The ketone body β-hydroxybutyrate ameliorates neurodevelopmental deficits in the GABAergic system of daf-18/PTEN Caenorhabditis elegans mutants
Figures
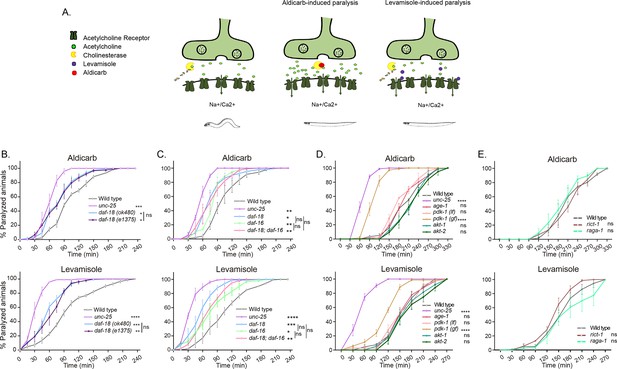
daf-18/PTEN mutants are hypersensitive to cholinergic drugs.
(A) Schematic representation of paralysis induced by aldicarb and levamisole. Aldicarb acts by inhibiting acetylcholinesterase, leading to an accumulation of acetylcholine at the neuromuscular junction, resulting in continuous stimulation of muscles and worm paralysis. Levamisole functions as an agonist at nicotinic acetylcholine receptors, causing prolonged depolarization and, also, muscle paralysis. (B–E) Quantification of paralysis induced by aldicarb (top) and levamisole (bottom). The assays were performed in nematode growth media (NGM) plates containing 2 mM aldicarb or 0.5 mM levamisole. Strains tested: N2 (wild-type) (B–E), CB1375 daf-18(e1375) (B), OAR144 daf-18(ok480) (B, C), GR1310 akt-1(mg144) (D), TJ1052 age-1(hx546) (D), VC204 akt-2(ok393) (D), VC222 raga-1(ok386) (E), and KQ1366 rict-1(ft7) (E). All of these strains carry loss-of-function mutations. Furthermore, the strains denoted as ‘pdk-1 (lf)’ and ‘(gf)’ correspond to JT9609 pdk-1(sa680), which possesses a loss-of-function mutation, and GR1318 pdk-1(mg142), which harbors a gain-of-function mutation in the pdk-1 gene, respectively. The strain CB156 unc-25(e156) was included as a strong GABA-deficient control (B–D). At least four independent trials for each condition were performed (n = 25–30 animals per trial). One-way analysis of variance (ANOVA) was used to test statistical differences in the area under the curve (AUC) among different strains. Post hoc analysis after one-way ANOVA was performed using Tukey’s multiple comparisons test (B, C) and Dunnet´s to compare against the wild-type strain (D, E) (ns p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
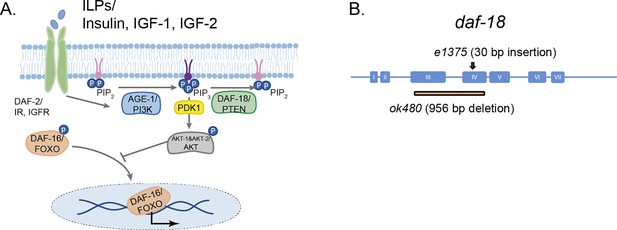
daf-18/PTEN is the negative modulator of the phosphatidylinositol 3-phosphate kinase (PI3K)/AKT pathway.
(A) daf-18/PTEN encodes a lipid and protein phosphatase that hydrolyzes phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to phosphatidylinositol-4,5-bisphosphate (PIP2). It is the main negative modulator of PDK and AKT activity. In daf-18/PTEN mutants, AKT is overactivated leading to high levels of DAF-16/FOXO phosphorylation that prevents the translocation of this transcription factor to the nucleus. (B) Gene structure of daf-18. Coding sequences are represented by blue boxes. The daf-18 (e1375) mutant allele inserts a 30-bp sequence in exon IV. This insertion occurs downstream of the phosphatase catalytic domain and causes a frameshift that leads to premature truncation of the protein. This e1375 mutation partially reduces DAF-18 function. The daf-18 (ok480) allele contains a 956-bp deletion that removes most of exons 3 and 4 and is generally considered to be a null allele.
