were selected as the knockout region. gRNAs are marked in blue or underlined and exons are marked in red. Cas9 mRNA and gRNAs were co-injected into fertilized eggs for KO mouse production. The pups will be genotyped by PCR followed by sequence analysis. F0 found animals were bred to wildtype mice to test germline transmission and F1 animal generation. Positive F1 animals were confirmed by PCR and Sanger sequencing (~3833 bp deletion containing exons 2~3 of Cfap52). Sequence of gRNAs, gel image, and Sanger sequencing were shown.
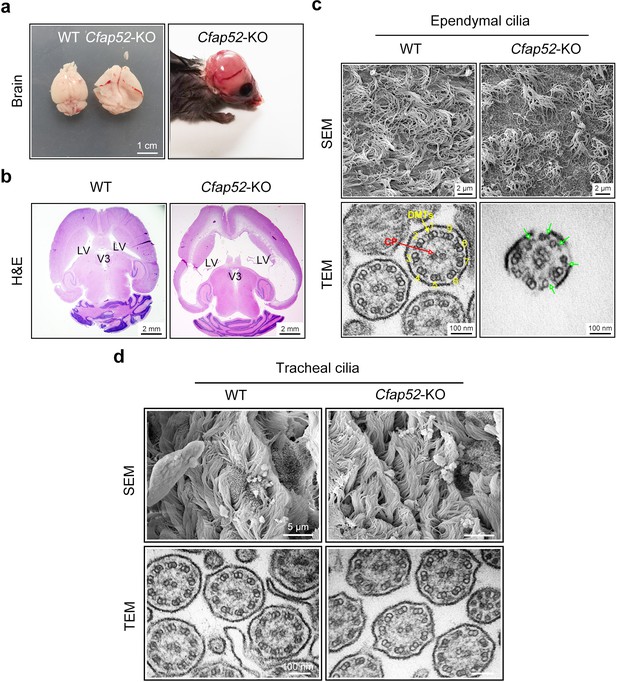
Cfap52-KO mice develop hydrocephalus.
(a) Enlarged brain hemispheres and hydrocephalus of Cfap52-KO mice. Scale bar, 1 cm. (b) Histological analysis of brain sections revealed enlarged lateral ventricles (LV) in 3-week-old Cfap52-KO mice. Scale bars, 2 mm. (c) Scanning electron microscope (SEM) images of the ependymal cilia from WT and Cfap52-KO brains, showing the sparse ependymal cilia in Cfap52-KO mice. Scale bars, 2 μm. Transmission electron microscope (TEM) images of ependymal cilia from WT and Cfap52-KO brains, showing the disrupted axonemal structures. Green arrows indicate a partial lack of DMT. CP, central pair; DMTs, doublet microtubules. Scale bars, 100 nm. (d) Scanning electron microscope (SEM) and transmission electron microscope (TEM) images of tracheal cilia from WT and Cfap52-KO mice, showing generally normal morphology and axonemal structure. Scale bars in SEM, 5 μm; Scale bars in TEM, 100 nm. All experiments were repeated three times with similar results.
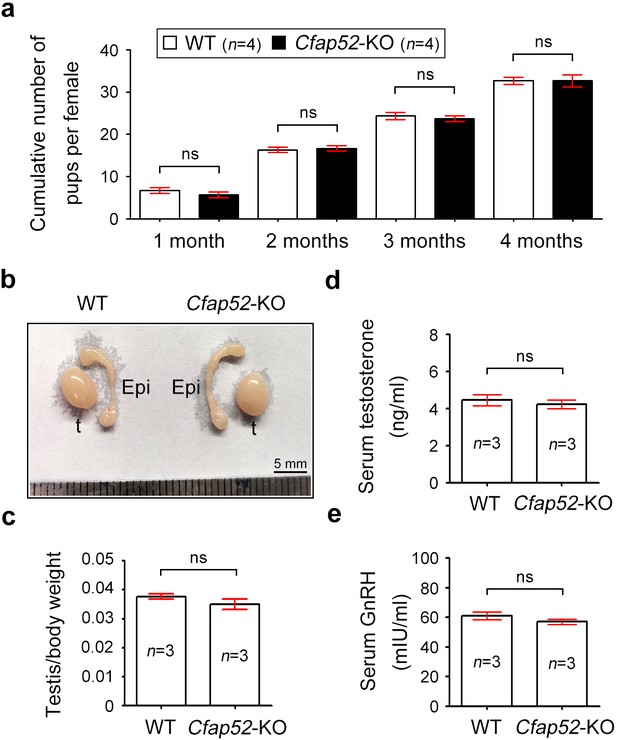
Detailed analysis of infertility in Cfap52-KO male and female mice.
