A neurotransmitter atlas of C. elegans males and hermaphrodites
Figures
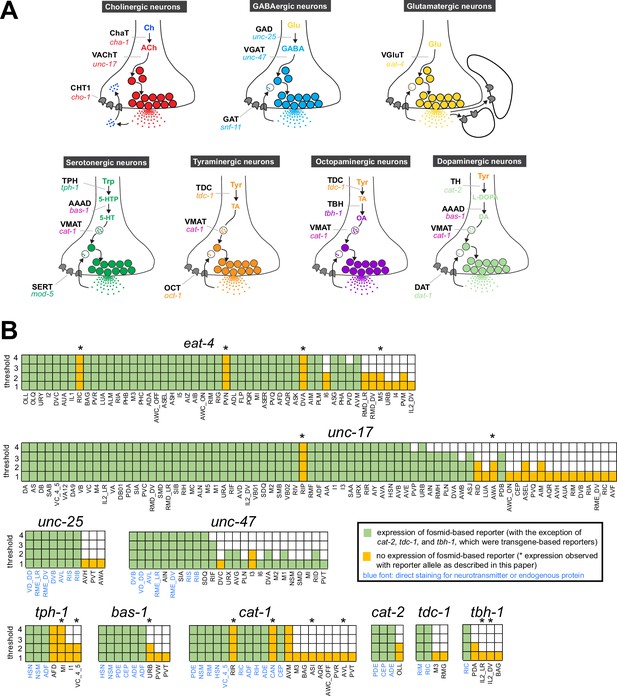
Background on genes examined in this paper.
(A) Neurotransmitter synthesis and transport pathways. TH = tyrosine hydroxylase; TDC = tyrosine decarboxylase; TBH = tyramine β-hydroxylase; TPH = tryptophan hydroxylase; GAD = glutamic acid decarboxylase; AAAD = aromatic amino acid decarboxylase; VMAT = vesicular monoamine transporter; VAChT = vesicular acetylcholine transporter; VGAT = vesicular γ-aminobutyric acid (GABA) transporter; Ch = choline; ACh = acetylcholine; TA = tyramine; OA = octopamine; DA = dopamine. CHT1 = choline uptake transporter; SERT = serotonin uptake transporter; OCT = organic cation transporter; DAT = dopamine uptake transporter; GAT = GABA uptake transporter. Taken and modified from Figure 6 of Hobert, 2013. (B) Graphic comparison of single-cell RNA (scRNA) expression data and previously reported reporter expression data. See Supplementary file 1 for a more comprehensive version that includes expression of reporter genes in cells that show no scRNA transcripts. Note that scRNA expression values for eat-4 and unc-47 can be unreliable because they were overexpressed to isolate individual neurons for scRNA analysis (Taylor et al., 2021).
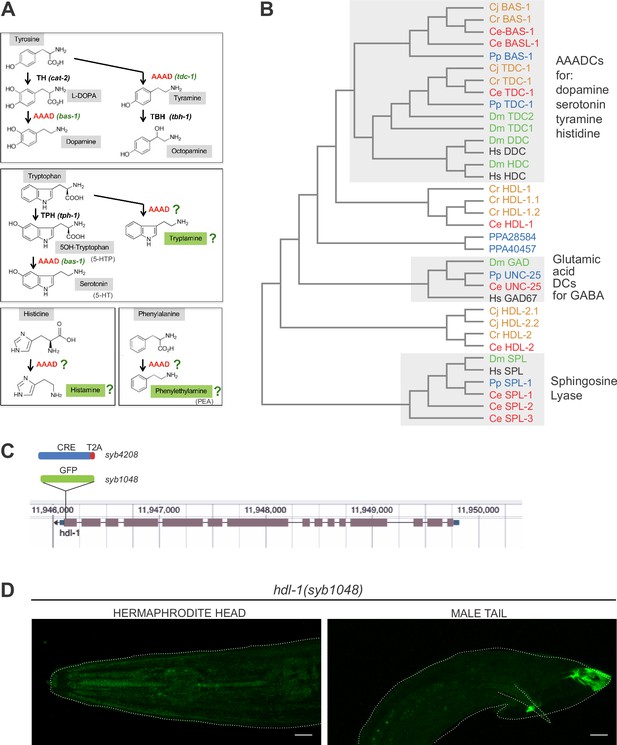
Use of aromatic amino acid decarboxylases (AAADs) in C. elegans.
(A) Biosynthesis of biogenic amines involve the use of AAADs. Modified from Figure 7 of Hobert, 2013. (B) Phylogenetic trees of amino acid decarboxylases. The only AAAD that displays reasonable sequence similarity to neurotransmitter-producing AAADs is the hdl-1 gene (Hare and Loer, 2004; Hobert, 2013). (C, D) We engineered a GFP reporter allele for hdl-1 (syb1048) (C) and did not detect any expression (D). We also attempted but failed at amplifying weak expression signals by using a Cre recombination strategy (C, syb4208, see Materials and methods).
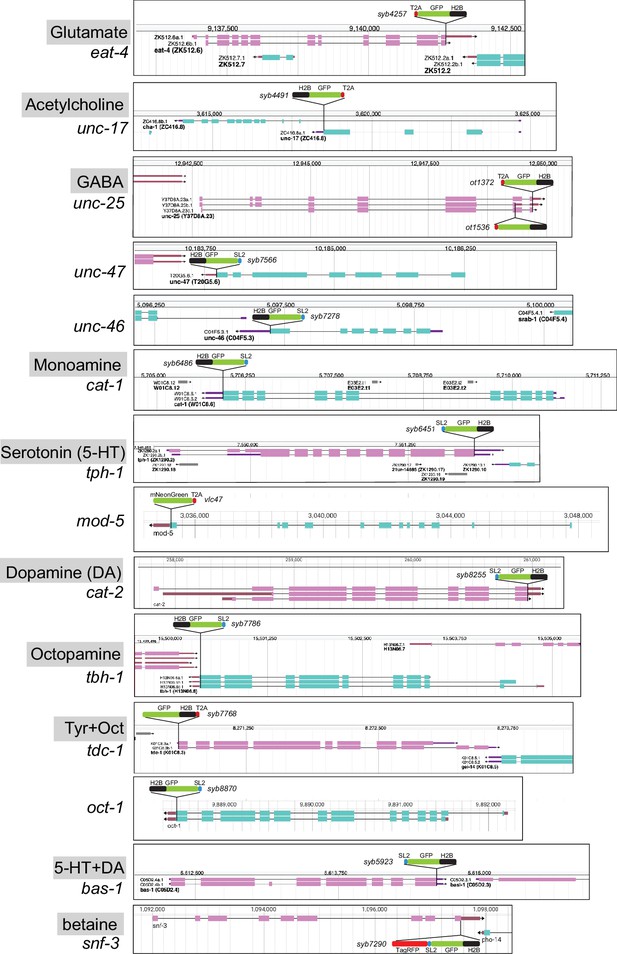
Schematics of reporter knock-in alleles.
Reporter alleles were generated by CRISPR/Cas9 genome engineering. The SL2- or T2A-based separation of the reporter from the coding sequence of the respective loci enables targeting of the reporter to the nucleus (via the H2B tag), which in turn facilitates the identification of the cell expressing a given reporter. Genome schematics are from WormBase (Davis et al., 2022). See Figure 1—figure supplement 1 for hdl-1 reporter alleles.