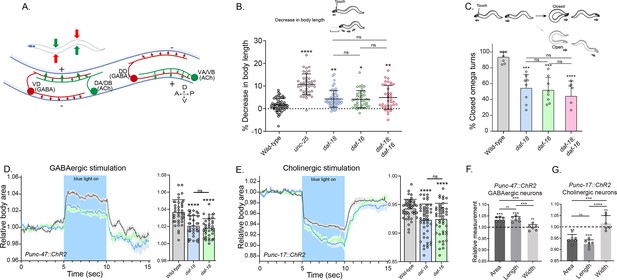
daf-18/PTEN mutants exhibit phenotypes typical of GABA-deficient animals.
(A) Schematic of C. elegans adult neuromuscular circuit. Red indicates GABAergic motor neurons (DD/VD) and green indicates cholinergic motor neurons (VA/VB and DA/DB). The VA and VB cholinergic motor neurons send synaptic inputs to the ventral body wall muscles and the DD GABAergic motor neurons. The release of ACh from VA/VB neurons leads to the contraction of the ventral body wall muscles and the activation of DD GABAergic motor neurons that release GABA on the opposite side of the worm, causing relaxation of the dorsal body wall muscles. Conversely, activation of the DA and DB cholinergic motor neurons produces contraction of the dorsal body wall muscles and activates the VD GABAergic motor neurons. The VD GABAergic motor neurons release GABA, causing relaxation of the ventral body wall muscles, and thus contralateral inhibition. (B) Quantification of body shortening in response to anterior touch. Data are represented as mean ± standard deviation (SD). n = 50–70 animals per genotype distributed across four independent experiments. Kruskal–Wallis analysis with Dunn’s post-test for multiple comparisons was performed. (C) (Top) Scheme of C. elegans escape response in nematode growth media (NGM) agar. After eliciting the escape response by an anterior gentle touch, the omega turns were classified as closed (head and tail are in contact) or open (no contact between head and tail). (Bottom) Quantification of % closed omega turns/total omega turns. At least six independent trials for each condition were performed (n = 20–25 animals per genotype/trial). Data are represented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s post-test for multiple comparisons was performed. (D-E) Light-evoked elongation/contraction of animals expressing Channelrhodopsin (ChR2) in GABAergic (D) and cholinergic (E) motorneurons. Animals were filmed before, during, and after a 5-s pulse of 470 nm light stimulus (15 frames/s). The body area in each frame was automatically tracked using a custom FIJI-ImageJ macro. The averaged area of each animal during the first 125 frames (0–5 s) established a baseline for normalizing light-induced body area changes. To compare the changes induced by optogenetic activity between different strains, the body area measurements for each animal were averaged from second 6 (1 s after the blue light was turned on) to second 9 (1 s before the light was turned off). These mean ± SD values are depicted in the bar graph shown to the right of each trace representation (n = 40–55 animals per genotype). Tukey’s multiple comparisons method following one-way ANOVA was performed for D, while Dunn’s multiple comparisons test after Kruskal–Wallis analysis was used in E. (F, G) Manual Measurement of body length and width upon optogenetic stimulation of GABAergic (F) and cholinergic (G) neurons. At the 2.5 s time point of light stimulation, we manually measured both the width and length of multiple animals and compared these measurements with the corresponding areas obtained from automated analysis (see Materials and methods). The Y-axis represents the ratio between measurements taken before and after blue light illumination. We performed an ANOVA with Tukey’s post hoc analysis for multiple comparisons using the normalized value (1.000) as the baseline. The area and length