Delivery of a Jagged1-PEG-MAL hydrogel with pediatric human bone cells regenerates critically sized craniofacial bone defects
Figures
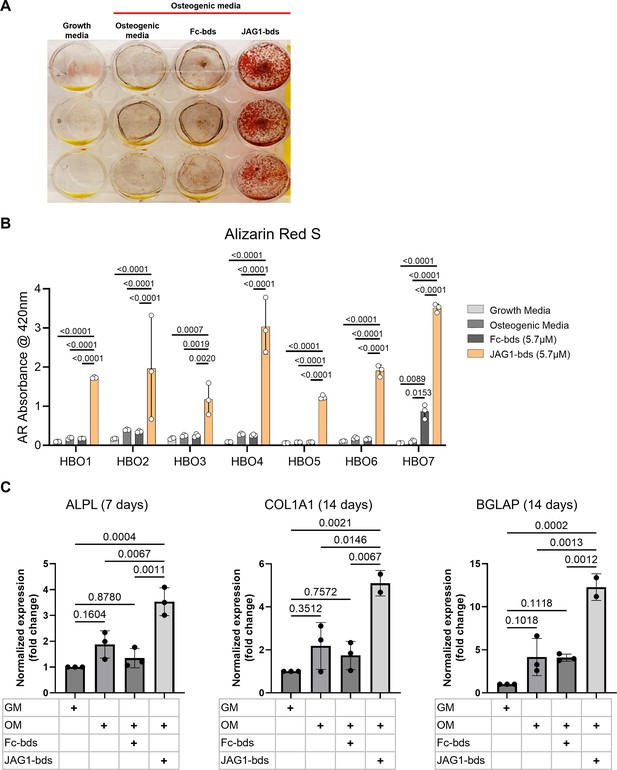
JAGGED1-induced mineralization and gene expression in HBO cells: seven HBO cell lines were treated with growth media alone, osteogenic media alone or with Fc-Dynabeads (5.7 μM) or JAG1-Dynabeads (5.7 μM).
The cells were half-fed every 5 days. On day 21 cells were fixed with 50% ethanol and thereafter, stained with 1% Alizarin Red S. (A) Representative image of HBO2. (B) Alizarin Red S dye was extracted from Alizarin Red S-stained cells using a 1:10 dilution of acetic acid and water, and the absorbance was read at 420 nm. Data represent the mean values of three technical replicates per cell line (mean ± standard deviation [SD], one-way analysis of variance [ANOVA] with Tukey post hoc). (C) HBO1 primary cell line was grown in triplicate and treated with growth media alone, osteogenic media alone or with Fc-Dynabeads (5.7 μM) or JAG1-Dynabeads (5.7 μM). The cells were half-fed every 5 days and collected at 7, 14, and 21 days. qRT-PCR was performed (see Methods). Data were normalized to growth media with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene. Data represent the mean values of three biological and two technical replicates per condition (mean ± SD, ordinary one-way ANOVA with Šídák’s multiple comparisons test, with single pooled variance).
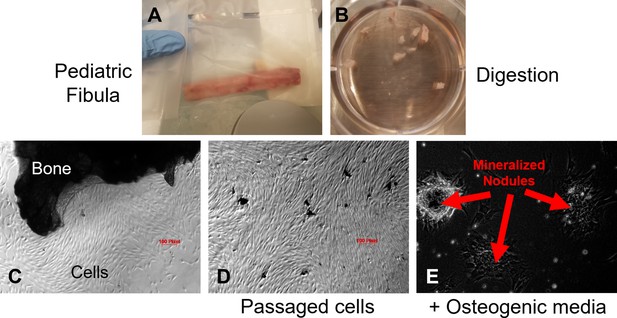
Method of processing human bone samples to produce primary human bone-derived osteoblast-like cell lines.
Human bone-derived osteoblast-like cells (HBO) were isolated by collagenase digestion of pediatric healthy fibular bones. (A) Pediatric fibula specimen (B) Bone is mechanically segmented into small pieces and digestion is accomplished with collagenase A. (C) Bone segments are placed in culture with DMEM plus 10% FBS supplemented with Primocin antibiotic, ascorbic acid, and dexamethasone. (D) Cultures are observed and media is changed every 5 days with osteoblast-like cells emerging by day 10. (E) With the addition of betaglycerophosphate disodium in the media, cells form mineralized nodules. See Methods.