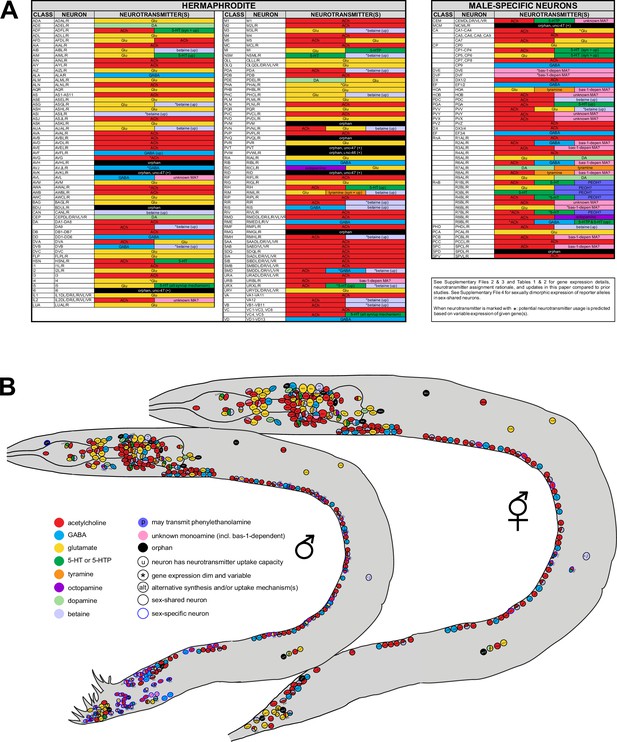
Summary of neurotransmitter usage and atlases.
See Table 1, Table 2, and Supplementary files 2–4 for individual gene expression, rationale for neurotransmitter assignments, and more detailed notes. (A) ACh=acetylcholine; Glu=glutamate; GABA=γ-aminobutyric acid; DA=dopamine; 5-HT=5-hydroxytryptamine, or serotonin; 5-HTP=5-hydroxytryptophan; PEOH?=the neuron has the potential to use β-hydroxyphenethylamine, or phenylethanolamine; bas-1-depen MA?=the neuron has the potential to use bas-1-dependent unknown monoamines (histamine, tryptamine, phenylethylamine [PEA]; also see Figure 1—figure supplement 1); unknown MA?=the neuron has the potential to use non-canonical monoamines; (up)=neurotransmitter uptake; (syn)=neurotransmitter synthesis; *=dim and variable expression of respective identity gene(s) is detected. Variability could be due to one of the following reasons: (1) the endogenous gene is indeed expressed in some but not all animals; (2) the endogenous gene is indeed expressed in every animal but the level of reporter expression is below detection threshold in some. Variability is detected only at low fluorescent intensity; at higher intensities, expression remains consistent. Results for anti-γ-aminobutyric acid (GABA) staining in SMD and anti-serotonin staining in VC4, VC5, CEM, I5, and URX are variable based on previous reports (see text for citations). (B) Information from (A) shown in the context of neuron positions in worm schematics. Note ‘unknown monoamine’ here includes both ‘bas-1-depen MA’ and ‘unknown MA’ in (A). Neurons marked with ‘u’ can uptake given neurotransmitters but not exclusively; some may also synthesize them, e.g., ADF can both synthesize and uptake serotonin.
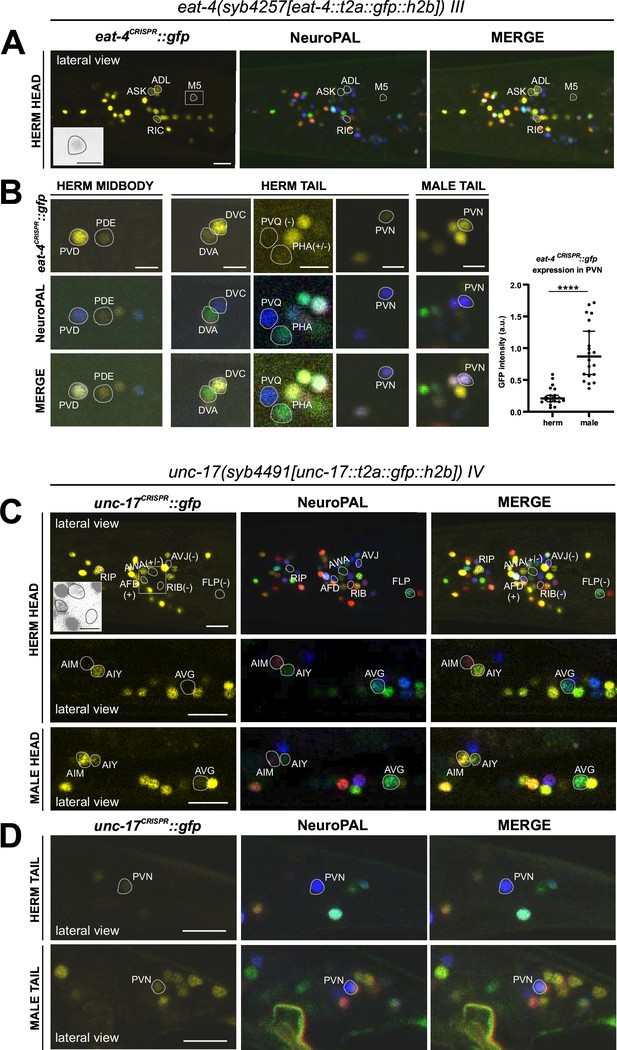
Expression of eat-4/VGLUT and unc-17/VAChT reporter alleles in the adult hermaphrodite.
Neuronal expression of eat-4(syb4257) and unc-17(syb4491) was characterized with landmark strain NeuroPAL (otIs696 and otIs669, respectively). Only selected neurons are shown for illustrating updates from previous reports. See Supplementary file 2 for a complete list of neurons. (A) Dim expression of eat-4(syb4257) in head neurons ASK and ADL is consistent with previous fosmid-based reporter expression. RIC expression is consistent with previous observation using the same reporter allele (Reilly et al., 2022). In addition, dim expression is detected in pharyngeal neuron M5 (also in grayscale inset), previously not detected with eat-4 GFP fosmid-based reporter (otIs388) but visible with eat-4 mCherry fosmid-based reporter (otIs518). (B) Previously uncharacterized eat-4 expression in PDE and DVA neurons is detected with the eat-4(syb4257) reporter allele. Variable expression in PHA is also occasionally detected. No expression is detected in PVQ. Expression in PVN is detected in both sexes but at a much higher level in the male. (C) In the head, prominent expression of unc-17(syb4491) in RIP and dim expression in AWA and AFD neurons are detected. There is no visible expression in RIB, FLP, or AVJ. Consistent with previous reports, AIM expresses unc-17 only in males and not hermaphrodites. In addition, very dim expression of AVG can be detected occasionally in hermaphrodites (representative image showing an animal with no visible expression) and slightly stronger in males (representative image showing an animal with visible expression). Inset, grayscale image showing dim expression for AWA and AFD and no expression for RIB. (D) In the tail, PVN expresses unc-17(syb4491) in both sexes, consistent with previous reports. Scale bars, 10 μm in color images in A, C, and D; 5 μm in B and all grayscale images. Quantification in B is done by normalizing fluorescent intensity of eat-4 GFP to that of the blue channel in the NeuroPAL background. Statistics, Mann-Whitney test.