show significant differences compared to the measurements prior to illumination and do not differ significantly from each other indicating they vary together (increasing for unc-47::ChR2 and decreasing for unc-17::ChR2 animals upon blue light stimulation). In contrast, the width values are not statistically different from the pre-stimulation measurements (p = 0.505 for unc-47::ChR2; p = 0.996 for unc-17::ChR2). This suggests that the changes in area detected by our automated method are due to variations in length. The six animals analyzed to validate the automated measurement were randomly selected from the optogenetic experiments. Data are shown as mean ± SD (ns p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
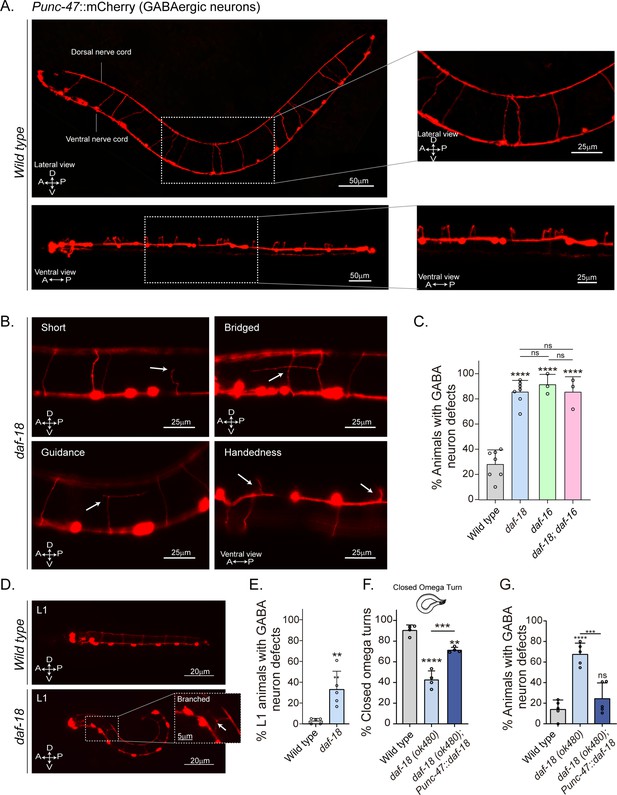
daf-18/PTEN mutants show neurodevelopmental defects in GABAergic motor neurons.
(A) Representative images of wild-type animals expressing mCherry in the GABAergic motor neurons are shown laterally (top) and ventrally (bottom). In the insets, commissures are depicted at a higher resolution. Note that in the ventral view, all the processes travel through the right side of the animal’s body. (B) Representative images of commissure defects observed in daf-18 (ok480) mutants (arrows). The defects shown are: Short, commissure length less than half of nematode width; Bridged, neighboring commissures linked by a neurite; Guidance, commissures that do not reach dorsal nerve cord; and Handedness, commissure running along the opposite side of the animal’s body. (C) Quantification of GABAergic system defects. Each bar represents the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test were used for statistics (ns p > 0.05; ****p ≤ 0.0001). At least three independent trials for each condition were performed (n: 20–25 animals per genotype/trial). (D) Representative image of L1 animals (1 hr post-thatch) expressing Punc-47::mCherry in wild-type (top) and daf-18(ok480) mutant (bottom) backgrounds. In this larval stage, only six GABAergic DD motor neurons are born. The inset shows a typical defective (branched) commissure. (E) Quantification of GABAergic system defects in L1s. Each bar represents the mean ± SD. Two-tailed unpaired Student’s t-test (**p ≤ 0.01). At least five independent trials for each condition were performed (n: ~20 animals per genotype/trial). (F, G) Quantification of closed omega turns/total omega turns and commissure defects in GABAergic neurons of animals expressing daf-18/PTEN solely in GABAergic neurons. One-way ANOVA and Tukey’s multiple comparisons test were used for statistics (ns p > 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001). At least four independent trials for each condition were performed (n: 15–20 animals per genotype/trial). A – anterior; P – posterior; D – dorsal; V – ventral.
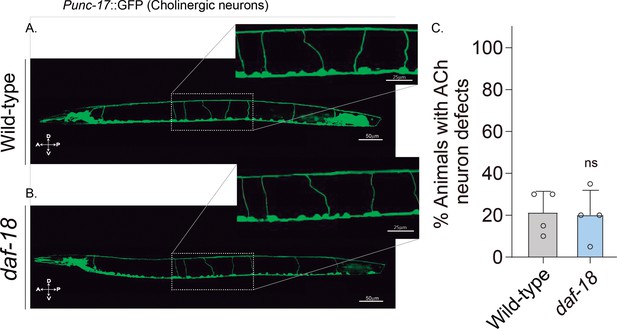
daf-18/PTEN mutations do not affect excitatory cholinergic motor-neuron morphology.
(A-B) Representative images of animals expressing GFP in the cholinergic neurons. In the insets, the commissural processes can be appreciated with higher resolution. (C) Quantification of cholinergic system defects. Each bar represents the mean ± standard deviation (SD) for at least four trials (~20 animals per trial). Statistical significance between the strains was determined by two-tailed unpaired Student’s t-test (ns p > 0.05). A – anterior; P – posterior; D – dorsal; V – ventral.