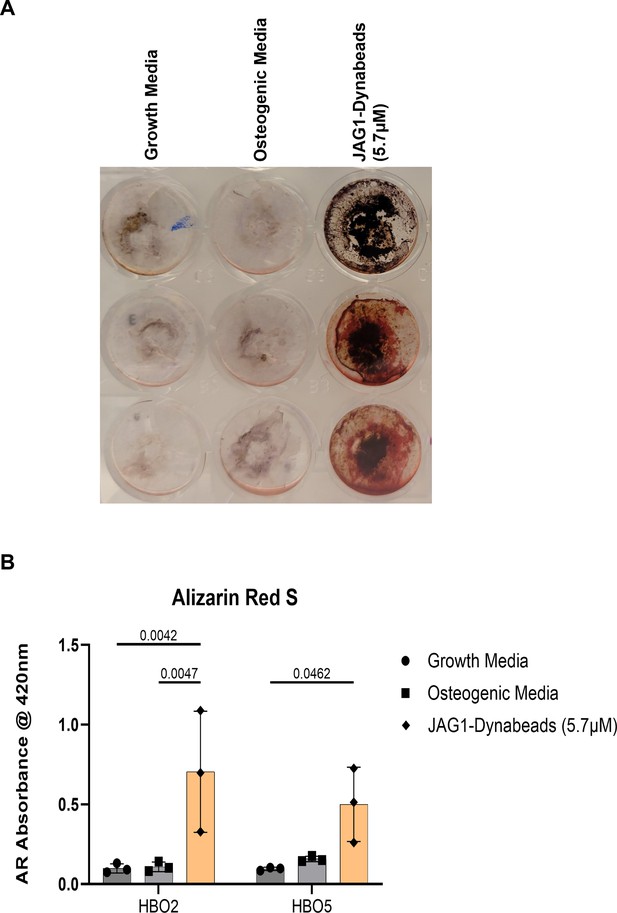
PEG-4MAL hydrogel-encapsulated JAGGED1-induced mineralization of HBO cells.
(A) HBO cells alone or in the presence of JAG1-Dynabeads complex (20 μM) were incorporated in 4% PEG-MAL hydrogels and grown in culture. The cells were half-fed every 5 days. On day 21 cells were fixed with 50% ethanol and thereafter, stained with 1% Alizarin Red S. (B) Alizarin Red S dye was extracted from stained cells using a 1:10 dilution of acetic acid and water, and the absorbance was read at 420 nm. Data represent mean ± standard deviation (SD) per cell line with p-values indicated, n = 3.
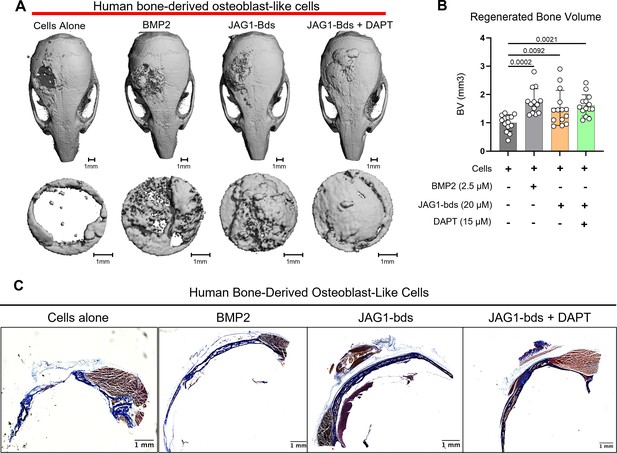
JAG1 delivery in a PEG hydrogel stimulates bone regeneration in a critical-sized bone defect mouse model.