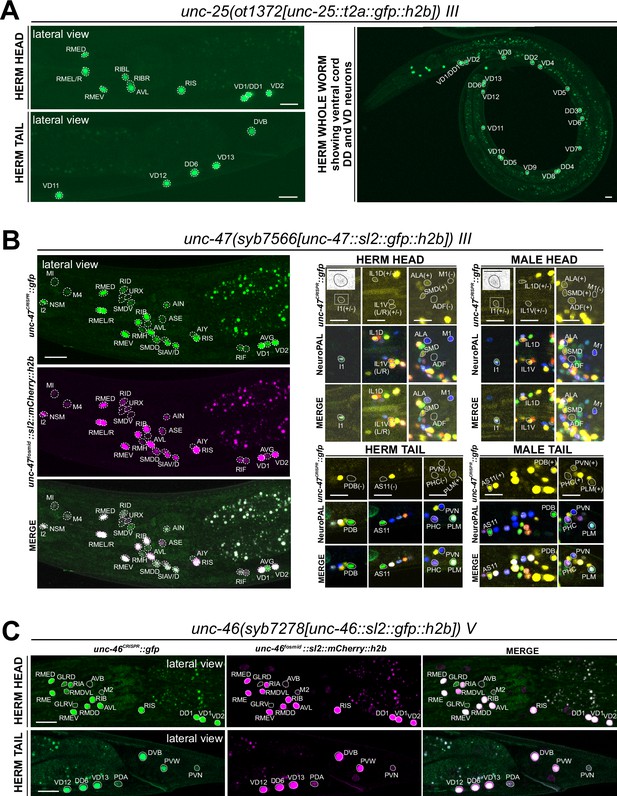
Expression of GABA pathway genes in the adult hermaphrodite.
(A) Expression of the unc-25/GAD reporter allele ot1372 is detected in the head, ventral nerve cord, and tail neurons. The expression pattern of this new T2A-based reporter allele is similar to that of a previously described SL2-based reporter allele, unc-25(ot867) (Gendrel et al., 2016). (B) Expression of unc-47/VGAT reporter allele syb7566. Left, the expression pattern of the reporter allele largely matches that of a previously described unc-47 mCherry fosmid-based reporter (otIs564) in the head. Right, a close-up view for the characterization of the reporter allele expression with landmark strain NeuroPAL (otIs669). In the head, consistent with previous reports of the unc-47 fosmid-based reporter (otIs564), dim expression of unc-47(syb7566) in SMD, ALA, and very dim and variable expression in IL1 is detected in both sexes, and unc-47(syb7566) is expressed in ADF only in the male and not hermaphrodite. In addition, the reporter allele is also expressed at a very dim level in the pharyngeal neuron I1 (also in inset) whereas no expression is detected in M1. In the tail, consistent with previous reports of the fosmid, sexually dimorphic expression of the unc-47(syb7566) reporter allele is also detected in PDB, AS11, PVN, and PHC only in the male and not the hermaphrodite. In addition, we also detected very dim expression of PLM in both sexes, confirming potential dim expression of the unc-47 mCherry fosmid-based reporter that was only readily visible after anti-mCherry staining in the past (Serrano-Saiz et al., 2017b). Scale bars, 5 μm for insets and 10 μm for all other images. (C) Expression of unc-46/LAMP reporter allele syb7278 is largely similar to that of the previously described unc-46/LAMP mCherry fosmid-based reporter (otIs568). We also observed expression of both the reporter allele and fosmid-based reporter in PVW, PVN, and very dimly in PDA. Scale bars, 10 μm.
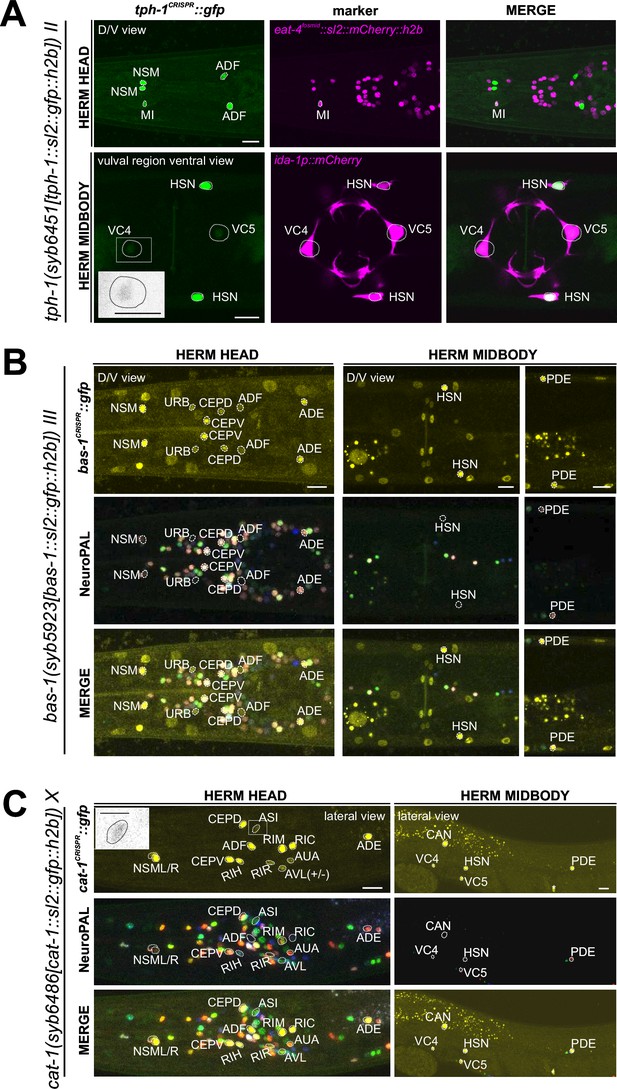
Expression of tph-1/TPH, bas-1/AAAD, and cat-1/VMAT reporter alleles in the adult hermaphrodite.
(A) Dorsoventral view of a hermaphrodite head and midbody expressing tph-1(syb6451). tph-1 expression is detected robustly in the MI neuron and dimly and variably in VC4 and VC5. Neuron identities for MI and VC4 and VC5 were validated using otIs518[eat-4(fosmid)::sl2::mCherry::h2b] and vsls269[ida-1::mCherry], respectively, as landmarks. Inset, grayscale image highlighting dim expression in VC4. Larval expression of this reporter allele is shown in Figure 6—figure supplement 1. (B) Neuronal expression of bas-1(syb5923) characterized with the landmark NeuroPAL (otIs669) strain in the head and midbody regions of young adult hermaphrodites. Dorsoventral view of the adult head shows bas-1/AAAD expression in left-right neuron pairs, including previously reported expression in NSM, CEP, ADF, and ADE (Hare and Loer, 2004). Additionally, we observed previously unreported expression in the URB neurons. Non-neuronal bas-1/AAAD expression is detected in other non-neuronal cell types as reported previously (Yu et al., 2023; also see Figure 14—figure supplement 1, Figure 14). (C) Lateral views of young adult hermaphrodite head and midbody expressing cat-1/VMAT (syb6486). Previously unreported cat-1/VMAT expression is seen in RIR, CAN, AUA, ASI (also in inset), and variably, AVL. Non-neuronal expression of cat-1/VMAT is detected in a single midbody cell in the gonad (also see Figure 14—figure supplement 1), marked with an asterisk. Scale bars, 10 μm for all color images; 5 μm for the inset in grayscale.