(a) Continuous breeding assay was performed with Cfap52-KO female mice and WT female mice (n=4 each). Female mice were crossed with WT male mice and the average cumulative number of pups per female was shown. Data are presented as the mean ± SEM (three biological repeats), student’s t-test; ns, not significance. (b) Representative reproductive system (testis and epididymis) of WT and Cfap52-KO mice, exhibiting similar morphology and size. Epi, epididymis; t, testis. Scale bar, 5 mm. (c) The testis/body weight ratio of WT and Cfap52-KO mice (n=3 each). Data are presented as the mean ± SEM, student’s t-test; ns, not significance. (d) Elisa measurement of serum testosterone by an ELISA kit (Beyotime, Shanghai, China). (e) The content of GnRH in serum was detected by an ELISA kit (Elabscience, Wuhan, China). Data are presented as the mean ± SEM (three biological repeats), student’s t-test; ns, not significance.
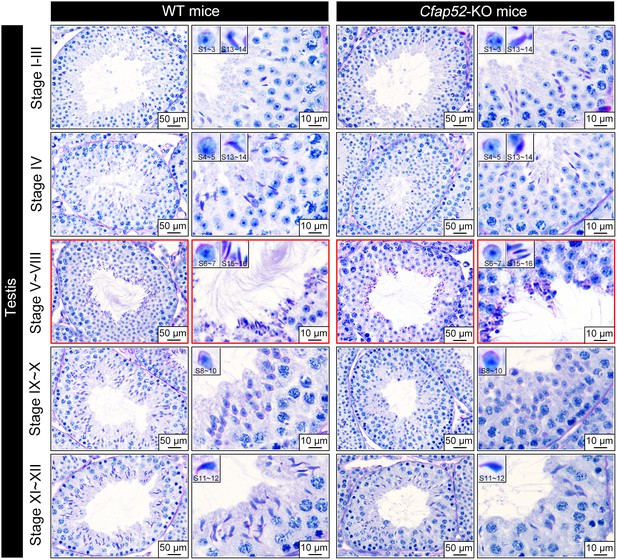
Periodic acid-Schiff staining of WT and Cfap52-KO testis sections.
Examination of seminiferous epithelium (stage I~XII) within testes revealed no difference in the population and differentiation of spermatogonia, spermatocyte and round spermatids between WT and Cfap52-KO mice. Acrosome biogenesis of spermatids was also normal in Cfap52-KO mice. An obvious defect was observed at stage V-VIII, showing abnormalities in spermatozoa flagella. Periodic acid-Schiff staining experiments were repeated three times with similar results. Scale bars, 50 μm or 10 μm.
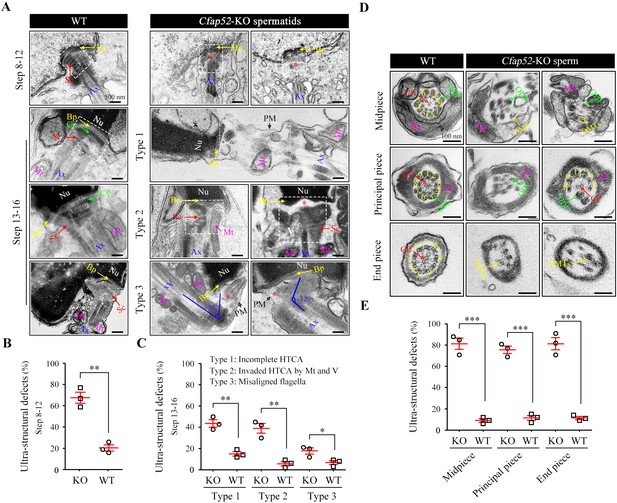
Impaired development of the connecting piece and the axoneme structure of spermatids/spermatozoa in Cfap52-KO mice.