HBO cells alone or in the presence of JAG1-Dynabeads complex (20 μM) ± DAPT and bone morphogenetic protein 2 (BMP2; 2.5 µM) + Fc-Dynabeads were incorporated in 4% PEG-MAL hydrogels and implanted into 4 mm critical-sized defects in the parietal bones of 6- to 8-week-old NOD SCID mice (n = 4–6 per HBO cell donor, 13–15 total) as two separate doses (Initial dose, week 4). After 8 weeks, we quantified differences in regenerated bone volume within the defect and compared them between experimental groups by micro computed tomography (μCT) analysis. (A) μCT reconstructions of defects. (B) Quantification of regenerated bone volume. Data are presented as mean (n = 13–15) ± standard deviation (SD) with p-values reported (one-way analysis of variance [ANOVA] with Šídák’s multiple comparisons test). (C) shows representative sections of the defect area on skulls from mice from all experimental groups stained with Masson trichrome stain.
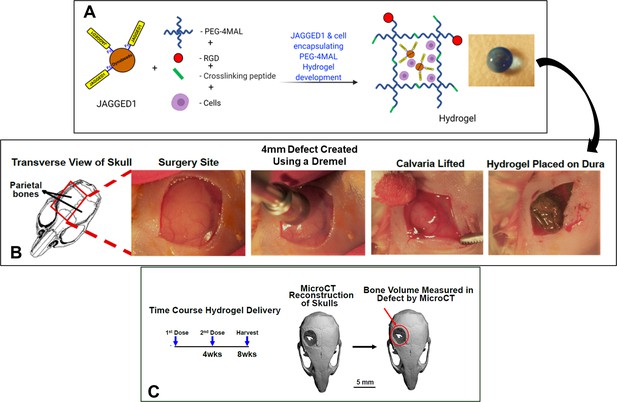
Visual depiction of calvarial defect studies.
(A) HBO cells alone or in the presence of other treatments were incorporated in 4% PEG-MAL hydrogels and (B) implanted into 4 mm critical-sized defects in the parietal bones of 6- to 8-week-old NOD SCID mice as two separate doses (Initial dose, week 4). (C) After 8 weeks, the regenerated bone volume within the defect was measured by micro computed tomography (μCT) and compared between experimental groups.
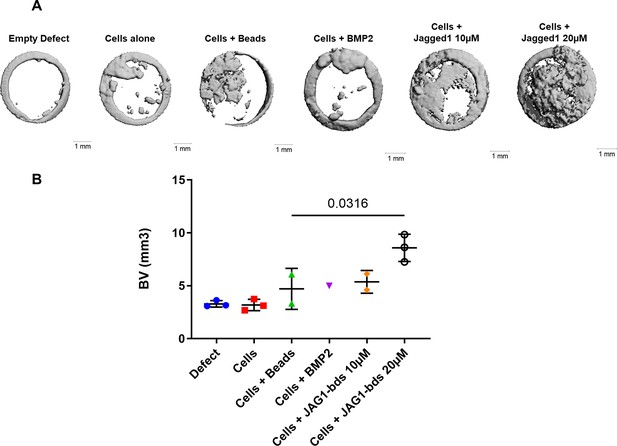
Pilot study of JAG1-bds delivery in a PEG hydrogel stimulates bone regeneration in a critical-sized bone defect mouse model.
No cells (Empty Defect) or HBO cells alone or in the presence of JAG1-Dynabeads complex (20 μM) ± DAPT and bone morphogenetic protein 2 (BMP2; 2.5 µM) + Fc-Dynabeads were incorporated in 4% PEG-MAL hydrogels and implanted into 4 mm critical-sized defects in the parietal bones of 6- to 8-week-old NOD SCID mice (n = 3) as two separate doses (Initial dose, week 4). After 8 weeks, we quantified differences in regenerated bone volume within the defect and compared them between experimental groups by micro computed tomography (μCT) analysis. (A) μCT reconstructions of defects. (B) Quantification of regenerated bone volume. Data are presented as mean (n is shown) ± standard deviation (SD) with p-values reported (ordinary one-way analysis of variance [ANOVA] with Tukey’s multiple comparisons test with a single pooled variance).