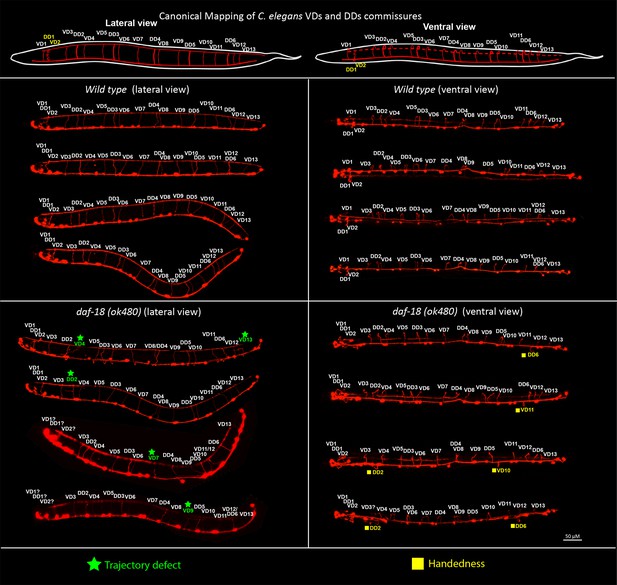
daf-18/PTEN deficiencies affect DDs and VDs GABAergic neurons.
Top, schematic representation of the location of the commissures belonging to the two types of GABAergic neurons in lateral and ventral views (based on Belew et al., 2023; Gujar et al., 2017). Below, representative images of wild-type and daf-18(ok 480) worms viewed laterally (left) and ventrally (right). Note that in both lateral and ventral views (handedness errors) defects appear in both DDs and VDs neurons. Errors in the first three commissures were not considered due to the difficulty of identifying the commissures corresponding to VD1, DD1, and VD2 neurons.
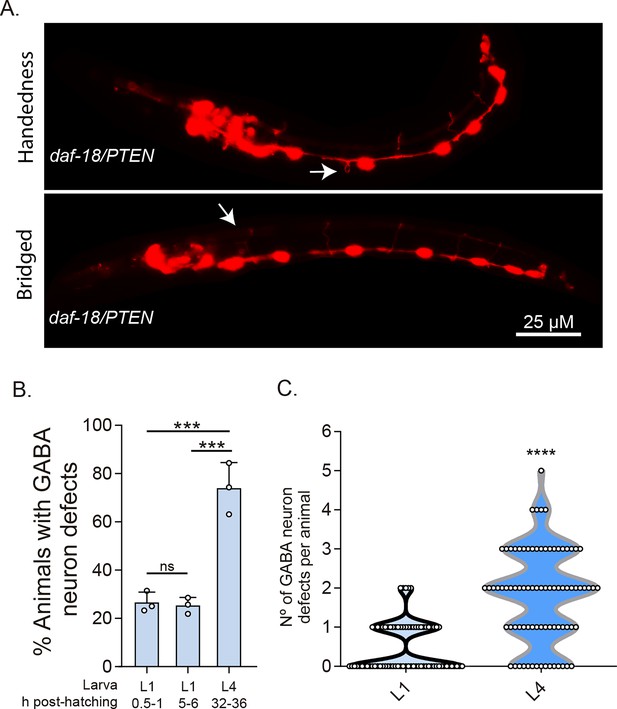
DD-GABAergic neurons show defects in recently hatched L1 animals of daf-18/PTEN mutants.
(A) Representative images of the most common errors observed in L1 animals (1 hr post-hatch) of daf-18 mutants. These types of defects, plus others such as short commissures and guidance defects, are also observed in L4 animals (Figure 3 and Figure 3—figure supplement 2). (B) GABAergic commissure defects were quantified in daf-18/PTEN mutants at various developmental stages: 0.5–1 hr post-hatching (early L1 larva), 5–6 hr post-hatching (mid-L1 larva), and L4 stage (32–36 hr post-hatching). Three independent trials for each condition were performed, with at least 30 animals per condition/trial. Results are presented as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) with Tukey’s post hoc test was used (ns p > 0.05; ***p ≤ 0.001; ****p ≤ 0.0001). (C) Quantification of the number of errors per animal in L1 and L4 larvae. Statistical significance was determined by Mann–Whitney test (****p ≤ 0.0001; n = 75–80).