(A) Longitudinal sections of elongating (steps 10–12) and elongated (steps 13–16) spermatids in WT mice and Cfap52-KO mice, showing the structure of the connecting piece. Sc, segmented columns; Cp, capitulum; Bp, basal plate; Mt, mitochondria; Ax, axoneme. V, vacuoles; Nu, nucleus: PM, plasma membrane. Scale bars, 500 nm. (B, C) The percentage of spermatids with ultrastructural defects in the connecting piece of elongating spermatids and elongated spermatids from WT mice and Cfap52-KO mice. At least 30 spermatids were counted for each mouse. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, *p<0.05, **p<0.01. HTCA, head-tail coupling apparatus. (D) Cross-sections showing the ultrastructure of the midpiece, principal piece, and end piece of spermatozoa from WT mice and Cfap52-KO mice. CP, central pair; DMT, doublet microtubule; ODF, outer dense fibres; MS; mitochondrial sheath; FS, fibrous sheath. Scale bars, 100 nm. (E) The ratio of ultrastructural defects of the flagellar axoneme in the midpiece, principal piece, and end piece of spermatozoa from WT mice and Cfap52-KO mice. At least 30 spermatozoa were counted for each mouse. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, ***p<0.001.
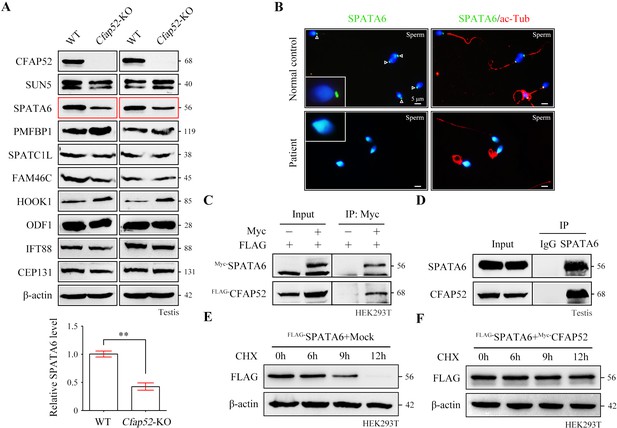
CFAP52 interacts with SPATA6 and regulates its expression.
(A) Expression of nine ASS-associated proteins was analysed by western blotting in testis lysates from WT mice and Cfap52-KO mice. β-actin served as a loading control. Western blotting experiments were repeated three times with similar results. ASS, acephalic spermatozoa syndrome. Bar graph representing band intensities of SPATA6 blots, and data represent the mean ± SEM of three biological replicates. Student’s t test, **p<0.01. (B) Coimmunofluorescence staining of SPATA6 (green) and ac-Tub (red) in spermatozoa from the CFAP52-mutant patient and a normal control. Scale bars, 5 μm. The staining experiments were repeated three times with similar results. (C) A coimmunoprecipitation assay showed that FLAG-tagged CFAP52 could be immunoprecipitated with Myc-tagged SPATA6 in HEK293T cell extracts. (D) An interaction between endogenous SPATA6 and CFAP52 was identified in mouse testis lysates. Rabbit IgG served as the negative control. Co-IP experiments were repeated three times with similar results. (E, F) The degradation of FLAG-tagged SPATA6 in HEK293T cells with or without Myc-tagged CFAP52. Protein samples were harvested at the indicated times after treatment with 100 μg/ml cycloheximide (CHX) to block new protein synthesis. β-actin served as a loading control. Western blotting experiments were repeated three times with similar results.
