A neurotransmitter atlas of C. elegans males and hermaphrodites
eLife assessment
This fundamental study reports the most comprehensive neurotransmitter atlas of any organism to date, using fluorescent knock-in reporter lines. The work is comprehensive, rigorous, and compelling. The tool will be used by broad audience of scientists interested in neuronal cell type differentiation and function, and could be a seminal reference in the field.
https://doi.org/10.7554/eLife.95402.3.sa0Fundamental: Findings that substantially advance our understanding of major research questions
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Compelling: Evidence that features methods, data and analyses more rigorous than the current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Mapping neurotransmitter identities to neurons is key to understanding information flow in a nervous system. It also provides valuable entry points for studying the development and plasticity of neuronal identity features. In the Caenorhabditis elegans nervous system, neurotransmitter identities have been largely assigned by expression pattern analysis of neurotransmitter pathway genes that encode neurotransmitter biosynthetic enzymes or transporters. However, many of these assignments have relied on multicopy reporter transgenes that may lack relevant cis-regulatory information and therefore may not provide an accurate picture of neurotransmitter usage. We analyzed the expression patterns of 16 CRISPR/Cas9-engineered knock-in reporter strains for all main types of neurotransmitters in C. elegans (glutamate, acetylcholine, GABA, serotonin, dopamine, tyramine, and octopamine) in both the hermaphrodite and the male. Our analysis reveals novel sites of expression of these neurotransmitter systems within both neurons and glia, as well as non-neural cells, most notably in gonadal cells. The resulting expression atlas defines neurons that may be exclusively neuropeptidergic, substantially expands the repertoire of neurons capable of co-transmitting multiple neurotransmitters, and identifies novel sites of monoaminergic neurotransmitter uptake. Furthermore, we also observed unusual co-expression patterns of monoaminergic synthesis pathway genes, suggesting the existence of novel monoaminergic transmitters. Our analysis results in what constitutes the most extensive whole-animal-wide map of neurotransmitter usage to date, paving the way for a better understanding of neuronal communication and neuronal identity specification in C. elegans.
Introduction
Understanding information processing in the brain necessitates the generation of precise maps of neurotransmitter deployment. Moreover, comprehending synaptic wiring diagrams is contingent upon decoding the nature of signaling events between anatomically connected neurons. Mapping of neurotransmitter identities onto individual neuron classes also presents a valuable entry point for studying how neuronal identity features become genetically specified during development and potentially modified in response to specific external factors (such as the environment) or internal factors (such as sexual identity or neuronal activity patterns).
The existence of complete synaptic wiring diagrams of the compact nervous system of male and hermaphrodite Caenorhabditis elegans nematodes raises questions about the molecular mechanisms by which individual neurons communicate with each other. C. elegans employs the main neurotransmitter systems that are used throughout the animal kingdom, including acetylcholine, glutamate, γ-aminobutyric acid (GABA), and several monoamines (Sulston et al., 1975; Horvitz et al., 1982; Loer and Kenyon, 1993; McIntire et al., 1993; Duerr et al., 1999; Lee et al., 1999; Duerr et al., 2001; Alkema et al., 2005; Duerr et al., 2008; Serrano-Saiz et al., 2013; Pereira et al., 2015; Gendrel et al., 2016; Serrano-Saiz et al., 2017b; Figure 1A). Efforts to map these neurotransmitter systems to individual cell types throughout the entire nervous system have a long history, beginning with the use of chemical stains that directly detected a given neurotransmitter (dopamine) (Sulston et al., 1975), followed by antibody staining of neurotransmitter themselves (serotonin and GABA) (Horvitz et al., 1982; McIntire et al., 1993) or antibody stains of biosynthetic enzymes or neurotransmitter vesicular transporters (acetylcholine and monoamines) (Loer and Kenyon, 1993; Duerr et al., 1999; Duerr et al., 2001; Alkema et al., 2005; Duerr et al., 2008; see Figure 1A for an overview of these enzymes and transporters).
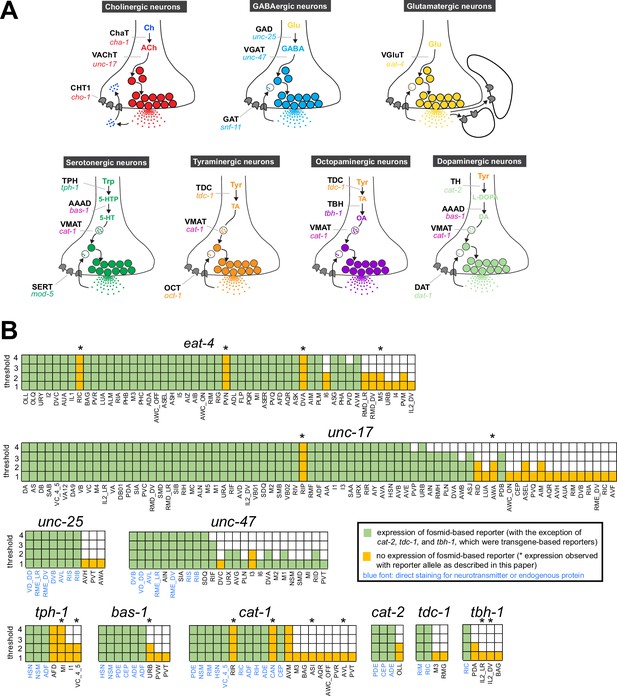
Background on genes examined in this paper.
(A) Neurotransmitter synthesis and transport pathways. TH = tyrosine hydroxylase; TDC = tyrosine decarboxylase; TBH = tyramine β-hydroxylase; TPH = tryptophan hydroxylase; GAD = glutamic acid decarboxylase; AAAD = aromatic amino acid decarboxylase; VMAT = vesicular monoamine transporter; VAChT = vesicular acetylcholine transporter; VGAT = vesicular γ-aminobutyric acid (GABA) transporter; Ch = choline; ACh = acetylcholine; TA = tyramine; OA = octopamine; DA = dopamine. CHT1 = choline uptake transporter; SERT = serotonin uptake transporter; OCT = organic cation transporter; DAT = dopamine uptake transporter; GAT = GABA uptake transporter. Taken and modified from Figure 6 of Hobert, 2013. (B) Graphic comparison of single-cell RNA (scRNA) expression data and previously reported reporter expression data. See Supplementary file 1 for a more comprehensive version that includes expression of reporter genes in cells that show no scRNA transcripts. Note that scRNA expression values for eat-4 and unc-47 can be unreliable because they were overexpressed to isolate individual neurons for scRNA analysis (Taylor et al., 2021).
While these early approaches proved successful in revealing neurotransmitter identities, they displayed several technical limitations. Since neurotransmitter-synthesizing or -transporting proteins primarily localize to neurites, the cellular identity of expressing cells (usually determined by assessing cell body position) often could not be unambiguously established in several, particularly cell- and neurite-dense regions of the nervous system. One example concerns cholinergic neurons, which are defined by the expression of the vesicular acetylcholine transporter UNC-17/VAChT and choline acetyltransferase CHA-1/ChAT. While mainly neurite-localized UNC-17 and CHA-1 antibody staining experiments could identify a subset of cholinergic neurons (Duerr et al., 2001; Duerr et al., 2008), many remained unidentified (Pereira et al., 2015). In addition, for GABA-producing neurons, it became apparent that antibody-based GABA detection was dependent on staining protocols, leading to the identification of ‘novel’ anti-GABA-positive neurons, i.e., GABAergic neurons, more than 20 years after the initial description of GABAergic neurons (McIntire et al., 1993; Gendrel et al., 2016).
An alternative approach to mapping neurotransmitter usage has been the use of reporter transgenes. This approach has the significant advantage of allowing the fluorophore to either fill the entire cytoplasm of a cell or to be targeted to the nucleus, thereby facilitating neuron identification. However, one shortcoming of transgene-based reporter approaches is that one cannot be certain that a chosen genomic region, fused to a reporter gene, indeed contains all cis-regulatory elements of the respective locus. In fact, the first report that described the expression of the vesicular glutamate transporter EAT-4, the key marker for glutamatergic neuron identity, largely underestimated the number of eat-4/VLGUT-positive and, hence, glutamatergic neurons (Lee et al., 1999). The introduction of fosmid-based reporter transgenes has largely addressed such concerns, as these reporters, with their 30–50 Kb size, usually cover entire intergenic regions (Sarov et al., 2012). Indeed, such fosmid-based reporters have been instrumental in describing the supposedly complete C. elegans glutamatergic nervous system, defined by the expression of eat-4/VGLUT (Serrano-Saiz et al., 2013), as well as the supposedly complete set of cholinergic (Pereira et al., 2015) and GABAergic neurons (Gendrel et al., 2016).
However, even fosmid-based reporters may not be the final word. In theory, they may still miss distal cis-regulatory elements. Moreover, the multicopy nature of transgenes harbors the risk of overexpression artifacts, such as the titrating of rate-limiting negative regulatory mechanisms. Also, RNAi-based silencing mechanisms triggered by the multicopy nature of transgenic reporter arrays have the potential to dampen the expression of reporter arrays (Nance and Frokjaer-Jensen, 2019). One way to get around these limitations, while still preserving the advantages of reporter gene approaches, is to generate reporter alleles in which an endogenous locus is tagged with a reporter cassette, using CRISPR/Cas9 genome engineering. Side-by-side comparisons of fosmid-based reporter expression patterns with those of knock-in reporter alleles indeed revealed several instances of discrepancies in expression patterns of homeobox genes (Reilly et al., 2022).