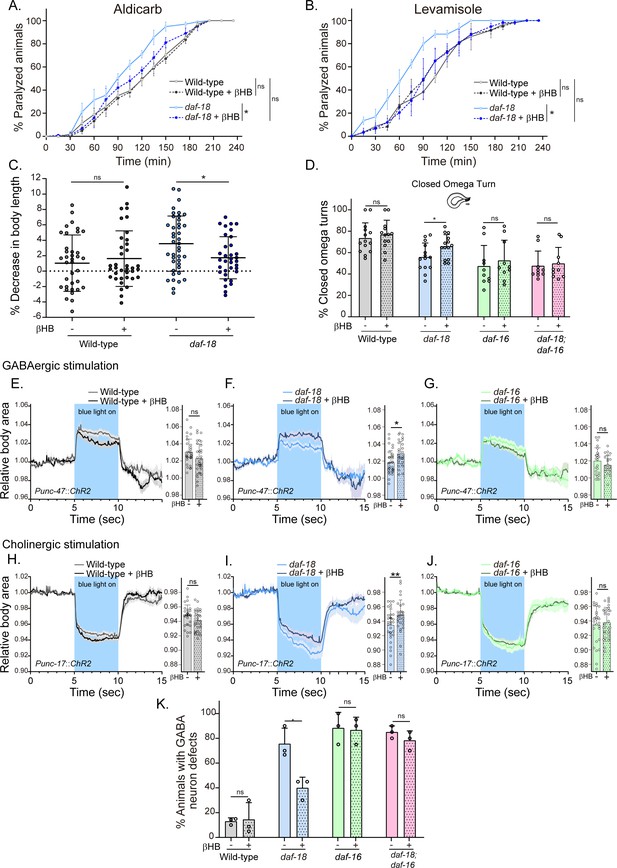
Dietary β-hydroxybutyrate (βHB) supplementation ameliorates GABAergic deficits in daf-18/PTEN mutants.
Animals were exposed to βHB (20 mM) throughout development (from embryo to L4/young adults). (A, B) Quantification of paralysis induced by cholinergic drugs. At least four independent trials for each condition were performed (n: 20–25 animals per genotype/trial). Two-tailed unpaired Student’s t-test (ns p > 0.05; *p ≤ 0.05; **p ≤ 0.01) was used to compare βHB treated and untreated animals. (C) Measurement of body length in response to anterior touch. n = 30–40 animals per genotype distributed across three independent experiments. Two-tailed unpaired Student’s t-test was used to compare βHB treated and untreated animals (ns p > 0.05; *p ≤ 0.05). (D) Quantification of closed omega turns/total omega turns during the escape response. At least eight independent trials for each condition were performed (n = 20 animals per genotype/trial). Results are presented as mean ± standard deviation (SD). Two-tailed unpaired Student’s t-test (ns p > 0.05; *p ≤ 0.05). Light-evoked elongation/contraction of animals expressing Channelrhodopsin (ChR2) in GABAergic (E–G) and cholinergic (H–J) motorneurons. The mean body area (mean ± SD) during 3 s of the light pulse is depicted in the bar graph shown to the right of each trace representation (see Figure 2) (n = 25–35 animals per condition). Two-tailed unpaired Student’s t-test (ns p > 0.05; *p ≤ 0.05; **p ≤ 0.01). (K) Quantification of commissure defects in GABAergic neurons. Results are presented as mean ± SD. Two-tailed unpaired Student’s t-test (ns p > 0.05; *p ≤ 0.05). At least three independent trials for each condition were performed (n = ~20 animals per genotype/trial).
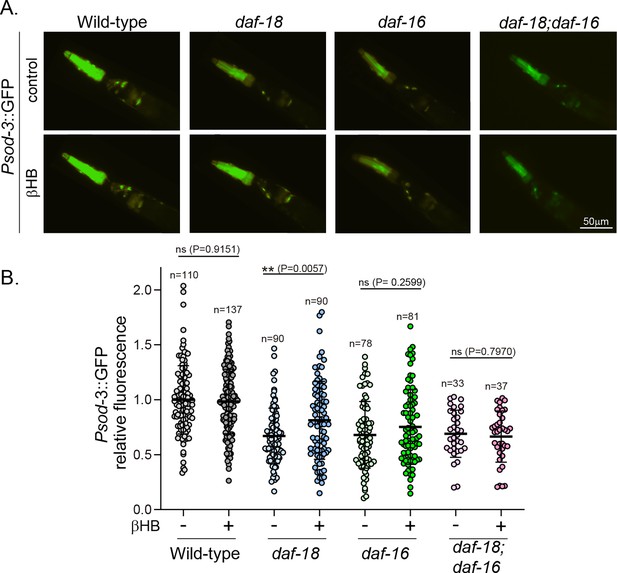
Exposure to β-hydroxybutyrate (βHB) induces sod-3 expression in daf-18/PTEN, but not in daf-16/FOXO mutants.