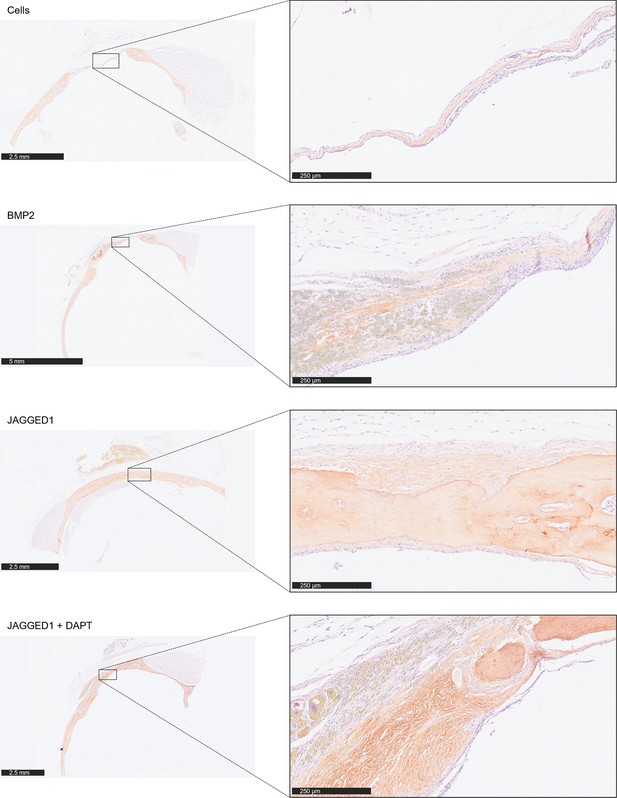
Immunohistochemical staining of calvarial defect tissue for Collagen 1.
Formalin-fixed paraffin-embedded (FFPE) tissue from the calvarial defect experiment shown in Figure 2 was sectioned and stained with an antibody for COL1A1 (Cell Signaling #72026S) and counterstained with hematoxylin. Slides were scanned with the Olympus Nanozoomer whole-slide scanner at 20×.
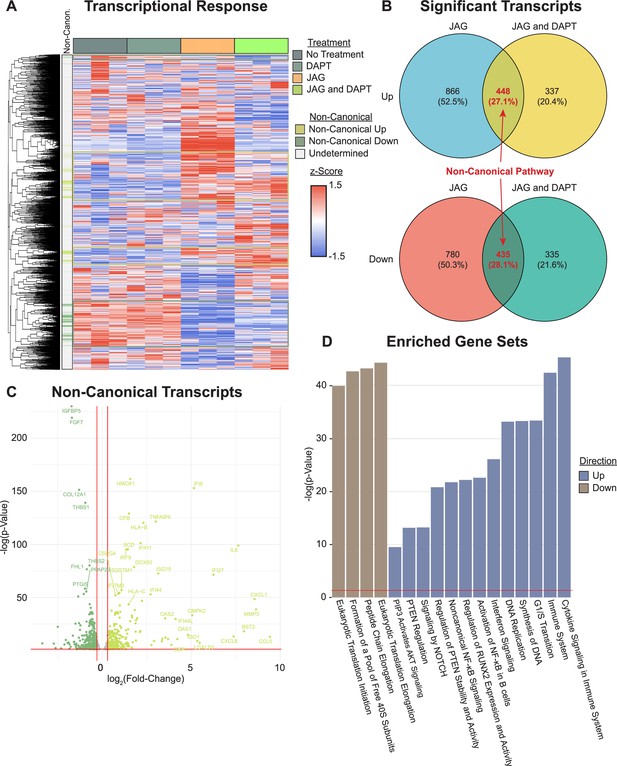
Transcriptional profiling reveals genes and pathways stimulated by non-canonical NOTCH signaling.
(A) RNAseq reveals clusters of genes associated with the non-canonical NOTCH pathway (rows are z-scored, side color bar identifies up- and downregulated differentially expressed genes (DEGs) stimulated by the non-canonical pathway). (B) Overlapping DEGs in the JAG1-bds vs no-treatment and JAG1-bds + DAPT vs no-treatment comparisons reveal the non-canonical pathway (DEseq2). (C) Overlapping DEGs from JAG1-bds + DAPT vs no-treatment comparison. (D) Gene ontology over-representation test reveals significantly enriched up- and downregulated pathways (false discovery rate adjusted).