-
Figure 6—source data 1
Primers for plasmid construction.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig6-data1-v1.zip
-
Figure 6—source data 2
Original blots for Figure 6A, C–F.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig6-data2-v1.zip
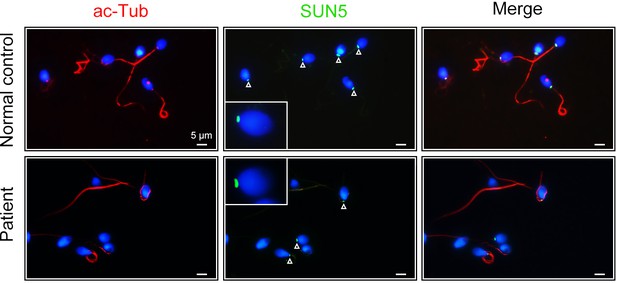
SUN5 staining in spermatozoa from the CFAP52-mutant patient and a healthy control.
Double immunofluorescence staining of SUN5 (green) and acetylated-tubulin (ac-Tub, red) in spermatozoa from the CFAP52-mutant patient and a normal control. Arrowheads indicated the SUN5 signals. Immunofluorescence staining experiments were repeated three times with similar results. Scale bars, 5 μm.
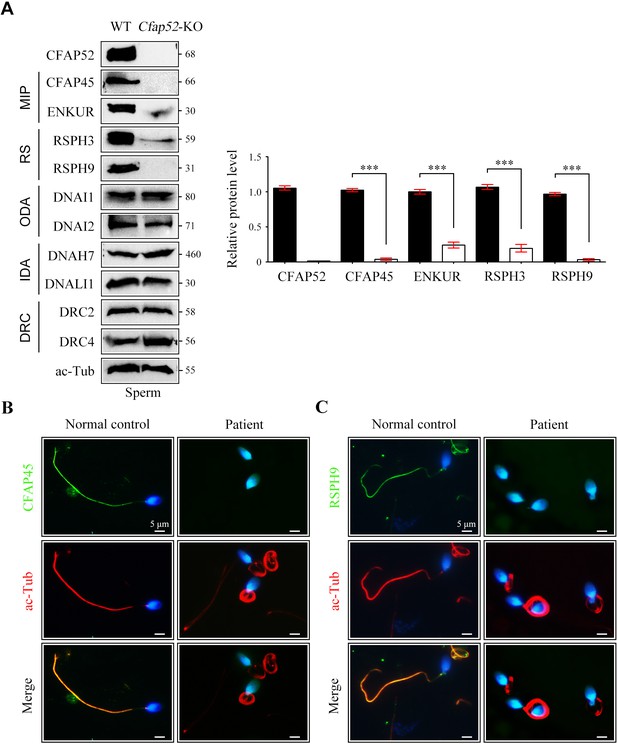
Reduced expression of components of MIP and RS in spermatozoa of Cfap52-KO mice.
(A) Immunoblots of components of MIP, RS, ODA, IDA, DRC in the sperm protein lysates of WT mice and Cfap52-KO mice. Acetylated-tubulin (ac-Tub) served as a loading control. Grey values of bands were analysed by ImageJ software. The relative protein levels of CFAP45, ENKUR, RSPH3, and RSPH9 in sperm samples from WT mice and Cfap52-KO mice. Data represent the mean ± SEM of three biological replicates, and band intensities were normalized to ac-tub. Student’s t test was performed, ***p<0.001. (B, C) Immunofluorescence staining of CFAP45 or RSPH9 (green) together with axonemal acetyl-tubulin (red) in spermatozoa from the Cfap52-mutant patient and a healthy control. The nuclei were counterstained with DAPI. Scale bars, 5 μm. The staining experiments were repeated three times with similar results.