An indication that previous neurotransmitter assignments may not have been complete was provided by recent single-cell RNA (scRNA) transcriptomic analyses of the hermaphrodite nervous system of L4 stage animals by the CeNGEN consortium (Taylor et al., 2021). As we describe in this paper in more detail, transcripts for several neurotransmitter-synthesizing enzymes or transporters were detected in a few cells beyond those previously described to express the respective reporter genes. This motivated us to use CRISPR/Cas9 engineering to fluorescently tag a comprehensive panel of genetic loci that code for neurotransmitter-synthesizing, -transporting, and -uptaking proteins (‘neurotransmitter pathway genes’). Using the landmark strain NeuroPAL for neuron identification (Yemini et al., 2021), we identified novel sites of expression of most neurotransmitter pathway genes. Furthermore, we used these reagents to expand and refine neurotransmitter maps of the entire nervous system of the C. elegans male, which contains almost 30% more neurons than the nervous system of the hermaphrodite yet lacks a reported scRNA transcriptome atlas. Together with the NeuroPAL cell-identification tool, these reporter alleles allowed us to substantially improve the previously described neurotransmitter map of the male nervous system (Serrano-Saiz et al., 2017b). Our analysis provides insights into the breadth of usage of each individual neurotransmitter system, reveals instances of co-transmitter use, indicates the existence of neurons that may entirely rely on neuropeptides instead of classic neurotransmitters, reveals sexual dimorphisms in neurotransmitter usage, and suggests the likely existence of presently unknown neurotransmitters.
Results
Comparing CeNGEN scRNA data to reporter gene data
To investigate the neurotransmitter identity of neurons throughout the entire C. elegans nervous system of both sexes, we consider here the expression pattern of the following 15 genetic loci (see also Figure 1A):
eat-4/VGLUT: expression of the vesicular glutamate transporter is alone sufficient to define glutamatergic neuron identity (Lee et al., 1999; Serrano-Saiz et al., 2013).
unc-17/VAChT: expression of the vesicular acetylcholine transporter, located in an operon together with the acetylcholine-synthesizing gene cha-1/ChAT (Alfonso et al., 1994), defines cholinergic neurons (Duerr et al., 2001; Duerr et al., 2008; Pereira et al., 2015).
unc-25/GAD, unc-47/VGAT, and its sorting co-factor unc-46/LAMP: expression of these three genes defines neurons that synthesize and release GABA (McIntire et al., 1993; McIntire et al., 1997; Jin et al., 1999; Schuske et al., 2007; Gendrel et al., 2016). Additional neurons that we classify as GABAergic are those that do not synthesize GABA (unc-25/GAD-negative), but take up GABA from other neurons (based on anti-GABA antibody staining) and are expected to release GABA based on unc-47/VGAT expression (Gendrel et al., 2016). unc-47/VGAT expression without any evidence of GABA synthesis or uptake (unc-25/GAD- and anti-GABA-negative) is indicative of an unknown transmitter being present in these cells and utilizing unc-47/VGAT for vesicular secretion.
tph-1/TPH and bas-1/AAAD: the co-expression of these two biosynthetic enzymes, together with the co-expression of the monoamine vesicular transporter cat-1/VMAT, defines all serotonin-synthesizing and -releasing neurons (Figure 1A; Horvitz et al., 1982; Duerr et al., 1999; Sze et al., 2000; Hare and Loer, 2004).
cat-2/TH and bas-1/AAAD: the co-expression of these two biosynthetic enzymes, together with the co-expression of the monoamine vesicular transporter cat-1/VMAT, defines all dopamine-synthesizing and -releasing neurons (Figure 1A; Sulston et al., 1975; Duerr et al., 1999; Lints and Emmons, 1999; Hare and Loer, 2004).
tdc-1/TDC: defines, together with cat-1/VMAT, all tyramine-synthesizing and -releasing neurons (Figure 1A; Alkema et al., 2005).
tbh-1/TBH: expression of this gene, in combination with that of tdc-1/TDC and cat-1/VMAT, defines octopamine-synthesizing and -releasing neurons (Figure 1A; Alkema et al., 2005).
cat-1/VMAT: expression of this vesicular monoamine transporter defines all four above-mentioned monoaminergic neurons (serotonin, dopamine, tyramine, octopamine) (Duerr et al., 1999), but as described and discussed below, it may also define additional sets of monoaminergic neurons.
hdl-1/AAAD: hdl-1, a previously uncharacterized gene, encodes the only other AAAD with sequence similarity to the bas-1 and tdc-1 AAAD enzymes that produce other bona fide monoamines (Figure 1—figure supplement 1; Hare and Loer, 2004). hdl-1 expression may therefore, in combination with cat-1/VMAT, identify neurons that produce and release trace amines of unknown identity.
snf-3/BGT1/SLC6A12: this gene encodes the functionally validated ortholog of the vertebrate betaine uptake transporter SLC6A12 (i.e. BGT1) (Peden et al., 2013). In combination with the expression of cat-1/VMAT, which synaptically transports betaine (Hardege et al., 2022), snf-3 expression may identify neurons that utilize betaine as a synaptically released neurotransmitter to gate betaine-gated ion channels, such as ACR-23 (Peden et al., 2013) or LGC-41 (Hardege et al., 2022).
mod-5/SERT: this gene codes for the functionally validated ortholog of the vertebrate serotonin uptake transporter SERT (Ranganathan et al., 2001), which defines neurons that take up serotonin independently of their ability to synthesize serotonin and, depending on their expression of cat-1/VMAT, may either re-utilize serotonin for synaptic signaling or serve as serotonin clearance neurons.
oct-1/OCT: this gene encodes the closest representative of the OCT subclass of SLC22 organic cation transporters (Zhu et al., 2015), several members of which are selective uptake transporters of tyramine (Breidert et al., 1998; Berry et al., 2016). Its expression or function in the nervous system had not previously been analyzed in C. elegans.
For all these 15 genetic loci, we compared scRNA transcriptome data from the CeNGEN scRNA atlas (at all four available stringency levels; Taylor et al., 2021) to previously published reporter and antibody staining data. As shown in Figure 1B and Supplementary file 1, such comparisons reveal the following: (a) scRNA data support the expression of genes in the vast majority of neurons in which those genes were found to be expressed with previous reporter gene approaches. In most cases, this is true even at the highest threshold levels for scRNA detection. (b) Vice versa, reporter gene expression supports scRNA transcriptome data for a specific neurotransmitter system in the great majority of cells. (c) In spite of this congruence, there were several discrepancies between reporter data and scRNA data. Generally, while valuable, scRNA transcriptome data cannot be considered the final word for any gene expression pattern assignments. Lack of detection of transcripts could be a sensitivity issue and, conversely, the presence of transcripts does not necessarily indicate that the respective protein is generated, due to the possibility of posttranscriptional regulation.
Hence, to consolidate and further improve neurotransmitter identity assignment throughout the entire C. elegans nervous system, and to circumvent potential limitations of multicopy, fosmid-based reporter transgenes on which previous neurotransmitter assignments have been based, we engineered and examined expression patterns of 16 knock-in reporter alleles of the 15 neurotransmitter synthesis, vesicular transport, and uptake loci listed above (Figure 1, Figure 2). For unc-17 and eat-4, we knocked-in a t2a::gfp::h2b (his-44) cassette right before the stop codon of the respective gene. For unc-25, we created two knock-in alleles with the t2a::gfp::h2b (his-44) cassette tagging isoforms a.1/c.1 and b.1 separately. For tdc-1, a gfp::h2b::t2a cassette was knocked into the N-terminus of the locus because of different C-terminal splice variants. The self-cleaving T2A peptide frees up GFP::H2B, which will be transported to the nucleus, thereby facilitating cell identification. For unc-46, unc-47, tph-1, bas-1, tbh-1, cat-1, cat-2, snf-3, and oct-1, we knocked-in a sl2::gfp::h2b cassette at the C-terminus of the locus. The SL2 sequence also provides for the separate production of GFP::H2B. Both types of reporter cassettes should capture posttranscriptional, 3’UTR-mediated regulation of each locus, e.g., by miRNAs and RNA-binding proteins (not captured by CeNGEN scRNA data). Since in each case the reporter is targeted to the nucleus, this strategy circumvents shortcomings associated with interpreting antibody staining patterns or dealing with too densely packed cytosolic signals. For mod-5, we analyzed a previously generated, non-nuclear reporter allele (Maicas et al., 2021). For all our neuronal cell identification, we utilized the neuronal landmark strain NeuroPAL (Tekieli et al., 2021; Yemini et al., 2021). The results of our neuronal expression pattern analysis are summarized in Figure 3 and detailed in Supplementary files 2 and 3. In the ensuing sections we describe these patterns in detail.
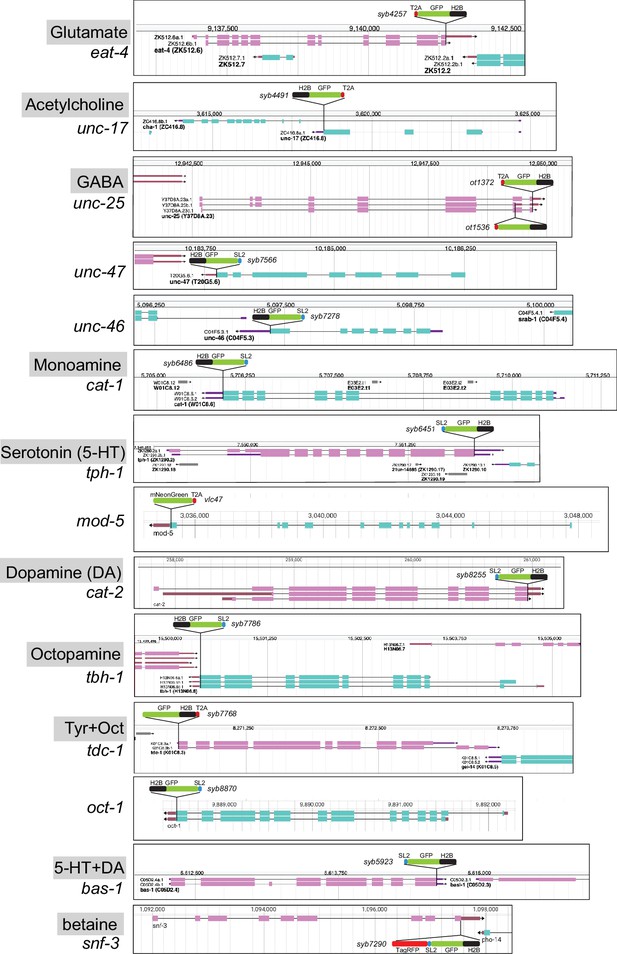
Schematics of reporter knock-in alleles.