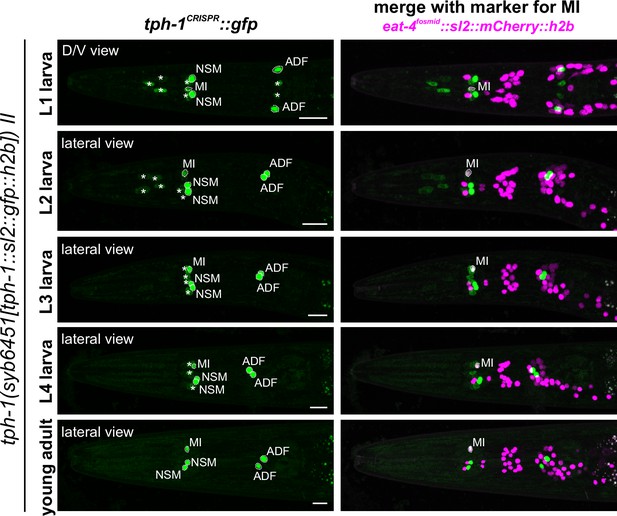
tph-1/TPH reporter allele expression in the hermaphrodite larvae.
Hermaphrodite heads from different larval stages (L1 to L4) and young adults expressing tph-1(syb6451). tph-1 expression in the NSML/R and ADFL/R neuron pairs and in the MI neuron was visible across all larval stages and during adulthood. MI expression was validated using otIs518[eat-4(fosmid)::SL2::mCherry::H2B], a reporter for the glutamatergic identity of MI. Non-neuronal expression of tph-1 (asterisks) could be detected in a subset of pharyngeal muscles in the L1 to L4 larval stages but very dim or no expression was detected in young adults. Scale bars, 10 μm.
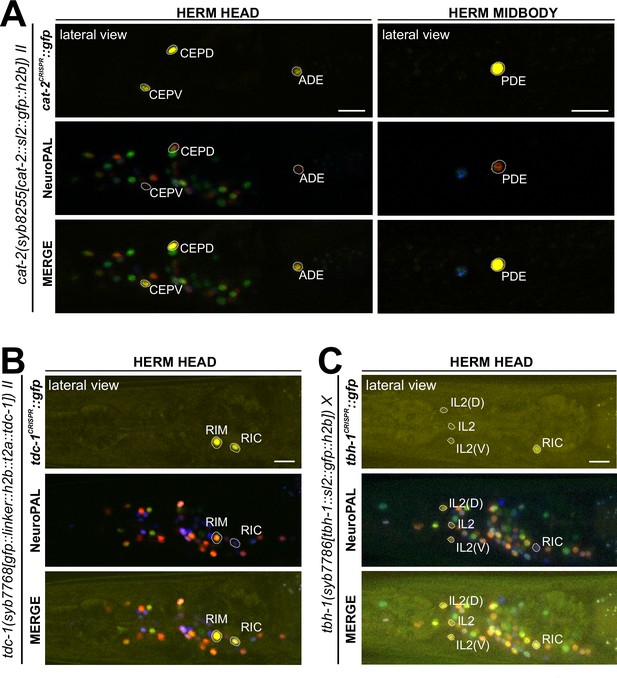
Expression of cat-2/TH, tdc-1/TDC, and tbh-1/TBH reporter alleles in the adult hermaphrodite.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). Lateral views of young adult hermaphrodites expressing reporter alleles for (A) cat-2(syb8255), (B) tbh-1(syb7786), and (C) tdc-1(syb7768). (A) cat-2/TH expression in CEP, ADE, and PDE match previously reported dopamine straining expression (Sulston et al., 1975). (B) and (C) Head areas are shown; no neuronal expression was detected in other areas. tdc-1 expression matches previous analysis (Alkema et al., 2005). We detected previously unreported expression of tbh-1 in all six IL2 neurons at low levels. Scale bars, 10 μm.
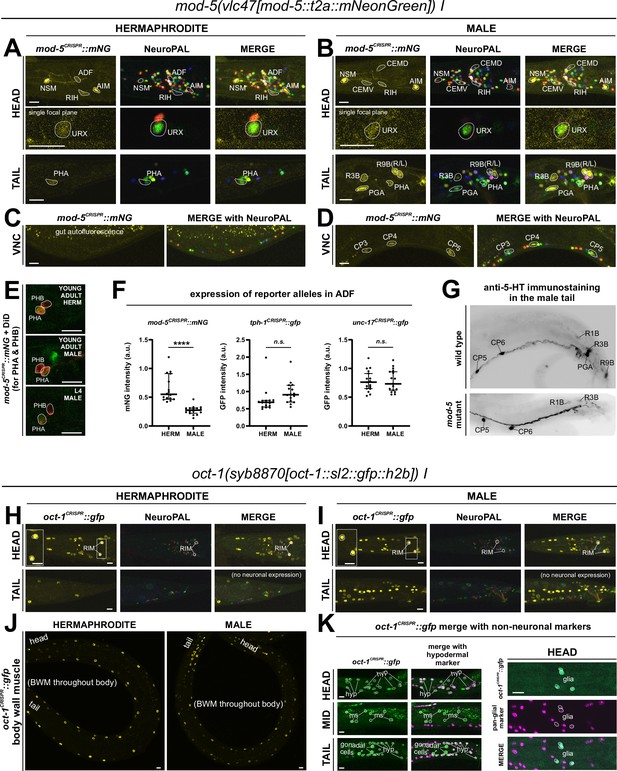
Expression of mod-5/SERT and oct-1/OCT reporter alleles in adult animals.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669) and DiD-filling. (A, C) In adult hermaphrodites, mod-5(vlc47) is expressed in sex-shared neurons NSM, ADF, RIH, AIM, consistent with previous reports (Jafari et al., 2011; Maicas et al., 2021). In addition, we also observed expression in the phasmid neuron PHA and dim and variable expression in URX. There is no visible expression in the ventral nerve cord (VNC). (B, D) In adult males, mod-5(vlc47) is visibly expressed in NSM, RIH, AIM, as well as the male-specific neurons CEM, PGA, R3B, R9B, and CP1 to CP6. Expression in ADF is often not detected (see F). (E) DiD-filling confirms mod-5(vlc47) expression in phasmid neuron class PHA, and not PHB, in young adults in both sexes (L4 male image is to facilitate neuron ID in adults, because the positions of the two neuron classes can change in males during the L4 to adult transition). (F) Expression of mod-5(vlc47) in ADF is stronger in hermaphrodites than in males. Each dot represents a single animal. Expression is not sexually dimorphic for the reporter alleles of either the serotonin-synthesizing enzyme tph-1 or the vesicular acetylcholine transporter unc-17. Expression was normalized against expression in other reporter-expressing neurons. Statistics, Mann-Whitney test. (G) In the tail region of wild type males, male-specific neurons PGA, R1B, R3B, and R9B are stained positive for serotonin. In a mod-5(n3314) mutant background, staining is completely lost in PGA (41/41 stained animals) and significantly affected for R9B (completely lost in 31/41 animals and much dimmer in the rest), while it remains in all 41 stained animals for R1B and R3B. The staining for CP1 to CP6 are also not affected in mod-5 mutant animals (remaining in 41/41 stained animals; image showing CP5 and CP6). (H, I) In adult animals, oct-1(syb8870) is expressed in the tyraminergic neuron class RIM in both sexes. Expression is not observed in any other neurons. (J, K) Outside the nervous system, oct-1(syb8870) is expressed in body wall muscle (BWM) throughout the worm (J) as well as hypodermal cells and selected head glia (K). Expression is also observed in gonadal cells in the male vas deferens (K). A pan-glial reporter otIs870[mir-228p::3xnls::TagRFP] and a dpy-7p::mCherry reporter stIs10166 [dpy-7p::his-24::mCherry+unc-119(+)] were used for glial and hypodermal identification, respectively. Scale bars, 10 μm.