-
Figure 7—source data 1
Antibodies used in this study.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig7-data1-v1.zip
-
Figure 7—source data 2
Original blots for Figure 7A.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig7-data2-v1.zip
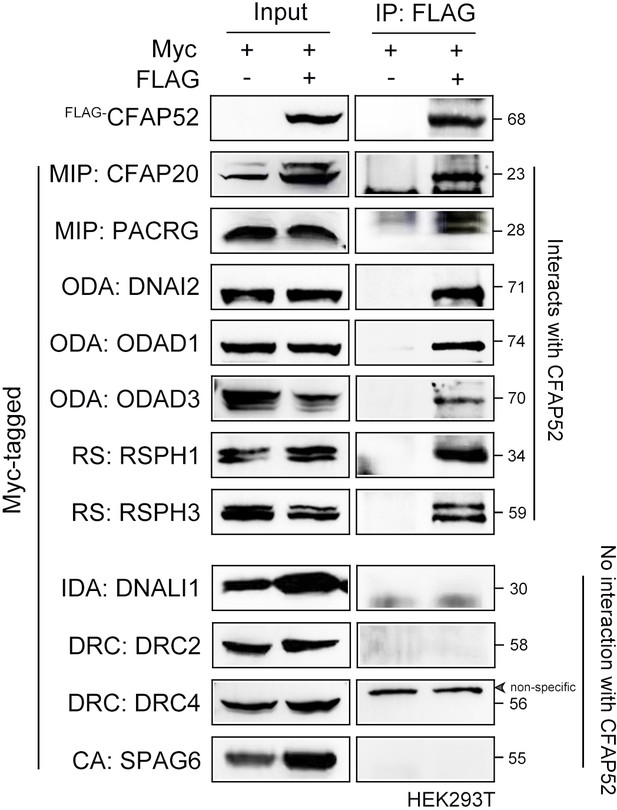
Axonemal interactors of CFAP52 as revealed by a coimmunoprecipitation assay.
FLAG-tagged CFAP52 interacted with Myc-tagged CFAP20, PACRG, DNAI2, ODAD1, ODAD3, RSPH1, and RSPH3 but not DNALI1, DRC2, DRC4, and SPAG6 in HEK293T cells. MIP, microtubule inner protein; ODA, outer dynein arm; RS, radial spoke; IDA, inner dynein arm; DRC, nexin–dynein regulatory complex; CA, central apparatus. co-IP experiments were repeated three times with similar results.
-
Figure 7—figure supplement 1—source data 1
Original blots for Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/92769/elife-92769-fig7-figsupp1-data1-v1.zip
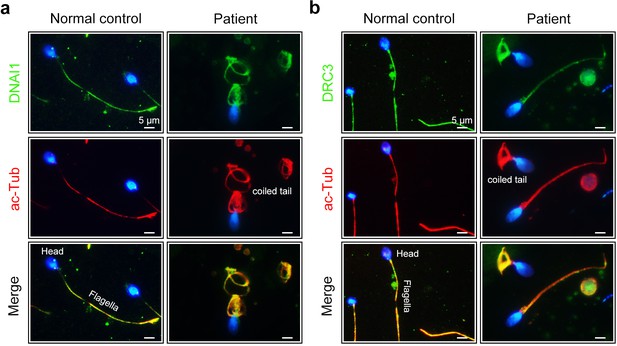
Expression of DNAI1 and DRC3 in spermatozoa of the CFAP52-mutant patient.
(a) Double immunofluorescence staining of DNAI1 (green) and acetylated tubulin (ac-Tub, red) in spermatozoa from CFAP52-mutant patient and a healthy control (blue, DAPI). (b) Double immunofluorescence staining of DRC3 (green) and acetylated tubulin (ac-Tub, red) in spermatozoa from CFAP52-mutant patient and a healthy control (blue, DAPI). Immunofluorescence staining experiments were repeated three times with similar results. Scale bars, 5 μm.
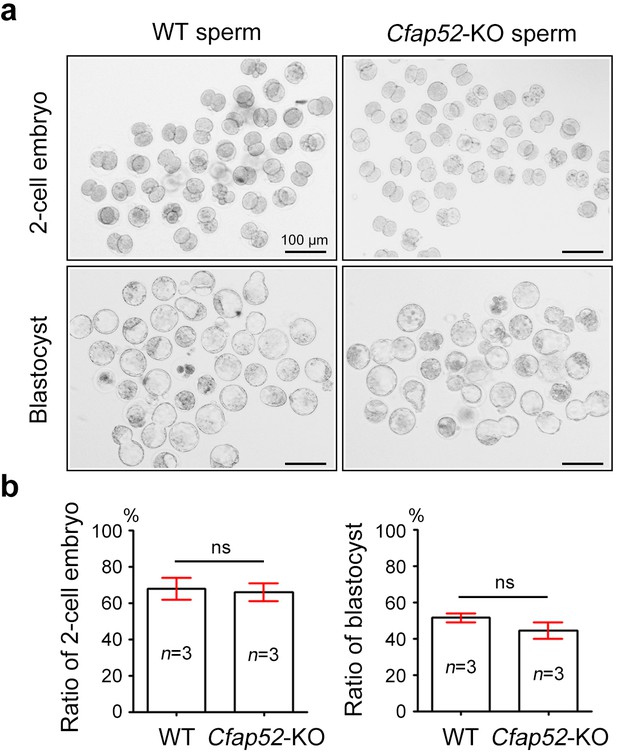
ICSI outcomes using spermatozoa from Cfap52-KO mice.