Reporter alleles were generated by CRISPR/Cas9 genome engineering. The SL2- or T2A-based separation of the reporter from the coding sequence of the respective loci enables targeting of the reporter to the nucleus (via the H2B tag), which in turn facilitates the identification of the cell expressing a given reporter. Genome schematics are from WormBase (Davis et al., 2022). See Figure 1—figure supplement 1 for hdl-1 reporter alleles.
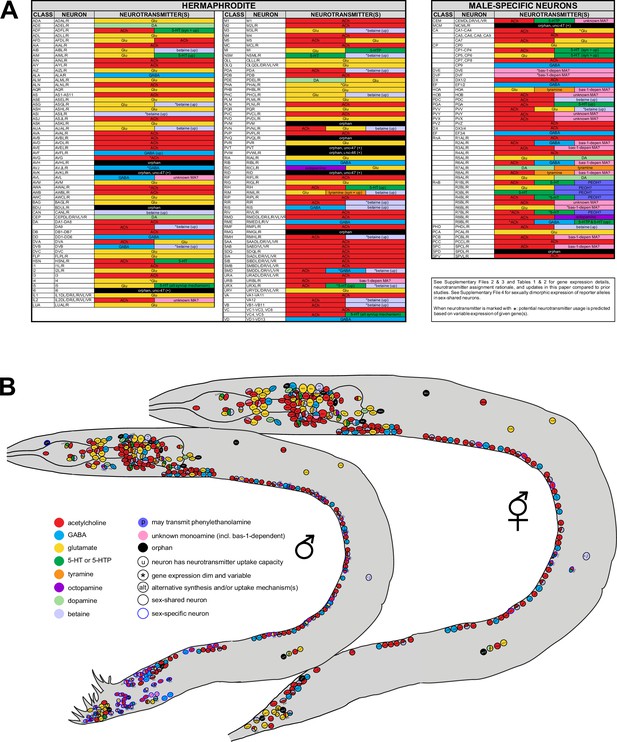
Summary of neurotransmitter usage and atlases.
See Table 1, Table 2, and Supplementary files 2–4 for individual gene expression, rationale for neurotransmitter assignments, and more detailed notes. (A) ACh=acetylcholine; Glu=glutamate; GABA=γ-aminobutyric acid; DA=dopamine; 5-HT=5-hydroxytryptamine, or serotonin; 5-HTP=5-hydroxytryptophan; PEOH?=the neuron has the potential to use β-hydroxyphenethylamine, or phenylethanolamine; bas-1-depen MA?=the neuron has the potential to use bas-1-dependent unknown monoamines (histamine, tryptamine, phenylethylamine [PEA]; also see Figure 1—figure supplement 1); unknown MA?=the neuron has the potential to use non-canonical monoamines; (up)=neurotransmitter uptake; (syn)=neurotransmitter synthesis; *=dim and variable expression of respective identity gene(s) is detected. Variability could be due to one of the following reasons: (1) the endogenous gene is indeed expressed in some but not all animals; (2) the endogenous gene is indeed expressed in every animal but the level of reporter expression is below detection threshold in some. Variability is detected only at low fluorescent intensity; at higher intensities, expression remains consistent. Results for anti-γ-aminobutyric acid (GABA) staining in SMD and anti-serotonin staining in VC4, VC5, CEM, I5, and URX are variable based on previous reports (see text for citations). (B) Information from (A) shown in the context of neuron positions in worm schematics. Note ‘unknown monoamine’ here includes both ‘bas-1-depen MA’ and ‘unknown MA’ in (A). Neurons marked with ‘u’ can uptake given neurotransmitters but not exclusively; some may also synthesize them, e.g., ADF can both synthesize and uptake serotonin.
Expression of a reporter allele of eat-4/VGLUT, a marker for glutamatergic identity, in the hermaphrodite
37 of the 38 previously reported neuron classes that express an eat-4 fosmid-based reporter (Serrano-Saiz et al., 2013) showed eat-4 transcripts in the CeNGEN scRNA atlas (Taylor et al., 2021) at all four thresholds of stringency, and 1/38 (PVD neuron) showed it in three out of the four threshold levels (Figure 1B, Supplementary file 1). However, scRNA transcripts were detected at all four threshold levels in three additional neuron classes, RIC, PVN, and DVA, for which no previous reporter data provided support. In a recent publication, we had already described that the eat-4 reporter allele syb4257 is expressed in RIC (Reilly et al., 2022) (confirmed in Figure 4A). We now also confirm expression of this reporter allele, albeit at low levels, in DVA and PVN (Figure 4B, Supplementary file 2).
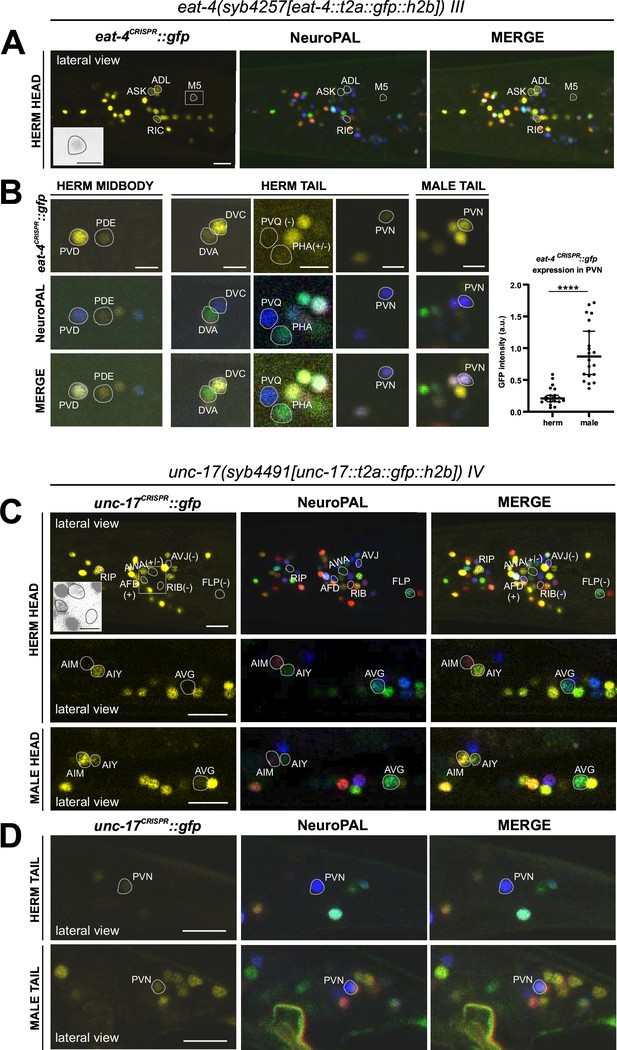
Expression of eat-4/VGLUT and unc-17/VAChT reporter alleles in the adult hermaphrodite.
Neuronal expression of eat-4(syb4257) and unc-17(syb4491) was characterized with landmark strain NeuroPAL (otIs696 and otIs669, respectively). Only selected neurons are shown for illustrating updates from previous reports. See Supplementary file 2 for a complete list of neurons. (A) Dim expression of eat-4(syb4257) in head neurons ASK and ADL is consistent with previous fosmid-based reporter expression. RIC expression is consistent with previous observation using the same reporter allele (Reilly et al., 2022). In addition, dim expression is detected in pharyngeal neuron M5 (also in grayscale inset), previously not detected with eat-4 GFP fosmid-based reporter (otIs388) but visible with eat-4 mCherry fosmid-based reporter (otIs518). (B) Previously uncharacterized eat-4 expression in PDE and DVA neurons is detected with the eat-4(syb4257) reporter allele. Variable expression in PHA is also occasionally detected. No expression is detected in PVQ. Expression in PVN is detected in both sexes but at a much higher level in the male. (C) In the head, prominent expression of unc-17(syb4491) in RIP and dim expression in AWA and AFD neurons are detected. There is no visible expression in RIB, FLP, or AVJ. Consistent with previous reports, AIM expresses unc-17 only in males and not hermaphrodites. In addition, very dim expression of AVG can be detected occasionally in hermaphrodites (representative image showing an animal with no visible expression) and slightly stronger in males (representative image showing an animal with visible expression). Inset, grayscale image showing dim expression for AWA and AFD and no expression for RIB. (D) In the tail, PVN expresses unc-17(syb4491) in both sexes, consistent with previous reports. Scale bars, 10 μm in color images in A, C, and D; 5 μm in B and all grayscale images. Quantification in B is done by normalizing fluorescent intensity of eat-4 GFP to that of the blue channel in the NeuroPAL background. Statistics, Mann-Whitney test.
Another neuron found to have some eat-4 transcripts, but only with the two lower threshold sets, is the I6 pharyngeal neuron. Consistent with our previous fosmid-based reporter data, we detected no I6 expression with our eat-4(syb4257) reporter allele. The eat-4 reporter allele also shows expression in the pharyngeal neuron M5, albeit very weakly (Figure 4A, Supplementary file 2), consistent with CeNGEN scRNA data. Weak expression of the eat-4 fosmid-based reporter in ASK and ADL remained weak, but clearly detectable with the eat-4(syb4257) reporter allele (Figure 4A, Supplementary file 2). Extremely dim expression in PHA can be occasionally detected. Whereas the PVQ neuron class displays eat-4 scRNA transcripts and was reported to show very dim eat-4 fosmid-based reporter expression, we detected no expression of the eat-4(syb4257) reporter allele in PVQ neurons (Figure 4B, Supplementary file 2). We also did not detect expression of eat-4(syb4257) in the GABAergic AVL and DVB neurons, in which a recent report describes expression of an eat-4 promoter fusion reporter (Li et al., 2023). An absence of eat-4(syb4257) expression in AVL and DVB is also consistent with the absence of scRNA transcripts in these neurons.