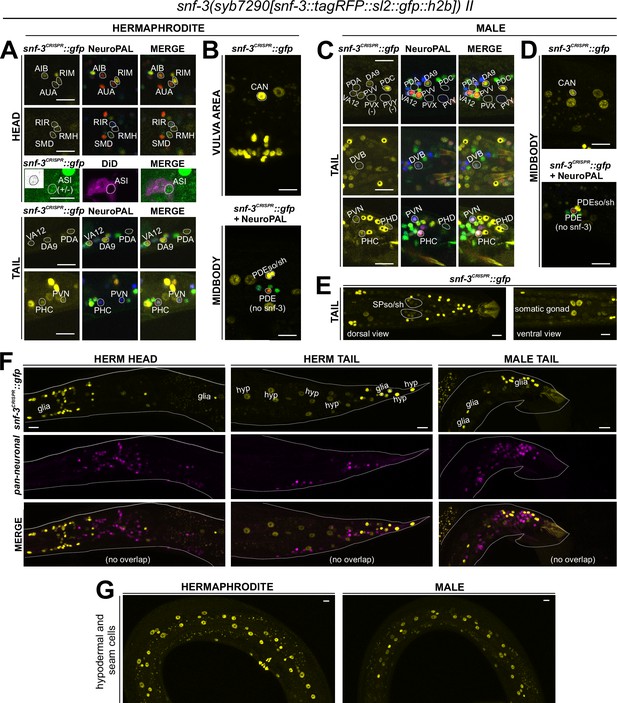
Expression of snf-3/BGT1/SLC6A12 in adult animals.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669) and DiD-filling. (A, B) In the adult hermaphrodite, neuronal expression of snf-3(syb7290) is detected in cat-1/VMAT-positive neurons AUA, CAN, and dimly and variably, RIR and ASI (confirmed with DiD-filling). In addition, it is also expressed in cat-1/VMAT-negative neurons AIB, RIM, RMH, SMD, VA12, DA9, PDA, PHC, PVN as labeled, as well as more neurons listed in Supplementary file 1. In the midbody, expression is not detected in PDE (dopaminergic, cat-1-positive) but is in its associated glial cells. It is also detected in multiple vulval support cells (B) and some epithelial cells near the somatic gonad. (C) In the adult male, in addition to its expression in sex-shared neurons as in hermaphrodites, snf-3(syb7290) is also expressed in male-specific neuron class PDC, as well as in PHD and variably in PVV. (D) Similarly to its expression in hermaphrodites, snf-3(syb7290) is detected in CAN and PDE-associated glial cells, but not PDE neurons, in males. (E) In the male tail, snf-3(syb7290) is expressed in a number of glial cells including the spicule sockets and/or sheath cells (dorsal view). It is also detected in the somatic gonad (ventral view). (F) snf-3(syb7290) is broadly expressed in most if not all glia in both sexes. Glial cell type is determined by cell location and the appearance of their nuclei in Normarski. To confirm they are not neurons, a pan-neuronal marker (UPN, or ‘uber pan-neuronal’, a component in NeuroPAL) is used to determine non-overlapping signals between the two reporters. Head expression in the male is very similar to that in the hermaphrodite and thus not shown. (G) snf-3(syb7290) is broadly expressed in hypodermal and seam cells in both sexes. Scale bars, 10 μm. Asterisks, non-neuronal expression.
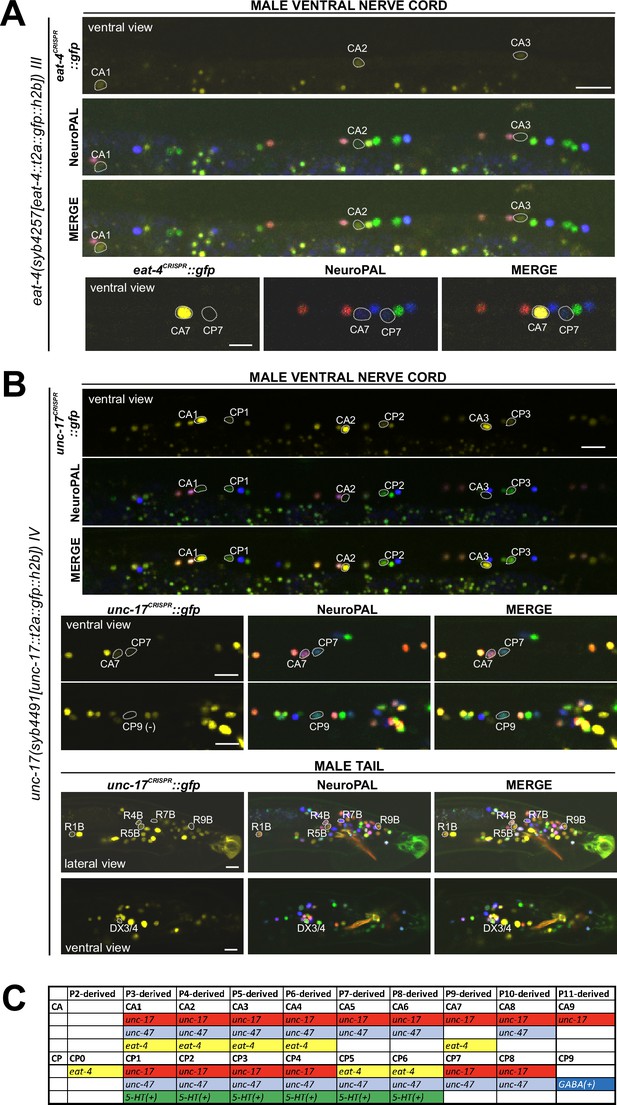
Expression of eat-4/VGLUT and unc-17/VAChT reporter alleles in the adult male.
Neuronal expression of eat-4(syb4257) and unc-17(syb4491) was characterized with landmark strain NeuroPAL (otIs696 and otIs669, respectively). Only selected neurons are shown to illustrate updates from previous studies. See Supplementary file 3 for a complete list of neurons. (A) eat-4(syb4257) expression. Top, long panels: CA1, CA2, and CA3 show visible, albeit very dim, novel expression of eat-4 (also expressed in CA4). Bottom panels: CA7 strongly expresses eat-4(syb4257), whereas CP7 does not. Neuron IDs for these two neurons were previously switched (Serrano-Saiz et al., 2017b). (B) unc-17(syb4491) expression. Top, long panels: ventral view of a male ventral nerve cord showing high levels of expression in CA1, CA2, and CA3 and previously unreported low levels of expression in CP1, CP2, and CP3. Middle panels: low levels of expression in CA7 and CP7. There is no visible expression in CP9. Bottom panels: lateral view of a male tail showing previously unreported dim expression in R1B, R4B, R5B, R7B, and R9B; ventral view of the preanal ganglion showing expression in DX3/4. Scale bars, 10 μm. (C) The updated neurotransmitter atlas underscores the molecular diversity of the male-specific ventral cord neuron class CA and CP. Based on their expression patterns for neurotransmitter genes, these neurons can be grouped into four CA and five CP subclasses.