(A) Representative fluorescence images (×20 magnification) of worms expressing Psod-3::GFP in different genetic backgrounds (wild-type, daf-18(ok480), daf-16(mgDf50) and daf-18(ok480); daf-16(mgDf50)) upon exposure to βHB (20 mM). (B) Corresponding quantification of the fluorescence intensity per animal in the head. Scatter dot plot (line at the median) with the relative expression of Psod-3::GFP normalized to naive wild-type animals. Statistical significance between the treatment and the corresponding control was determined by Mann–Whitney test (ns p > 0.05; **p ≤ 0.01, n = 40–90).
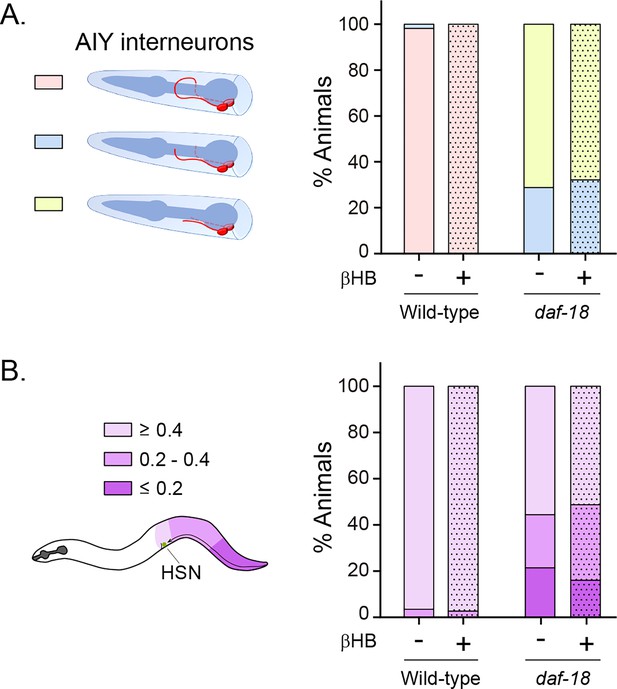
β-Hydroxybutyrate (βHB) does not prevent neurodevelopmental defects in AIY and HSN neurons.
(A) AIY processes were visualized in transgenic animals expressing cytoplasmic GFP in AIY neurons (Pttx-3b::GFP) in wild-type and daf-18(ok480) mutant backgrounds. AIY neuronal growth defects were quantified as described before (Christensen et al., 2011). Left: Scheme of AIY morphology and location in the nematode nerve ring. Blue, pharynx; red, AIY interneurons; pink, wild-type AIY morphology. The two interneurons meet at the dorsal midline. Light blue and yellow: denote different levels of AIY neurite truncation. Right: Percentage of animals with truncated neurites in wild-type and daf-18(ok480) mutants under exposure (or not) to βHB (20 mM). (B) HSN were visualized in transgenic animals expressing GFP in serotonergic neurons (Ptph-1::GFP) in wild-type and daf-18(ok480) mutant backgrounds. HSN under-migration defects were identified as described before (Kennedy et al., 2013). Left: Schematic representation of the HSN migratory route during embryogenesis and the corresponding location of the HSN (green circle) in a young adult animal. Only one of two bilaterally symmetric HSNs is illustrated. Colors show information about the position of HSNs: Light purple: complete migration (≥0.4), middle purple: intermediated migration (>0.2 to <0.4), dark purple: unmigrated (≤0.2). Right: Quantification of the percentage of animals with different HSN migration positions (the most under-migrated neuron of each animal is considered). Bars represent the mean values of at least three independent experiments. Note that there is no significant effect with βHB treatment compared to controls.