A few neurons were found to express eat-4 transcripts by the CeNGEN atlas, but only with lower threshold levels, including, for example, the RMD, PVM, and I4 neurons (Figure 1B, Supplementary file 1). We failed to detect reporter allele expression in RMD or PVM neurons, but occasionally observed very dim expression in I4. Lastly, we identified a novel site of eat-4 expression in the dopaminergic PDE neuron (Figure 4B, Supplementary file 2). While such expression was neither detected with previous reporters nor scRNA transcripts, we detected it very consistently but at relatively low levels.
Expression of a reporter allele of unc-17/VAChT, a marker for cholinergic identity, in the hermaphrodite
41 of previously described 52 neuron classes that show unc-17 fosmid-based reporter expression (Pereira et al., 2015) showed transcripts in the CeNGEN scRNA atlas at four out of four threshold levels, another seven neuron classes at three out of four threshold levels, and one at the lowest two threshold levels (Taylor et al., 2021). Only one neuron class, RIP, displayed scRNA levels at all four thresholds, but showed no corresponding unc-17 fosmid-based reporter expression (Figure 1B, Supplementary file 1). Using the unc-17(syb4491) reporter allele (Figure 1A), we confirmed expression in RIP (Figure 4C, Supplementary file 2). Of the additional neuron classes that show unc-17 expression at the lower stringency transcript detection levels (Figure 1B, Supplementary file 1), we were able to detect unc-17 reporter allele expression only in AWA (Figure 4C, Supplementary file 2).
Conversely, a few neurons display weak expression with previous multicopy, fosmid-based reporter constructs (RIB, AVG, PVN) (Pereira et al., 2015), but show no CeNGEN scRNA support for such expression (Taylor et al., 2021). The unc-17(syb4491) reporter allele confirmed weak but consistent expression in the PVN neurons as well as variable, borderline expression in AVG (Figure 4C and D). However, we failed to detect unc-17(syb4491) reporter allele expression in the RIB neurons.
We detected another novel site of unc-17 expression, albeit dim, in the glutamatergic AFD neurons (Figure 4C, Supplementary file 2). This expression was not reported with previous fosmid-based reporter or CeNGEN scRNA data. Consistent with AFD and PVN being potentially cholinergic, scRNA transcript reads for cha-1/ChAT, the ACh-synthesizing choline acetyltransferase, were also detected in AFD and PVN (Supplementary file 1).
Lastly, another notable observation is the lack of any unc-17 reporter expression or CeNGEN scRNA transcripts in the interneuron AVJ, but presence of CeNGEN scRNA transcript reads for cha-1/ChAT (Supplementary file 1), which shares exons with the unc-17/VAChT locus (Alfonso et al., 1994). Although no reporter data is available for cha-1/ChAT, such interesting mismatch between available unc-17 and cha-1/ChAT expression data could provide a hint to potential non-vesicular cholinergic transmission in the AVJ neurons in C. elegans, potentially akin to reportedly non-vesicular release of acetylcholine in the visual system of Drosophila (Yang and Kunes, 2004).
Expression of reporter alleles for GABAergic pathway genes in the hermaphrodite
Expression of unc-25/GAD
The most recent analysis of GABAergic neurons identified GABA-synthesizing cells by anti-GABA staining and an SL2-based unc-25/GAD reporter allele that monitors expression of the rate-limiting step of GABA synthesis, generated by CRISPR/Cas9 engineering (Gendrel et al., 2016). The CeNGEN scRNA atlas shows robust support for these assignments at all four threshold levels (Figure 1B, Supplementary file 1). unc-25 scRNA signals (but no reporter signals) were detected at several orders of magnitude lower levels in three additional neuron classes (AWA, AVH, PVT), but only with the least robust threshold level.
In this study we generated another unc-25/GAD reporter allele, using a t2a::gfp::h2b cassette (ot1372) (Figure 2). This allele showed the same expression pattern as the previously described SL2-based unc-25(ot867) reporter allele (Figure 5A, Supplementary file 2). This includes a lack of expression in a number of neurons that stain with anti-GABA antibodies (SMD, AVA, AVB, AVJ, ALA, and AVF) and GLR glia, corroborating the notion that these neurons and glia take up GABA from other cells (indeed, a subset of those cells do express the GABA uptake reporter SNF-11; Gendrel et al., 2016).
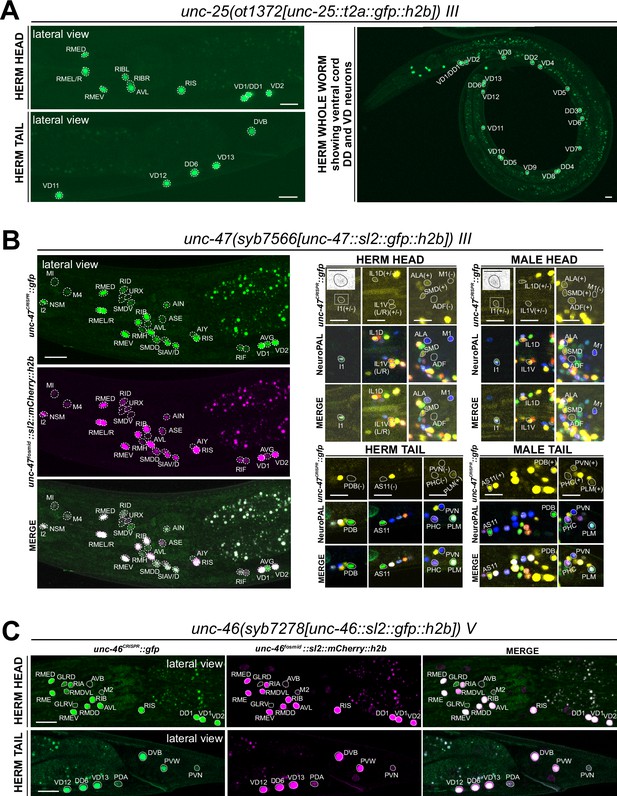
Expression of GABA pathway genes in the adult hermaphrodite.
(A) Expression of the unc-25/GAD reporter allele ot1372 is detected in the head, ventral nerve cord, and tail neurons. The expression pattern of this new T2A-based reporter allele is similar to that of a previously described SL2-based reporter allele, unc-25(ot867) (Gendrel et al., 2016). (B) Expression of unc-47/VGAT reporter allele syb7566. Left, the expression pattern of the reporter allele largely matches that of a previously described unc-47 mCherry fosmid-based reporter (otIs564) in the head. Right, a close-up view for the characterization of the reporter allele expression with landmark strain NeuroPAL (otIs669). In the head, consistent with previous reports of the unc-47 fosmid-based reporter (otIs564), dim expression of unc-47(syb7566) in SMD, ALA, and very dim and variable expression in IL1 is detected in both sexes, and unc-47(syb7566) is expressed in ADF only in the male and not hermaphrodite. In addition, the reporter allele is also expressed at a very dim level in the pharyngeal neuron I1 (also in inset) whereas no expression is detected in M1. In the tail, consistent with previous reports of the fosmid, sexually dimorphic expression of the unc-47(syb7566) reporter allele is also detected in PDB, AS11, PVN, and PHC only in the male and not the hermaphrodite. In addition, we also detected very dim expression of PLM in both sexes, confirming potential dim expression of the unc-47 mCherry fosmid-based reporter that was only readily visible after anti-mCherry staining in the past (Serrano-Saiz et al., 2017b). Scale bars, 5 μm for insets and 10 μm for all other images. (C) Expression of unc-46/LAMP reporter allele syb7278 is largely similar to that of the previously described unc-46/LAMP mCherry fosmid-based reporter (otIs568). We also observed expression of both the reporter allele and fosmid-based reporter in PVW, PVN, and very dimly in PDA. Scale bars, 10 μm.
We carefully examined potential unc-25/GAD reporter allele expression in the AMsh glia, which were reported to generate GABA through unc-25/GAD (Duan et al., 2020; Fernandez-Abascal et al., 2022). We did not detect visible unc-25(ot867) or unc-25(ot1372) reporter allele expression in AMsh, consistent with the failure to directly detect GABA in AMsh through highly sensitive anti-GABA staining (Gendrel et al., 2016). Since these reporters do not capture an alternatively spliced isoform b.1 (https://www.wormbase.org), we generated another reporter allele, unc-25(ot1536), to specifically target this isoform. However, we did not observe any discernible fluorescent reporter expression from this allele. Hence, it is unlikely that an alternative isoform could contribute to expression in additional cell types.
Expression of unc-47/VGAT
While promoter-based transgenes for the vesicular transporter for GABA, unc-47/VGAT, had shown expression patterns that precisely match that of unc-25/GAD (Eastman et al., 1999), we had noted in our previous analysis of the GABA system that a fosmid-based reporter showed much broader expression in many additional neuron classes that showed no sign of GABA usage (Gendrel et al., 2016). In several of these neuron classes both the fosmid-based reporter and the CeNGEN scRNA data indicate very robust expression (e.g. AIN, SIA, SDQ), while in many others scRNA transcripts are only evident at looser thresholds and, correspondingly, fosmid-based reporter expression in these cells is often weak (Supplementary file 1; Gendrel et al., 2016). To investigate this matter further, we CRISPR/Cas9-engineered a gfp-based reporter allele for unc-47, syb7566, and first crossed it with an mCherry-based unc-47 fosmid-based reporter (otIs564) as a first-pass assessment for any obvious overlaps and mismatches of expression patterns between the two (Figure 5B, left side panels). The vast majority of neurons exhibited overlapping expression between syb7566 and otIs564. There were also many notable similarities in the robustness of expression of the fosmid-based reporter and the reporter allele (Supplementary file 1). In a few cases where the fosmid-based reporter expression was so dim that it is only detectable via antibody staining against its fluorophore (mCherry) (Gendrel et al., 2016; Serrano-Saiz et al., 2017b), the reporter allele expression was readily visible (Supplementary file 1).