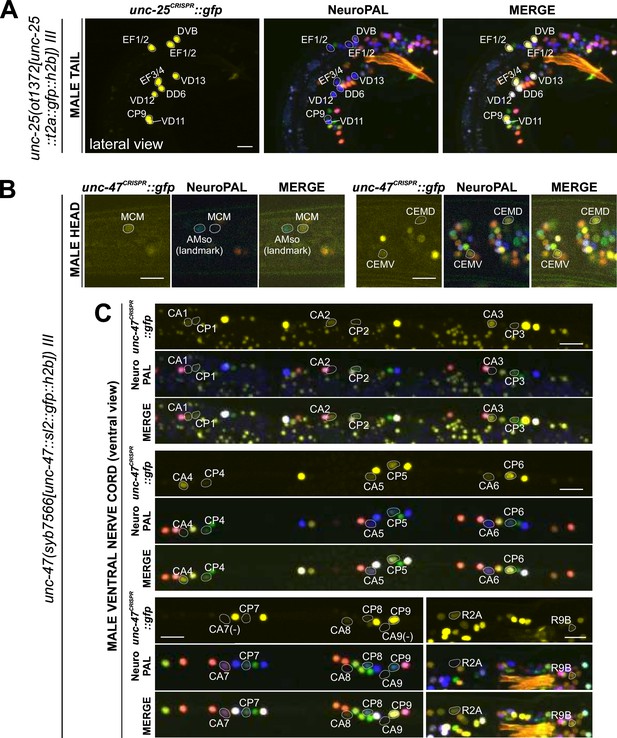
Expression of GABAergic reporter alleles in the adult male.
Neuronal expression of unc-25(ot1372) and unc-47(syb7566) reporter alleles was characterized with landmark strain NeuroPAL (otIs669). Only selected neurons are shown to illustrate updates from previous reports. See Supplementary file 3 for a complete list of neurons. (A) unc-25(ot1372) is expressed in male-specific CP9 and EF neurons as well as a few sex-shared neurons, all consistent with previous reports (Gendrel et al., 2016; Serrano-Saiz et al., 2017b). (B) unc-47(syb7566) shows expression in male head neuron classes MCM and CEM, the former previously undetected and the latter consistent with fosmid-based reporter otIs564. (C) unc-47(syb7566) shows expression in a number of ventral cord CA and CP neurons, largely consistent with reported otIs564 fosmid-based reporter expression except for no visible expression of syb7566 in CA7 (due to its initial confusion with CP7, described in Figure 10) and presence of very dim expression in CP7. The syb7566 reporter allele is also not visible in CA9. Scale bars, 10 μm.
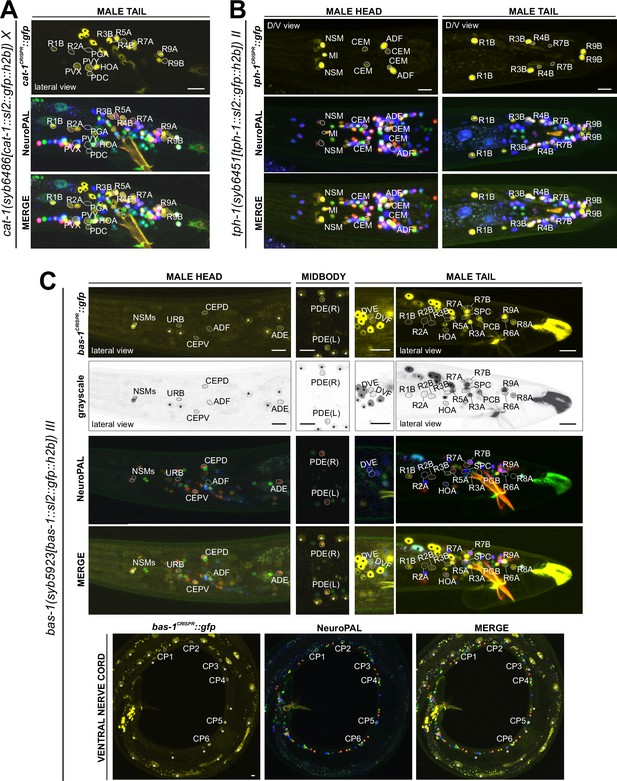
Expression of the cat-1/VMAT, tph-1/TPH, and bas-1/AAAD reporter alleles in the adult male.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). (A) Novel cat-1(syb6486) expression is seen in male-specific neurons PDC, PVY, PVX, R2A, and R4B. Consistent with previous reports, it is also expressed in HOA, PGA, R5A, R7A, R9A, R1B, and R8B. Its expression in ventral cord neurons CP1 to CP6 is consistent with earlier studies. (B) tph-1(syb6451) is expressed in male-specific head neuron class CEM and sex-shared neurons ADF, NSM, and MI. Similar to its expression in hermaphrodites, tph-1 in MI was previously undetected. In the tail, in addition to previously determined expression in R1B, R3B, and R9B, tph-1(syb6451) is also expressed at very low levels in R4B and R7B. Ventral cord expression of tph-1(syb6451) in CP1 to CP6 is consistent with previous reports and thus not shown here. (C) bas-1(syb5923) is expressed in previously identified NSM, ADE, PDE, and CEP neurons. In addition, we detected weak expression in URB as in the hermaphrodite. We also updated bas-1/AAAD expression in 39 male-specific neurons (see Supplementary file 3 for complete list). Neurons are also shown in grayscale for clearer visualization in some cases. Scale bars, 10 μm. Asterisks, non-neuronal expression, also see Figure 14 and Figure 14—figure supplement 1.
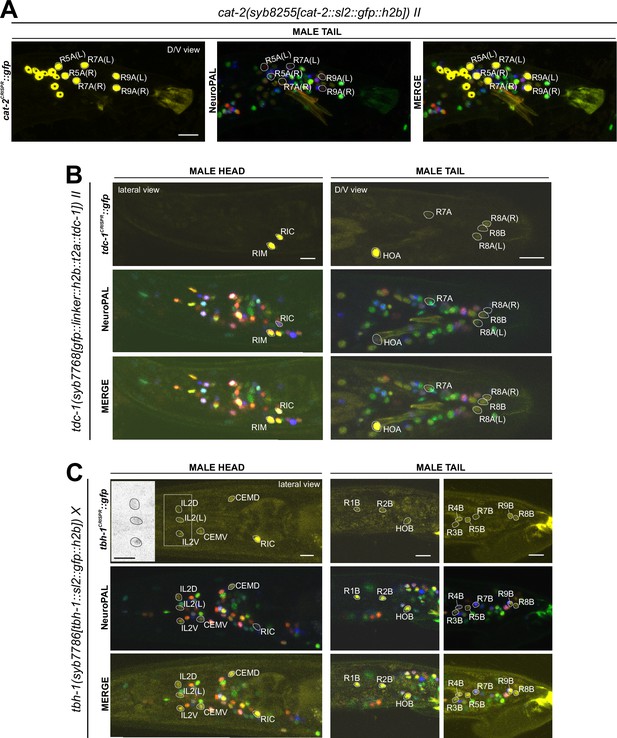
Expression of cat-2/TH, tdc-1/TDC, and tbh-1/TBH reporter alleles in the adult male.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). (A) cat-2(syb8255) is expressed in male-specific neurons R4A, R7A, and R9B. This expression, as well as its expression in sex-shared neurons PDE, CEP, and ADE, is consistent with previous reports (Sulston et al., 1975; Sulston et al., 1980; Lints and Emmons, 1999). (B) tdc-1(syb7768) is expressed in sex-shared neurons RIM and RIC and male-specific neurons HOA, R8A, and R8B, all consistent with previous studies (Serrano-Saiz et al., 2017b). We also detected weak expression in R7A. (C) tbh-1(syb7786) is expressed in RIC, consistent with its previously reported expression in hermaphrodites. As in hermaphrodites, we also detected tbh-1(syb7786) in IL2 neurons of the male. In male-specific neurons, previously unreported expression is detected in CEM, HOB, and all type B ray neurons except for R6B. Intriguingly, this expression pattern resembles that of pkd-2 and lov-1, both genes essential for male mating functions (Barr and Sternberg, 1999; Barr et al., 2001). Inset, grayscale image showing dim expression for IL2 neurons. Scale bars, 10 μm. Asterisks, non-neuronal expression, also see Figure 14 and Figure 14—figure supplement 1.