(a) An intracytoplasmic sperm injection (ICSI) assay was performed using spermatozoa from Cfap52-KO and wild-type mice. Two-cell embryos and blastocysts were calculated at 20 hr and 96 hr postfertilization, respectively. Scale bars, 100 μm. (b) The bar chart showed the percentage of two-cell embryos and blastocysts in ICSI experiments using spermatozoa from Cfap52-KO and wild-type mice, respectively (n=3 males for each group). Data are represented as mean ± SEM and statistical analysis was analyzed by Student’s t-test; ns, not significant.
Tables
Semen analysis in the patient.
| Semen parameters | Patient | Reference limits* |
|---|---|---|
| Sperm volume (ml) | 5.5 | ≥ 1.5 |
| Sperm concentration (106 /ml) | 11.0 | ≥ 15 |
| Motility (A+B, %) | 6.0 | ≥ 40 |
| Vitality (%) | 68.0 | ≥ 58 |
| Normal spermatozoa (%) | 0.5 | ≥ 4 |
| Defective spermatozoa (%) | 99.5 | - |
| Defective sperm flagella (%) | 50.7 | |
| Short flagella (%) | 6.0 | - |
| Coiled flagella (%) | 30.0 | - |
| Absent flagella (%) | 10.5 | - |
| Sperm decapitation (%) | 34.0 | - |
-
*
Reference limits according to the WHO standards.
Variant analysis in the patient.
| M1 | M2 | ||
|---|---|---|---|
| Variants | cDNA mutation* | c.203G>T | c.1128G>A |
| Predicted protein changes | p.S68I | p.W376* | |
| Validate protein changes | p.D24Vfs*5 | p.W376* | |
| Mutation type | Splicing | Nonsense | |
| Genotype | Heterozygous | Heterozygous | |
| Allele frequency | ExAC Browser | 0 | 0 |
| GnomAD | 0 | 0.00000397709 | |
| 1000 Genomes Project | 0 | 0 | |
| Function prediction | dpsi_zscore† | –2.706 | –3.021 |
| SpliceAI score‡ | 0.86 | - |
-
M1 refers to mutation 1; M2 refers to mutation 2.
-
*
NCBI reference sequence number of CFAP52 is NM_001080556.2 (https://www.ncbi.nlm.nih.gov/genbank/).
-
†
Absolute values of the score >2 are considered to be deleterious.
-
‡
Scores >0.5 are suggested to affect splicing.
Clinical features of the patient’s spouse with ICSI treatment.
| Spouse of the patient | ||
|---|---|---|
| Age(y) | 31 | |
| Length of primary infertility history (y) | 2 | |
| BMI | 20.5 | |
| Basal hormones | FSH (IU/l) | 7.1 |
| LH (IU/l) | 4.7 | |
| E2 (pg/ml) | 43.8 | |
| Progesterone (ng/ml) | 0.26 | |
| Cycle 1 | Protocol | Antagonist |
| E2 level on the trigger day (pg/ml) | 8101 | |
| No. of follicles ≥14 mm on the trigger day | 6 | |
| No. of follicles ≥18 mm on the trigger day | 4 | |
| No. of oocytes retrieved | 31 | |
| ICSI progress | Oocytes injected | 21 |
| Fertilization rate (%) | 95.2 (20/21) | |
| Cleavage rate (%) | 100 (20/20) | |
| Available D3 embryos | 20 | |
| Blastocyst formation rate (%) | 70 (14/20) | |
| 4AA | 3 | |
| 4AB | 2 | |
| 4BC | 5 | |
| 4BB | 3 | |
| 3BB | 1 | |