The very few mismatches of expression of the fosmid-based reporter and the reporter allele included the pharyngeal neuron M1, which expresses no visible unc-47(syb7566) reporter allele but weak fosmid-based reporter expression, and the pharyngeal neuron I1, which expresses dim syb7566 but no fosmid-based reporter (Figure 5B, right side panels). AVJ shows very dim and variable unc-47(syb7566) reporter allele expression but no fosmid-based reporter expression. Since AVJ stains with anti-GABA antibodies (Gendrel et al., 2016), this neuron likely engages in vesicular relase of GABA, even though its source of GABA remains unclear since it neither expresses conventional GABA synthesis machinery (UNC-25/GAD) nor GABA uptake machinery (SNF-11). Other neurons previously shown to stain with anti-GABA antibodies and to express the unc-47 fosmid-based reporter (ALA and SMD) (Gendrel et al., 2016) still show expression of the unc-47 reporter allele.
In addition, while the reporter allele of unc-47/VGAT, in conjunction with CeNGEN scRNA data, corroborates the notion that unc-47/VGAT is expressed in all GABA-synthesizing and most GABA uptake neurons, there is a substantial number of unc-47-positive neurons that do not show any evidence of GABA presence. This suggests that UNC-47/VGAT may transport another unidentified neurotransmitter (see Discussion) (Gendrel et al., 2016).
Expression of unc-46/LAMP
In all GABA-synthesizing neurons, the UNC-47/VGAT protein requires the LAMP-like protein UNC-46 for proper localization (Schuske et al., 2007). A previously analyzed fosmid-based reporter confirmed unc-46/LAMP expression in all ‘classic’ GABAergic neurons (i.e. anti-GABA and unc-25/GAD-positive neurons), but also showed robust expression in GABA- and unc-47-negative neurons, such as RMD (Gendrel et al., 2016). This non-GABAergic neuron expression is confirmed by CeNGEN scRNA data (Taylor et al., 2021; Supplementary file 1). We generated an unc-46/LAMP reporter allele, syb7278, and found its expression to be largely similar to that of the fosmid-based reporter and to the scRNA data (Figure 5C, Supplementary file 1), therefore corroborating the non-GABAergic neuron expression of unc-46/LAMP. We also detected previously unreported expression in the PVW and PVN neurons in both the reporter allele and fosmid-based reporter (Figure 5C), thereby further corroborating CeNGEN data. In addition, we also detected very dim expression in PDA (Figure 5C), which shows no scRNA transcript reads (Supplementary file 1). With one exception (pharyngeal M2 neuron class), the sites of non-GABAergic neuron expression of unc-46/LAMP expression do not show any overlap with the sites of unc-47/VGAT expression, indicating that these two proteins have functions independent of each other.
Expression of reporter alleles for serotonin biosynthetic enzymes, tph-1/TPH and bas-1/AAAD, in the hermaphrodite
tph-1/TPH and bas-1/AAAD code for enzymes required for serotonin (5-HT = 5-hydroxytryptamine) synthesis (Figure 1A). scRNA transcripts for tph-1 and bas-1 are detected in previously defined serotonergic neurons at all four threshold levels (HSN, NSM, ADF) (Figure 1, Supplementary file 1). In addition to these well-characterized sites of expression, several of the individual genes show scRNA-based transcripts in a few additional cells: tph-1 at all four threshold levels in AFD and MI. Neither of these cells display scRNA transcripts for bas-1/AAAD, the enzyme that metabolizes the TPH-1 product 5-HTP (5-hydroxytryptophan) into serotonin (5-HT) (Figure 1A). To further investigate these observations, we generated reporter alleles for both tph-1 and bas-1 (Figure 2). Expression of the tph-1 reporter allele syb6451 confirmed expression in the previously well-described neurons that stained positive for serotonin, namely NSM, HSN, and ADF, matching CeNGEN data. While expression in AFD (seen at all four threshold levels in the CeNGEN scRNA atlas) could not be confirmed with the reporter allele, expression in the pharyngeal MI neurons could be confirmed (Figure 6A, Figure 6—figure supplement 1, Supplementary file 2).
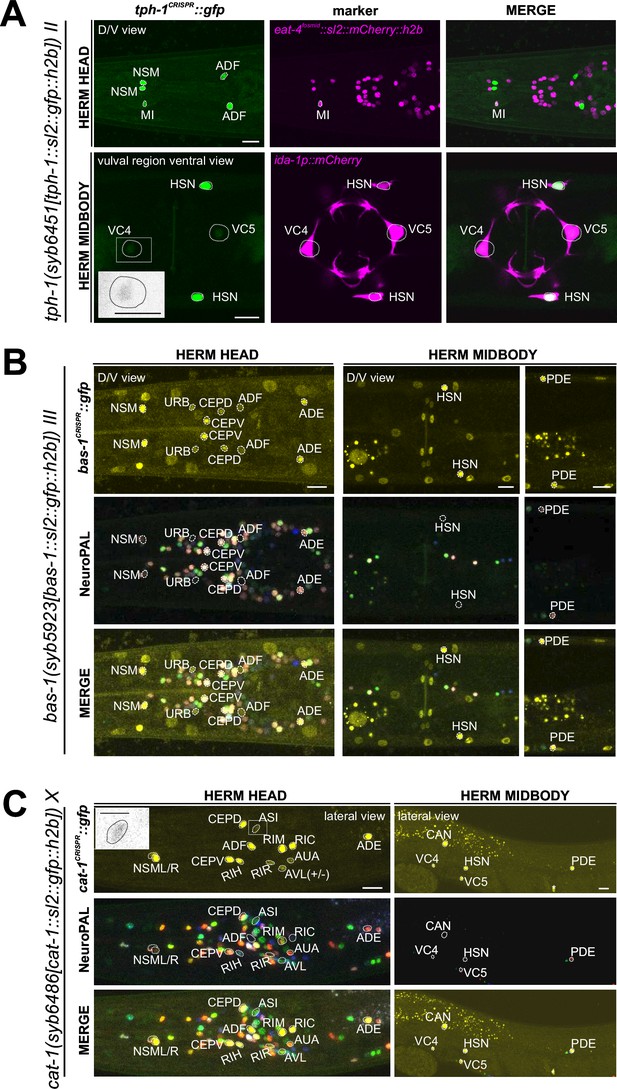
Expression of tph-1/TPH, bas-1/AAAD, and cat-1/VMAT reporter alleles in the adult hermaphrodite.
(A) Dorsoventral view of a hermaphrodite head and midbody expressing tph-1(syb6451). tph-1 expression is detected robustly in the MI neuron and dimly and variably in VC4 and VC5. Neuron identities for MI and VC4 and VC5 were validated using otIs518[eat-4(fosmid)::sl2::mCherry::h2b] and vsls269[ida-1::mCherry], respectively, as landmarks. Inset, grayscale image highlighting dim expression in VC4. Larval expression of this reporter allele is shown in Figure 6—figure supplement 1. (B) Neuronal expression of bas-1(syb5923) characterized with the landmark NeuroPAL (otIs669) strain in the head and midbody regions of young adult hermaphrodites. Dorsoventral view of the adult head shows bas-1/AAAD expression in left-right neuron pairs, including previously reported expression in NSM, CEP, ADF, and ADE (Hare and Loer, 2004). Additionally, we observed previously unreported expression in the URB neurons. Non-neuronal bas-1/AAAD expression is detected in other non-neuronal cell types as reported previously (Yu et al., 2023; also see Figure 14—figure supplement 1, Figure 14). (C) Lateral views of young adult hermaphrodite head and midbody expressing cat-1/VMAT (syb6486). Previously unreported cat-1/VMAT expression is seen in RIR, CAN, AUA, ASI (also in inset), and variably, AVL. Non-neuronal expression of cat-1/VMAT is detected in a single midbody cell in the gonad (also see Figure 14—figure supplement 1), marked with an asterisk. Scale bars, 10 μm for all color images; 5 μm for the inset in grayscale.
We detected co-expression of the bas-1 reporter allele, syb5923, with tph-1(syb6451) in NSM, HSN, and ADF, in accordance with the previous reporter and scRNA data (Figure 6B, Supplementary file 2). However, bas-1(syb5923) is not co-expressed with tph-1 in MI (Figure 6A and B), nor is there CeNGEN-transcript evidence for bas-1/AAAD in MI (Figure 1, Supplementary file 1). Hence, TPH-1-synthesized 5-HTP in MI is not metabolized into 5-HT (serotonin), consistent with the lack of serotonin-antibody staining in MI (Horvitz et al., 1982; Sze et al., 2000).
We also detected tph-1(syb6451) reporter allele expression in the serotonergic VC4 and VC5 neurons (Figure 6A, Supplementary file 2), consistent with scRNA data (Figure 1, Supplementary file 1) and previous reporter transgene data (Mondal et al., 2018). This suggests that these neurons are capable of producing 5-HTP. However, there is no bas-1(syb5923) expression in VC4 or VC5, consistent with previous data showing that serotonin is taken up, but not synthesized by them (Duerr et al., 2001) (more below on monoamine uptake; Tables 1 and 2).
Neurons that uptake monoaminergic neurotransmitters.