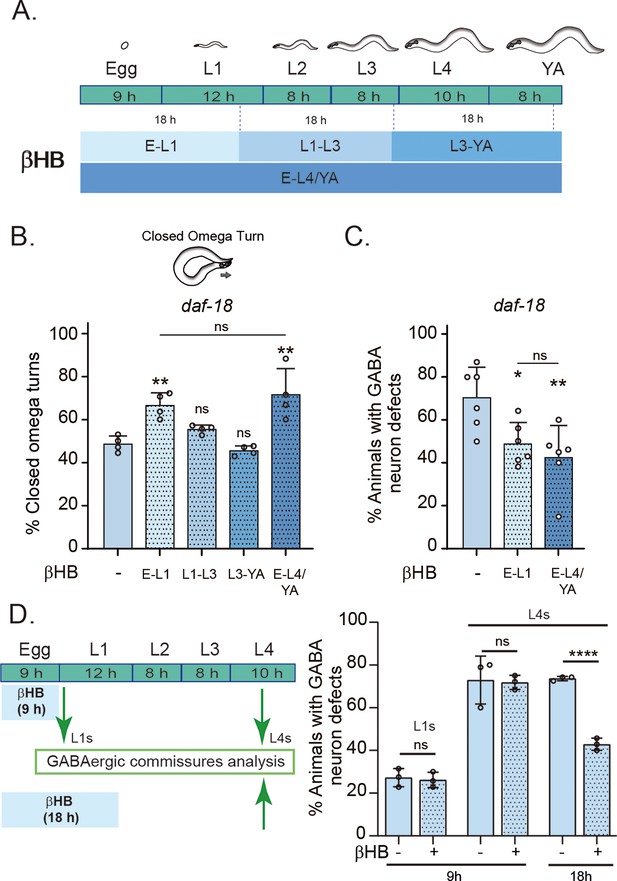
Early developmental stages are critical for β-hydroxybutyrate (βHB) modulation of GABAergic signaling.
(A) Animals were exposed to βHB-enriched diet for 18 hr periods at different developmental stages: (1) E-L1 covered ex utero embryonic development (~9 hr) and the first 8–9 hr of the L1 stage; (2) L1–L3 covered the latter part of the L1 stage (~3–4 hr), the entire L2 stage (~8 hr), and most of the L3 stage (~6–7 hr); (3) L3-YA (Young Adult) spanned the latter part of the L3 stage (~1–2 hr), the entire L4 stage (~10 hr), and the first 6–7 hr as adults, and (4) E-L4/YA implies exposure throughout development (from embryo to Young Adult). Quantification of closed omega turns/total omega turns in daf-18/PTEN (B) and GABAergic commissure defects (C) in daf-18/PTEN mutants exposed to βHB at different developmental intervals. Four and six independent trials for each condition were performed in B and C, respectively (n = 20–25 animals per genotype/trial). Results are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s post-test for multiple comparisons was performed (ns p > 0.05; *p ≤ 0.05; **p ≤ 0.01). (D) βHB does not prevent neurodevelopmental defects in GABAergic neurons when applied exclusively during ex utero embryonic development. Quantification of GABAergic commissure defects in L4-stage of daf-18/PTEN mutant animals exposed to βHB during the first 9 hr post-egg laying (just before hatching) and 18 hr post-egg laying. The animals were then transferred to control plates without βHB and maintained until GABAergic commissures analysis. Scoring was performed in 0.5–1 hr post-hatching (early L1 larva) and L4 animals (green arrows). A two-tailed unpaired Student’s t-test was used for statistical analysis. Data represent three independent trials with at least 20 worms per trial. Results are presented as mean ± SD. (ns P>0.05; **** P≤0.0001).
Videos
A wild-type animal performing a full omega turn in response to anterior touch.
After a prolonged backward movement, the animal makes a pronounced ventral turn, with its head making contact with and gliding along the ventral side of its body, before resuming forward movement in the direction opposite to its original path.
A daf-18 mutant animal does not execute a complete omega turn in response to an anterior touch.
Following a long reversal, the animal’s head fails to touch the ventral side of the body.
Optogenetic activation of an animal expressing Channelrhodopsin (ChR2) in GABAergic neurons.
Upon stimulation of the GABAergic neurons with blue light, the massive relaxation of the body wall muscles leads to an elongation of its body length.
Optogenetic activation of an animal expressing Channelrhodopsin (ChR2) in cholinergic neurons.
Upon stimulation of the cholinergic neurons with blue light, the massive contraction of the body wall muscles leads to a shortening of its body length.