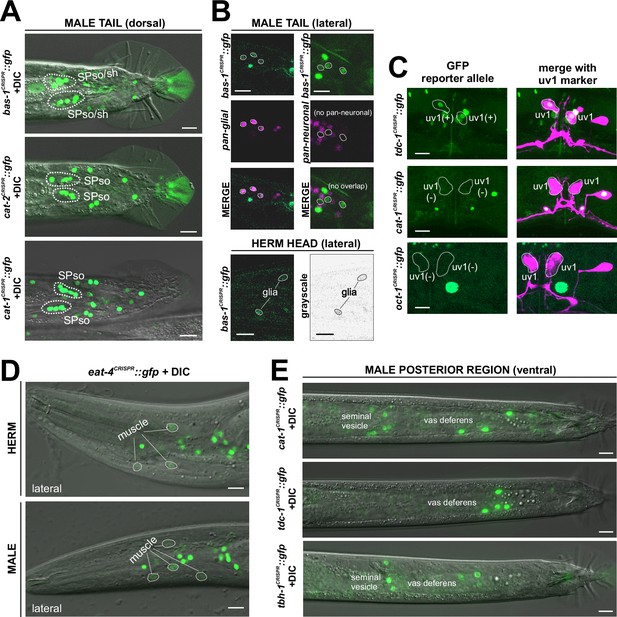
Expression of neurotransmitter pathway genes in non-neuronal cell types.
Multiple neurotransmitter pathway genes show expression in glial cells (A, B) and other non-neuronal cell types (C–E). Also see Figure 14—figure supplement 1 for whole-worm views that capture more non-neuronal expression. (A) bas-1(syb5923), cat-2(syb8255), and cat-1(syb6486) reporter alleles exhibit expression in the male spicule glial cell types, largely consistent with previous reports (Lints and Emmons, 1999; Hare and Loer, 2004; LeBoeuf et al., 2014). (B) Top 6 panels: bas-1(syb5923) is expressed in additional, multiple glial cell types in the male tail. Left 3 panels: bas-1(syb5923) crossed into a pan-glial reporter otIs870[mir-228p::3xnls::TagRFP], confirming its expression in glial cells; right 3 panels: bas-1(syb5923) shows no overlap with the pan-neuronal marker component in NeuroPAL (otIs669). Bottom 2 panels: bas-1(syb5923) also shows expression in at least two glial cells in the head. A hermaphrodite head is shown here. Expression is similar in the male. (C) In the hermaphrodite vulval region, tdc-1(syb7768) is expressed in uv1, consistent with previous reports (Alkema et al., 2005). This expression in uv1 is not observed for either cat-1(syb6486) or oct-1(syb8870). An ida-1p::mCherry integrant vsls269[ida-1::mCherry] was used for identifying uv1. (D) Detection of eat-4(syb4257) expression in muscle cells in both sexes, most prominently in the head. (E) cat-1(syb6486), tdc-1(syb7768), and tbh-1(syb7786) are expressed in the male somatic gonad. All three have expression in the vas deferens; additionally, cat-1 and tbh-1 are also expressed in the seminal vesicle.
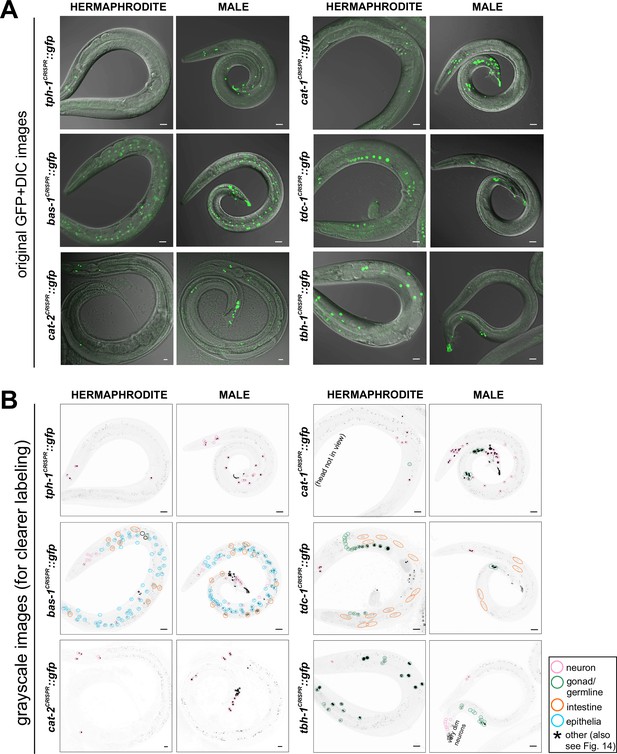
Whole-worm images showing monoaminergic pathway gene expression in different tissue types.
Monoaminergic neurotransmitter reporters show abundant expression outside of the nervous system. Lateral views of entire worms expressing the tph-1/TPH (syb6451), bas-1/AAAD (syb5923), cat-2/TH (syb8255), cat-1/VMAT (syb6486), tdc-1/TDC (syb7768), and tbh-1/TBH (syb7786) reporter alleles. (A) GFP and DIC views. (B) Grayscale views of the GFP signal with tissue types labeled as noted on the figure. We note that tdc-1 reporter allele expression appears to localize to oocytes in the gonad, while the tbh-1 reporter allele appears to be expressed in somatic gonadal sheath cells. This is consistent with previous antibody staining patterns showing distinctive localization of TDC-1 and TBH-1 within the gonad (Alkema et al., 2005). Scale bars, 20 μm. For more details, see Figure 14.
Tables
Neurons that uptake monoaminergic neurotransmitters.
+: presence of reporter allele expression; -: lack of visible reporter allele expression; +/-: dim and variable expression (variability is only detected when reporter fluorescent intensity is low); m: anti-serotonin staining observed in males; *: sex-specific neurons; **: variable/very dim antibody staining reported in previous publications. ***N/A=not presently applicable because betaine is provided by diet, in addition to possible endogenous synthesis. See text for citations.
| Uptake | Synthesis | Release | ||
|---|---|---|---|---|
| Serotonin | Neuron | mod-5 | tph-1 | cat-1 |
| ADF | + | + | + | |
| AIM | + | - | - | |
| I5** | - | - | +/- | |
| NSM | + | + | + | |
| PVW(m)** | - | - | - | |
| RIH | + | - | + | |
| URX** | +/- | - | - | |
| *HSN | - | + | + | |
| *VC4-5** | - | +/- | + | |
| *CEM** | + | + | - | |
| *CP1-6 | + | + | + | |
| *PGA | + | - | + | |
| *R1B | - | + | + | |
| *R3B | + | + | + | |
| *R9B | + | + | + | |
| Tyramine | Neuron | oct-1 | tdc-1 | cat-1 |
| RIM | + | + | + | |
| Betaine | Neuron | snf-3 | N/A*** | cat-1 |
| AUA | + | + | ||
| CAN | + | + | ||
| NSM | +/- | + | ||
| RIM | + | + | ||
| RIR | +/- | + | ||
| ASI | +/- | + | ||
| M3 | +/- | - | ||
| AIB | + | - | ||
| DVB | +/- | - | ||
| SMD | +/- | - | ||
| RIS | + | - | ||
| URX | +/- | - | ||
| PDA | +/- | - | ||
| ASG | +/- | - | ||
| DA9 | +/- | - | ||
| VB1-11 | +/- | - | ||
| PHC | + | - | ||
| PVN | + | - | ||
| VA12 | +/- | - | ||
| RMH | +/- | - | ||
| *PDC | + | + | ||
| *PHD | + | - | ||
| *PVV | +/- | - |
Categories of neuronal expression patterns for monoaminergic neurotransmitter pathway genes.