+: presence of reporter allele expression; -: lack of visible reporter allele expression; +/-: dim and variable expression (variability is only detected when reporter fluorescent intensity is low); m: anti-serotonin staining observed in males; *: sex-specific neurons; **: variable/very dim antibody staining reported in previous publications. ***N/A=not presently applicable because betaine is provided by diet, in addition to possible endogenous synthesis. See text for citations.
| Uptake | Synthesis | Release | ||
|---|---|---|---|---|
| Serotonin | Neuron | mod-5 | tph-1 | cat-1 |
| ADF | + | + | + | |
| AIM | + | - | - | |
| I5** | - | - | +/- | |
| NSM | + | + | + | |
| PVW(m)** | - | - | - | |
| RIH | + | - | + | |
| URX** | +/- | - | - | |
| *HSN | - | + | + | |
| *VC4-5** | - | +/- | + | |
| *CEM** | + | + | - | |
| *CP1-6 | + | + | + | |
| *PGA | + | - | + | |
| *R1B | - | + | + | |
| *R3B | + | + | + | |
| *R9B | + | + | + | |
| Tyramine | Neuron | oct-1 | tdc-1 | cat-1 |
| RIM | + | + | + | |
| Betaine | Neuron | snf-3 | N/A*** | cat-1 |
| AUA | + | + | ||
| CAN | + | + | ||
| NSM | +/- | + | ||
| RIM | + | + | ||
| RIR | +/- | + | ||
| ASI | +/- | + | ||
| M3 | +/- | - | ||
| AIB | + | - | ||
| DVB | +/- | - | ||
| SMD | +/- | - | ||
| RIS | + | - | ||
| URX | +/- | - | ||
| PDA | +/- | - | ||
| ASG | +/- | - | ||
| DA9 | +/- | - | ||
| VB1-11 | +/- | - | ||
| PHC | + | - | ||
| PVN | + | - | ||
| VA12 | +/- | - | ||
| RMH | +/- | - | ||
| *PDC | + | + | ||
| *PHD | + | - | ||
| *PVV | +/- | - |
Categories of neuronal expression patterns for monoaminergic neurotransmitter pathway genes.
Criteria for monoaminergic neurotransmitter assignment and a summary for neurons with updated identities are presented here. The categories represent our best assessments based on available data; in every category there is a possibility for the existence of non-canonical synthesis and/or uptake mechanisms that are yet to be discovered. +: presence of reporter allele expression (incl. dim); -: lack of visible reporter allele expression; bas-1-dependent unknown monoamine?=bas-1-dependent unknown monoamine (histamine, tryptamine, PEA; see Figure 1—figure supplement 1A and Discussion); unknown monoamine?=potentially non-canonical monoamines; see Discussion and Results sections on specific gene expression patterns; 5-HT=5-hydroxytryptamine, or serotonin; 5-HTP=5-hydroxytryptophan; PEOH = β-hydroxyphenethylamine, or phenylethanolamine; *: The expression of tph-1 in VC4-5, bas-1 in R4B and R6B, cat-1 in AVL, and snf-3 in NSM, RIR, ASI, URX, M3, DVB, SMD, PDA, ASG, DA9, VA12, VB1-11, RMH, and PVV is dim and variable (this study; variability is only detected when reporter fluorescent intensity is low); anti-5-HT staining in VC4, VC5, CEM, I5, URX, and PVW (male) is variable in previous reports (see text for citations). ** indicates that R4B and R7B express 5-HT synthesis machinery (tph-1 and bas-1), but do not stain with 5-HT antibodies.
| Synthesis (and/or uptake) | cat-1 | tph-1 | cat-2 | bas-1 | tdc-1 | tbh-1 | mod-5 | snf-3 | oct-1 | Direct staining | Sex-specific neurons | Sex-shared neurons |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyramine+bas-1-dependent unknown monoamine? | + | - | - | + | + | - | - | - | - | HOA | ||
| Tyramine+bas-1-dependent unknown monoamine? | - | - | - | + | + | - | - | - | - | R8A | ||
| Tyramine+dopamine | + | - | + | + | + | - | - | - | - | Dopamine | R7A | |
| Tyramine (+uptake)+betaine (uptake) | + | - | - | - | + | - | - | + | + | RIM | ||
| bas-1-dependent unknown monoamine? | + | - | - | + | - | - | - | - | - | R2A | ||
| bas-1-dependent unknown monoamine? | - | - | - | + | - | - | - | - | - | R3A, R6A, R6B*, PCB, SPC, DVE, DVF | URB | |
| Octopamine | + | - | - | - | + | + | - | - | - | RIC | ||
| Octopamine | - | - | - | - | + | + | - | - | - | R8B | ||
| Dopamine | + | - | + | + | - | - | - | - | - | Dopamine | R5A, R9A | ADE, CEP, PDE |
| 5-HTP (synthesis)+5-HT (alternative synthesis/uptake mechanism?)+unknown monoamine? | - | + | - | - | - | + | - | - | - | 5-HT | CEM* | |
| 5-HTP | - | + | - | - | - | - | - | - | - | MI | ||
| PEOH? | - | - | - | + | - | + | - | - | - | R2B | ||
| 5-HT+PEOH? | - | + | - | + | - | + | - | - | - | R7B** | ||
| 5-HT+PEOH? | + | + | - | + | - | + | - | - | - | 5-HT | R1B | |
| 5-HT+PEOH? | + | + | - | + | - | + | + | - | - | 5-HT | R3B | |
| 5-HT+PEOH? | + | + | - | + | - | + | - | - | - | R4B** | ||
| 5-HT (uptake) | + | - | - | - | - | - | + | - | - | 5-HT | PGA | RIH |
| 5-HT (uptake) | - | - | - | - | - | - | + | - | - | 5-HT | AIM | |
| 5-HT (uptake)+betaine (uptake) | - | - | - | - | - | - | + | + | - | 5-HT | URX* | |
| 5-HT (& uptake) | + | + | - | + | - | - | + | - | - | 5-HT | CP1-6 | ADF |
| 5-HT (alternative synthesis/uptake mechanism?) | - | - | - | - | - | - | - | - | - | 5-HT | I5*, PVW (male only) | |
| 5-HT | + | + | - | + | - | - | - | - | - | 5-HT | HSN | |
| 5-HTP (synthesis) and 5-HT (uptake) | + | + | - | - | - | + | + | - | - | 5-HT | R9B | |
| 5-HTP (synthesis) and 5-HT (alternative synthesis/uptake mechanism?) | + | + | - | - | - | - | - | - | - | 5-HT | VC4-5* | |
| Unknown monoamine? | + | - | - | - | - | - | - | - | - | PVX, PVY | AVL* | |
| Unknown monoamine? | - | - | - | - | - | + | - | - | - | HOB, R5B | IL2 | |
| 5-HT+betaine (uptake) | + | + | - | + | - | - | - | + | - | 5-HT | NSM* | |
| Betaine (uptake) | + | - | - | - | - | - | - | + | - | PDC | AUA, CAN, RIR*, ASI* | |
| Betaine (uptake) | - | - | - | - | - | - | - | + | - | PHD, PVV* | M3*, AIB, DVB*, SMD*, RIS, PDA*, ASG*, DA9*, PHC, PVN, VA12*, VB1-11*, RMH* |
As expected from the role of bas-1/AAAD in dopamine synthesis (Hare and Loer, 2004), bas-1(syb5923) is also expressed in dopaminergic neurons PDE, CEP, and ADE. In addition, it is also expressed weakly in URB, consistent with scRNA data. We did not detect visible expression in PVW or PVT, both of which showed very low levels of scRNA transcripts (Figure 1, Supplementary file 1). Expression of bas-1/AAAD in URB may suggest that URB generates a non-canonical monoamine (e.g. tryptamine, phenylethylamine [PEA], or histamine), but since URB expresses no vesicular transporter (cat-1/VMAT, see below), we consider it unlikely that any such monoamine would be secreted via canonical vesicular synaptic release mechanisms.
Expression of a reporter allele of cat-2/TH, a dopaminergic marker, in the hermaphrodite
The CeNGEN scRNA atlas shows transcripts for the rate-limiting enzyme of dopamine synthesis encoded by cat-2/TH (Figure 1B, Supplementary file 1) at all four threshold levels in all three previously described dopaminergic neuron classes in the hermaphrodite, ADE, PDE, and CEP (Sulston et al., 1975; Sulston et al., 1980; Lints and Emmons, 1999). At lower threshold levels, transcripts can also be detected in the OLL neurons. A CRISPR/Cas9-engineered reporter allele for cat-2/TH, syb8255, confirmed expression in ADE, PDE, and CEP in adult hermaphrodites (Figure 7A, Supplementary file 2). As expected and described above, all three neuron classes also expressed bas-1/AAAD (Figure 6B) and cat-1/VMAT (Figure 6C, see below) (Supplementary file 2). We did not detect visible expression of cat-2(syb8255) in OLL. The OLL neurons also display no scRNA transcripts or reporter allele expression of bas-1/AAAD or cat-1/VMAT. No additional sites of expression of cat-2(syb8255) were detected in the adult hermaphrodite.
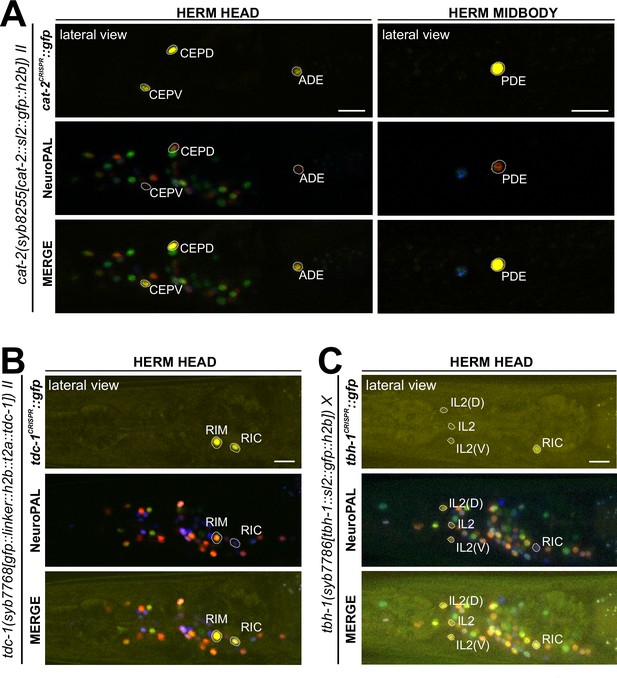
Expression of cat-2/TH, tdc-1/TDC, and tbh-1/TBH reporter alleles in the adult hermaphrodite.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). Lateral views of young adult hermaphrodites expressing reporter alleles for (A) cat-2(syb8255), (B) tbh-1(syb7786), and (C) tdc-1(syb7768). (A) cat-2/TH expression in CEP, ADE, and PDE match previously reported dopamine straining expression (Sulston et al., 1975). (B) and (C) Head areas are shown; no neuronal expression was detected in other areas. tdc-1 expression matches previous analysis (Alkema et al., 2005). We detected previously unreported expression of tbh-1 in all six IL2 neurons at low levels. Scale bars, 10 μm.