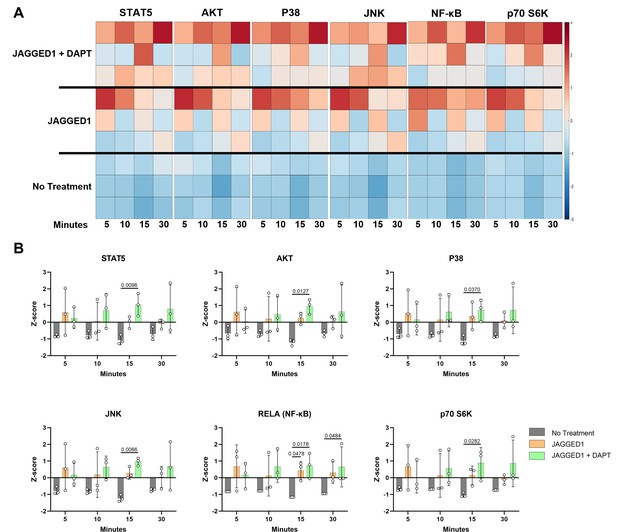
JAGGED1 induces a non-canonical NOTCH pathway in HBO cells.
HBO cells undergo mineralization through a non-canonical pathway. Luminex analysis of lysates obtained from three HBO cell lines untreated or treated with Dynabead-bound recombinant JAG1-Fc fragment (5.7 μM) ± DAPT (15 μM), a NOTCH canonical pathway inhibitor in a time course manner (5, 10, 15, and 30 min), (A) Heatmaps and (B) z-Scores plotted on graphs. Each data point represents mean n = 3 ± standard deviation (SD) per cell line with p-values reported.
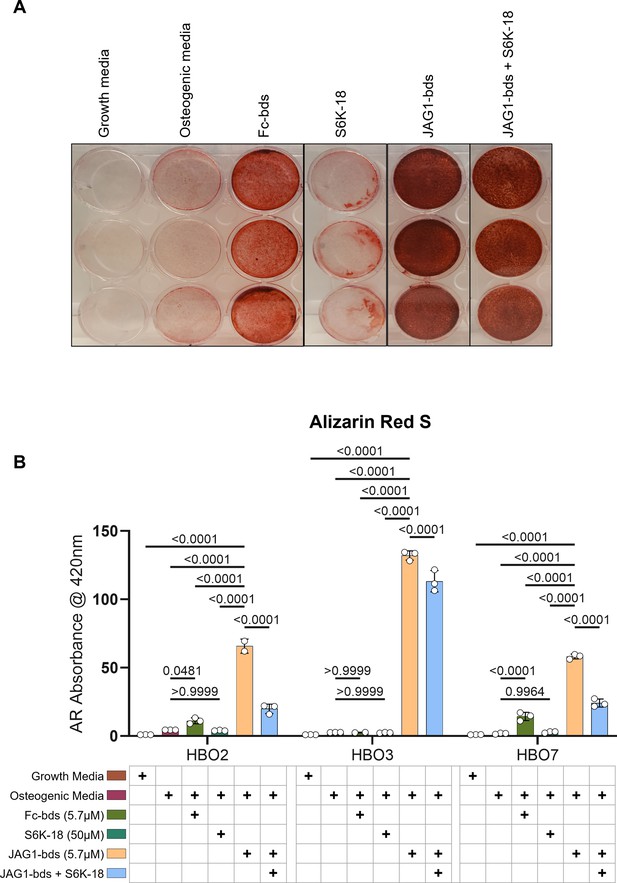
p70 S6K is an essential target during JAGGED1-induced mineralization of HBO cells: HBO cells were treated with growth media alone, osteogenic media alone or with Fc-Dynabeads (5.7 μM), S6K-18 alone (a p70 S6K phosphorylation inhibitor) (50 μM), and JAG1-Dynabeads (5.7 μM) alone or in combination with S6K-18 (50 μM).