Criteria for monoaminergic neurotransmitter assignment and a summary for neurons with updated identities are presented here. The categories represent our best assessments based on available data; in every category there is a possibility for the existence of non-canonical synthesis and/or uptake mechanisms that are yet to be discovered. +: presence of reporter allele expression (incl. dim); -: lack of visible reporter allele expression; bas-1-dependent unknown monoamine?=bas-1-dependent unknown monoamine (histamine, tryptamine, PEA; see Figure 1—figure supplement 1A and Discussion); unknown monoamine?=potentially non-canonical monoamines; see Discussion and Results sections on specific gene expression patterns; 5-HT=5-hydroxytryptamine, or serotonin; 5-HTP=5-hydroxytryptophan; PEOH = β-hydroxyphenethylamine, or phenylethanolamine; *: The expression of tph-1 in VC4-5, bas-1 in R4B and R6B, cat-1 in AVL, and snf-3 in NSM, RIR, ASI, URX, M3, DVB, SMD, PDA, ASG, DA9, VA12, VB1-11, RMH, and PVV is dim and variable (this study; variability is only detected when reporter fluorescent intensity is low); anti-5-HT staining in VC4, VC5, CEM, I5, URX, and PVW (male) is variable in previous reports (see text for citations). ** indicates that R4B and R7B express 5-HT synthesis machinery (tph-1 and bas-1), but do not stain with 5-HT antibodies.
| Synthesis (and/or uptake) | cat-1 | tph-1 | cat-2 | bas-1 | tdc-1 | tbh-1 | mod-5 | snf-3 | oct-1 | Direct staining | Sex-specific neurons | Sex-shared neurons |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyramine+bas-1-dependent unknown monoamine? | + | - | - | + | + | - | - | - | - | HOA | ||
| Tyramine+bas-1-dependent unknown monoamine? | - | - | - | + | + | - | - | - | - | R8A | ||
| Tyramine+dopamine | + | - | + | + | + | - | - | - | - | Dopamine | R7A | |
| Tyramine (+uptake)+betaine (uptake) | + | - | - | - | + | - | - | + | + | RIM | ||
| bas-1-dependent unknown monoamine? | + | - | - | + | - | - | - | - | - | R2A | ||
| bas-1-dependent unknown monoamine? | - | - | - | + | - | - | - | - | - | R3A, R6A, R6B*, PCB, SPC, DVE, DVF | URB | |
| Octopamine | + | - | - | - | + | + | - | - | - | RIC | ||
| Octopamine | - | - | - | - | + | + | - | - | - | R8B | ||
| Dopamine | + | - | + | + | - | - | - | - | - | Dopamine | R5A, R9A | ADE, CEP, PDE |
| 5-HTP (synthesis)+5-HT (alternative synthesis/uptake mechanism?)+unknown monoamine? | - | + | - | - | - | + | - | - | - | 5-HT | CEM* | |
| 5-HTP | - | + | - | - | - | - | - | - | - | MI | ||
| PEOH? | - | - | - | + | - | + | - | - | - | R2B | ||
| 5-HT+PEOH? | - | + | - | + | - | + | - | - | - | R7B** | ||
| 5-HT+PEOH? | + | + | - | + | - | + | - | - | - | 5-HT | R1B | |
| 5-HT+PEOH? | + | + | - | + | - | + | + | - | - | 5-HT | R3B | |
| 5-HT+PEOH? | + | + | - | + | - | + | - | - | - | R4B** | ||
| 5-HT (uptake) | + | - | - | - | - | - | + | - | - | 5-HT | PGA | RIH |
| 5-HT (uptake) | - | - | - | - | - | - | + | - | - | 5-HT | AIM | |
| 5-HT (uptake)+betaine (uptake) | - | - | - | - | - | - | + | + | - | 5-HT | URX* | |
| 5-HT (& uptake) | + | + | - | + | - | - | + | - | - | 5-HT | CP1-6 | ADF |
| 5-HT (alternative synthesis/uptake mechanism?) | - | - | - | - | - | - | - | - | - | 5-HT | I5*, PVW (male only) | |
| 5-HT | + | + | - | + | - | - | - | - | - | 5-HT | HSN | |
| 5-HTP (synthesis) and 5-HT (uptake) | + | + | - | - | - | + | + | - | - | 5-HT | R9B | |
| 5-HTP (synthesis) and 5-HT (alternative synthesis/uptake mechanism?) | + | + | - | - | - | - | - | - | - | 5-HT | VC4-5* | |
| Unknown monoamine? | + | - | - | - | - | - | - | - | - | PVX, PVY | AVL* | |
| Unknown monoamine? | - | - | - | - | - | + | - | - | - | HOB, R5B | IL2 | |
| 5-HT+betaine (uptake) | + | + | - | + | - | - | - | + | - | 5-HT | NSM* | |
| Betaine (uptake) | + | - | - | - | - | - | - | + | - | PDC | AUA, CAN, RIR*, ASI* | |
| Betaine (uptake) | - | - | - | - | - | - | - | + | - | PHD, PVV* | M3*, AIB, DVB*, SMD*, RIS, PDA*, ASG*, DA9*, PHC, PVN, VA12*, VB1-11*, RMH* |
Additional files
-
Supplementary file 1
Single-cell RNA (scRNA) data for neurotransmitters in the hermaphrodite.
Here, we show expression of previous reporters and reporter alleles used in this study, compared to scRNA data. Note that scRNA expression values for eat-4 and unc-47 can be unreliable because they were overexpressed to isolate individual neurons for scRNA analysis (Taylor et al., 2021).
- https://cdn.elifesciences.org/articles/95402/elife-95402-supp1-v1.xlsx
-
Supplementary file 2
Updated expression patterns of neurotransmitter pathway genes in hermaphrodites.
- https://cdn.elifesciences.org/articles/95402/elife-95402-supp2-v1.xlsx
-
Supplementary file 3
Updated expression patterns of neurotransmitter pathway genes in male-specific neurons.
- https://cdn.elifesciences.org/articles/95402/elife-95402-supp3-v1.xlsx
-
Supplementary file 4
Summary of sexually dimorphic use of neurotransmitter pathway genes in sex-shared neurons.
- https://cdn.elifesciences.org/articles/95402/elife-95402-supp4-v1.xlsx
-
Supplementary file 5
Summary of updates to expression patterns of classic neurotransmitter pathway genes.
- https://cdn.elifesciences.org/articles/95402/elife-95402-supp5-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/95402/elife-95402-mdarchecklist1-v1.docx