Expression of reporter alleles of tdc-1/TDC and tbh-1/TBH, markers for tyraminergic and octopaminergic neurons, in the hermaphrodite
The invertebrate analogs of adrenaline and noradrenaline, tyramine and octopamine, are generated by tdc-1 and tbh-1 (Figure 1A; Alkema et al., 2005). Previous work had identified expression of tdc-1 in the hermaphrodite RIM and RIC neurons and tbh-1 in the RIC neurons (Alkema et al., 2005). Transcripts in the CeNGEN atlas match those sites of expression for both tdc-1 (scRNA at four threshold levels in RIM and RIC neurons) and tbh-1 (scRNA at four threshold levels in RIC neurons) (Figure 1B, Supplementary file 1). Much lower transcript levels are present in a few additional, non-overlapping neurons (Figure 1B). CRISPR/Cas9-engineered reporter alleles confirmed tdc-1 expression in RIM and RIC and tbh-1 expression in RIC (Figure 7B and C, Supplementary file 2). In addition, we also detected dim expression of tbh-1(syb7786) in all six IL2 neurons, corroborating scRNA transcript data (Figure 7C, Supplementary files 1 and 2). However, IL2 neurons do not exhibit expression of the reporter allele of tdc-1, which acts upstream of tbh-1 in the octopamine synthesis pathway, or of cat-1/VMAT, the vesicular transporter for octopamine (Figure 6C, see below). Hence, the IL2 neurons are unlikely to produce or synaptically release octopamine, but they may produce another monoaminergic signal (Table 2).
Expression of a reporter allele of cat-1/VMAT, a marker for monoaminergic identity, in the hermaphrodite
As the vesicular monoamine transporter, cat-1/VMAT is expected to be expressed in all neurons that synthesize serotonin, dopamine, tyramine, and octopamine (Figure 1A). Both scRNA data and a CRISPR/Cas9-engineered reporter allele, syb6486, confirm expression in all these cells (Figure 6C, Supplementary file 2). In addition, based on antibody staining and previous fosmid-based reporters, cat-1/VMAT is known to be expressed in neurons that do not synthesize serotonin but are nevertheless positive for serotonin antibody staining (VC4, VC5, and RIH) (Duerr et al., 1999; Duerr et al., 2001; Serrano-Saiz et al., 2017b). Again, both scRNA data and a CRISPR/Cas9-engineered reporter allele, syb6486, confirm expression in these cells (Figure 6C, Supplementary file 2).
In addition to these canonical monoaminergic neurons, the CeNGEN scRNA data shows cat-1/VMAT expression at all four threshold levels in RIR, CAN, AVM and, at a much lower threshold, eight additional neuron classes (Figure 1B, Supplementary file 1). Our cat-1/VMAT reporter allele, syb6486, corroborates expression in RIR and CAN, but not in AVM (Figure 6C, Supplementary file 2). We also observed expression of the cat-1 reporter allele in two of the neuron classes with scRNA transcripts at the lowest threshold level, ASI and variably, AVL (Figure 6C, Supplementary file 1). Interestingly, AVL does not express any other monoaminergic pathway genes (Supplementary file 2), therefore it may be transporting a new amine yet to be discovered. This scenario also applies for two male-specific neurons (more below). As previously mentioned, we detected no cat-1/VMAT expression in the tph-1/TPH-positive MI or the cat-2/TH-positive OLL neurons.
The cat-1/VMAT reporter allele revealed expression in an additional neuron class, the AUA neuron pair (Figure 6C, Supplementary file 2). Expression in this neuron is not detected in scRNA data; however, such expression may be consistent with previous CAT-1/VMAT antibody staining data (Duerr et al., 1999). These authors found the same expression pattern as we detected with cat-1/VMAT reporter allele, except for the AIM neuron, which Duerr et al. identified as CAT-1/VMAT antibody-staining positive. However, neither our reporter allele, nor a fosmid-based cat-1/VMAT reporter, nor scRNA data showed expression in AIM, and we therefore think that the neurons identified by Duerr et al. as AIM may have been the AUA neurons instead (see also Serrano-Saiz et al., 2017b). Additionally, a cat-1-positive neuron pair in the ventral ganglion, unidentified but mentioned by Duerr et al., 1999, is likely the tyraminergic RIM neuron pair, based on our reporter allele and CeNGEN scRNA data.
Expression of reporter alleles of monoamine uptake transporters in the hermaphrodite
In addition to or in lieu of synthesizing monoamines, neurons can uptake them from their surroundings. To investigate the cellular sites of monoamine uptake in more detail, we analyzed fluorescent protein expression from engineered reporter alleles for the uptake transporter of serotonin (mod-5/SERT(vlc47)), the predicted uptake transporter for tyramine (oct-1/OCT(syb8870)), and that for betaine (snf-3/BGT1(syb7290)).
Serotonin/5-HT uptake
Using a promoter-based transgene and antibody staining, previous work had shown expression of the serotonin uptake transporter mod-5/SERT in NSM, ADF, RIH, and AIM (Jafari et al., 2011; Maicas et al., 2021). This matched the observations that RIH and AIM do not synthesize serotonin (i.e. do not express tph-1), but stain positive with a serotonin antibody (Jafari et al., 2011). In mod-5 mutants or wild type worms treated with serotonin reuptake inhibitors (such as the SSRI fluoxetine), RIH and AIM lose serotonin immunoreactivity (Jafari et al., 2011). We analyzed a CRISPR-based reporter allele, mod-5(vlc47) (Maicas et al., 2021), and confirmed expression in the four neuron classes NSM, ADF, RIH, and AIM (Figure 8). Because only NSM, ADF, and RIH, but not AIM, express the reporter allele of the monoamine transporter CAT-1/VMAT (Figure 6), AIM likely functions as a serotonin uptake/clearance neuron (Tables 1 and 2; see also Discussion). In addition, we also detected dim mod-5/SERT expression in the phasmid neuron class PHA and very dim, variable signals in URX (Figure 8A, B, E) consistent with scRNA data (Supplementary file 1). The results for anti-serotonin-staining from previous reports are variable in a few neurons, possibly due to differences in staining methods (including URX, I5, VC4, VC5, and PVW Loer and Kenyon, 1993; Rand and Nonet, 1997; Duerr et al., 1999; Serrano-Saiz et al., 2017b). In light of its mod-5/SERT reporter expression, URX may acquire serotonin via mod-5, akin to AIM (Tables 1 and 2).
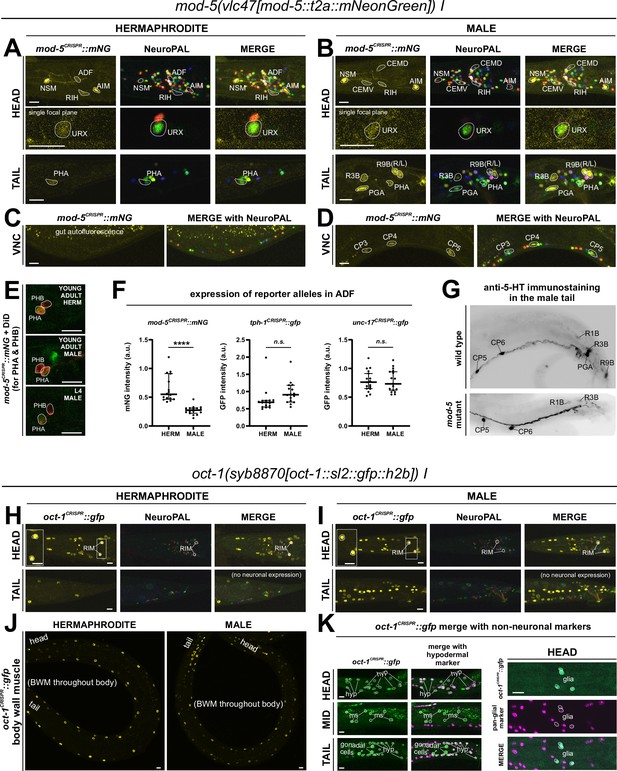
Expression of mod-5/SERT and oct-1/OCT reporter alleles in adult animals.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669) and DiD-filling. (A, C) In adult hermaphrodites, mod-5(vlc47) is expressed in sex-shared neurons NSM, ADF, RIH, AIM, consistent with previous reports (Jafari et al., 2011; Maicas et al., 2021). In addition, we also observed expression in the phasmid neuron PHA and dim and variable expression in URX. There is no visible expression in the ventral nerve cord (VNC). (B, D) In adult males, mod-5(vlc47) is visibly expressed in NSM, RIH, AIM, as well as the male-specific neurons CEM, PGA, R3B, R9B, and CP1 to CP6. Expression in ADF is often not detected (see F). (E) DiD-filling confirms mod-5(vlc47) expression in phasmid neuron class PHA, and not PHB, in young adults in both sexes (L4 male image is to facilitate neuron ID in adults, because the positions of the two neuron classes can change in males during the L4 to adult transition). (F) Expression of mod-5(vlc47) in ADF is stronger in hermaphrodites than in males. Each dot represents a single animal. Expression is not sexually dimorphic for the reporter alleles of either the serotonin-synthesizing enzyme tph-1 or the vesicular acetylcholine transporter unc-17. Expression was normalized against expression in other reporter-expressing neurons. Statistics, Mann-Whitney test. (G) In the tail region of wild type males, male-specific neurons PGA, R1B, R3B, and R9B are stained positive for serotonin. In a mod-5(n3314) mutant background, staining is completely lost in PGA (41/41 stained animals) and significantly affected for R9B (completely lost in 31/41 animals and much dimmer in the rest), while it remains in all 41 stained animals for R1B and R3B. The staining for CP1 to CP6 are also not affected in mod-5 mutant animals (remaining in 41/41 stained animals; image showing CP5 and CP6). (H, I) In adult animals, oct-1(syb8870) is expressed in the tyraminergic neuron class RIM in both sexes. Expression is not observed in any other neurons. (J, K) Outside the nervous system, oct-1(syb8870) is expressed in body wall muscle (BWM) throughout the worm (J) as well as hypodermal cells and selected head glia (K). Expression is also observed in gonadal cells in the male vas deferens (K). A pan-glial reporter otIs870[mir-228p::3xnls::TagRFP] and a dpy-7p::mCherry reporter stIs10166 [dpy-7p::his-24::mCherry+unc-119(+)] were used for glial and hypodermal identification, respectively. Scale bars, 10 μm.