The cells were half-fed every 5 days. On day 21 cells are fixed with 50% ethanol and thereafter, stained with 1% Alizarin Red S.(A) Representative image of HBO7. (B) Alizarin Red S dye was extracted from stained cells using a 1:10 dilution of acetic acid and water, and the absorbance was read at 420 nm. Data represent mean n = 3 ± standard deviation (SD) per cell line with p-values indicated.
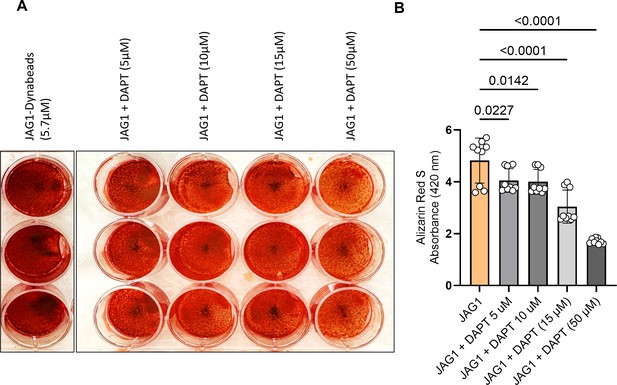
Mineralization assay with DAPT inhibition of the NOTCH canonical pathway.
HBO1 cells were treated with JAG1-Dynabeads (5.7 μM) alone or in combination with increasing concentrations of DAPT in a dose–response test (50 μM). The cells were half-fed every 5 days. On day 21 cells were fixed with 50% ethanol and thereafter, stained with 1% Alizarin Red S. (A) Representative image of HBO1. (B) Alizarin Red S dye was extracted from stained cells using a 1:10 dilution of acetic acid and water, and the absorbance was read at 420 nm. Data represent mean ± standard deviation (SD) per cell line with p-values indicated, n = 9. Ordinary one-way ANOVA with Dunnett's multiple comparisons test, with a single pooled variance.
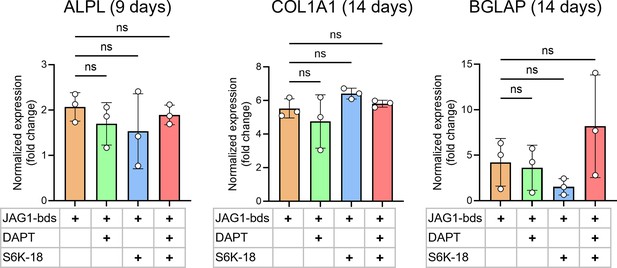
Inhibition of JAGGED1-induced mineralization of HBO cells with inhibitors of NOTCH and p70 S6K.
HBO cells were treated with JAG1-bds (5.7 μM) alone or in combination with DAPT (15 µM), S6K-18 (a p70 S6K phosphorylation inhibitor) (50 μM) or S6K-18 (50 μM) + DAPT (15 μM). The cells were half-fed every 5 days. Mineralization assays were conducted in triplicate, and cells were collected at days 9, 14, and 21. RNA was subsequently assessed by qRT-PCR as described in Methods. Data were normalized to growth media with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene. These charts compare normalized expression of genes with JAGGED1 stimulation and with inhibitors. Data represent the mean values of three biological and two technical replicates per condition (mean ± standard deviation [SD], ordinary one-way analysis of variance [ANOVA] with Šídák’s multiple comparisons test, with single pooled variance).
Additional files
-
Supplementary file 1
List in excel sheet attached shows differentially expressed genes (DEGs) in JAG1 and JAG1 + DAPT groups compared to no treatment.
Analysis revealed a total of 448 upregulated genes and 435 downregulated genes in the non-canonical pathway.
- https://cdn.elifesciences.org/articles/92925/elife-92925-supp1-v1.xlsx
-
Supplementary file 2
Primer sequences used for qRT-PCR.
- https://cdn.elifesciences.org/articles/92925/elife-92925-supp2-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/92925/elife-92925-mdarchecklist1-v1.pdf