In the hermaphrodite-specific neurons HSN, VC4, and VC5, we did not observe expression of the mod-5/SERT reporter allele (Tables 1 and 2). Since VC4 and VC5 do not express the complete synthesis pathway for serotonin, we infer that the anti-serotonin staining in these neurons is a result of alternative serotonin uptake or synthesis mechanisms. A similar scenario holds for the pharyngeal neuron I5, which was previously reported to stain weakly for serotonin (Serrano-Saiz et al., 2017b).
Tyramine uptake
Biochemical studies in vertebrates have shown that the SLC22A1/2/3 (aka OCT-1/2/3) organic cation transporters can uptake monoaminergic neurotransmitters (Nigam, 2018), with SLC22A2 being apparently selective for tyramine (Berry et al., 2016). oct-1 is the ortholog of the OCT subclass of SLC22 family members (Zhu et al., 2015), but neither its expression nor function in the nervous system had been previously reported. We tagged the endogenous oct-1 locus with an sl2::gfp::h2b cassette (syb8870) and, within the nervous system, observed exclusive expression in the RIM neuron (Figure 8H and I), indicating that RIM is likely capable of uptaking tyramine in addition to synthesizing it via tdc-1/TDC. This is consistent with RIM being the only neuron showing oct-1 scRNA transcripts at all four threshold levels in the CeNGEN atlas (Supplementary file 1).
Betaine uptake
Notably, four CAT-1/VMAT-expressing neuron classes, CAN, AUA, RIR, and ASI, do not express biosynthetic enzymes for synthesis or uptake transporters of the four conventional monoaminergic transmitters known to be employed in C. elegans (serotonin, dopamine, octopamine, or tyramine). Hence, these neuron classes might instead synthesize or uptake another transmitter for ensuing synaptic release via CAT-1/VMAT. We considered the putative neurotransmitter betaine as a possible candidate, since CAT-1/VMAT is also able to package betaine (Peden et al., 2013; Hardege et al., 2022). Betaine is synthesized endogenously, within the nervous system mostly in the cat-1/VMAT-positive RIM neuron (Hardege et al., 2022), but it is also available in the bacterial diet of C. elegans (Peden et al., 2013). In vertebrates, dietary betaine is taken up by the betaine transporter BGT1 (aka SLC6A12). To test whether cat-1/VMAT-positive neurons may acquire betaine via BGT1-mediated uptake, we CRISPR/Cas9-engineered a reporter allele for snf-3/BGT1, syb7290. We detected expression in the betaine-synthesizing (and also tyraminergic) RIM neuron (Figure 9, Tables 1 and 2). In addition, snf-3 is indeed expressed in all the four cat-1/VMAT-positive neuron classes that do not synthesize a previously known monoaminergic transmitter (CAN, AUA, and variably, RIR and ASI) (Figure 9A and B). These neurons may therefore take up betaine and synaptically release it via CAT-1/VMAT. The snf-3(syb7290) reporter allele is also expressed in the serotonergic neuron NSM (albeit variably) (Tables 1 and 2), thus NSM could also be a betaine uptake neuron. In addition, we also detected snf-3(syb7290) expression in several other neurons that do not express cat-1(syb6486) (Supplementary file 1). Expression was also observed in a substantial number of non-neuronal cell types (Figure 9E–G, Table 2, Supplementary file 1). These neurons and non-neuronal cells may serve to clear betaine (see Discussion, Neurotransmitter synthesis versus uptake). snf-3(syb7290) is not expressed in the inner and outer labial neuron classes as previously suggested (Peden et al., 2013); these cells were likely misidentified in the previous study and are in fact inner and outer labial glial cells (as discussed further below).
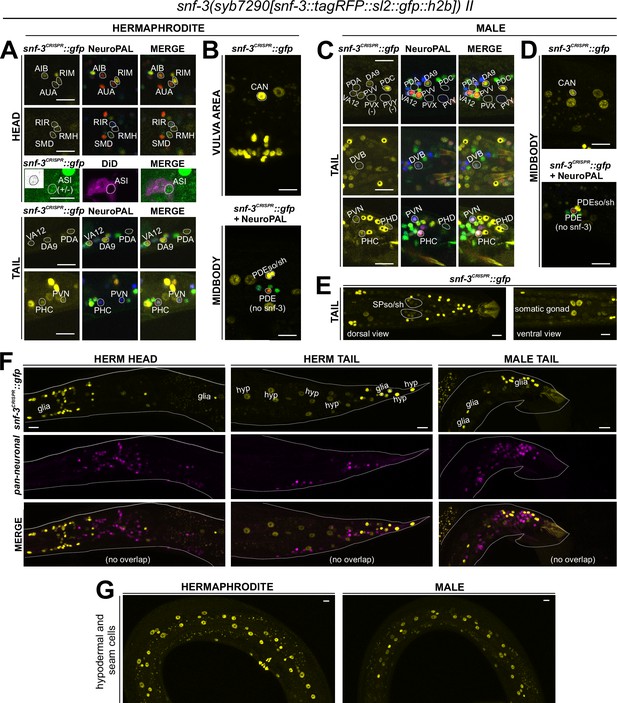
Expression of snf-3/BGT1/SLC6A12 in adult animals.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669) and DiD-filling. (A, B) In the adult hermaphrodite, neuronal expression of snf-3(syb7290) is detected in cat-1/VMAT-positive neurons AUA, CAN, and dimly and variably, RIR and ASI (confirmed with DiD-filling). In addition, it is also expressed in cat-1/VMAT-negative neurons AIB, RIM, RMH, SMD, VA12, DA9, PDA, PHC, PVN as labeled, as well as more neurons listed in Supplementary file 1. In the midbody, expression is not detected in PDE (dopaminergic, cat-1-positive) but is in its associated glial cells. It is also detected in multiple vulval support cells (B) and some epithelial cells near the somatic gonad. (C) In the adult male, in addition to its expression in sex-shared neurons as in hermaphrodites, snf-3(syb7290) is also expressed in male-specific neuron class PDC, as well as in PHD and variably in PVV. (D) Similarly to its expression in hermaphrodites, snf-3(syb7290) is detected in CAN and PDE-associated glial cells, but not PDE neurons, in males. (E) In the male tail, snf-3(syb7290) is expressed in a number of glial cells including the spicule sockets and/or sheath cells (dorsal view). It is also detected in the somatic gonad (ventral view). (F) snf-3(syb7290) is broadly expressed in most if not all glia in both sexes. Glial cell type is determined by cell location and the appearance of their nuclei in Normarski. To confirm they are not neurons, a pan-neuronal marker (UPN, or ‘uber pan-neuronal’, a component in NeuroPAL) is used to determine non-overlapping signals between the two reporters. Head expression in the male is very similar to that in the hermaphrodite and thus not shown. (G) snf-3(syb7290) is broadly expressed in hypodermal and seam cells in both sexes. Scale bars, 10 μm. Asterisks, non-neuronal expression.
Together with the expression pattern of the uptake transporters, all cat-1/VMAT-positive neurons in the hermaphrodite can be matched with an aminergic neurotransmitter. We nevertheless wondered whether another presently unknown monoaminergic transmitter, e.g., histamine or other trace amine, could be synthesized by a previously uncharacterized AAAD enzyme encoded in the C. elegans genome, hdl-1 (Figure 1—figure supplement 1A; Hare and Loer, 2004). We CRISPR/Cas9-engineered an hdl-1 reporter allele, syb1048, but detected no expression of this reporter in the animal (Figure 1—figure supplement 1C and D). Attempts to amplify weak expression signals by insertion of Cre recombinase into the locus failed [hdl-1(syb4208)] (see Materials and methods). CeNGEN scRNA data also shows no strong transcript expression in the hermaphrodite nervous system and only detected notable expression in sperm (Taylor et al., 2021).
Reporter alleles and NeuroPAL-facilitated neuron class-identification reveal novel expression patterns of neurotransmitters in the male-specific nervous system
No comprehensive scRNA atlas has yet been reported for the nervous system of the male. Based on the expression of fosmid-based reporters, we had previously assembled a neurotransmitter atlas of the C. elegans male nervous system in which individual neuron classes are notoriously difficult to identify (Serrano-Saiz et al., 2017b). We have since established a NeuroPAL landmark strain that permits more reliable identification of gene expression patterns in both the hermaphrodite and male-specific nervous system (Tekieli et al., 2021; Yemini et al., 2021). We used NeuroPAL to facilitate the analysis of the expression profiles of our CRISPR/Cas9-engineered reporter alleles in the male, resulting in updated expression profiles for 11 of the 16 knock-in reporter alleles analyzed. As in the hermaphrodite, reasons for these updates vary. In addition to the improved accuracy of neuron identification provided by NeuroPAL, in some cases there are true differences of expression patterns between the fosmid-based reporters and reporter alleles. We elaborate on these updates for individual reporter alleles below.
Expression of reporter alleles of Glu/ACh/GABA markers in the male-specific nervous system
We analyzed eat-4/VGLUT (syb4257), unc-17/VAChT (syb4491), unc-25/GAD (ot1372), and unc-47/VGAT (syb7566) expression in the male-specific nervous system using NeuroPAL landmark strains (otIs696 for eat-4 and otIs669 for all others)(Figures 10 and 11). Of all those reporter alleles, unc-25/GAD (ot1372) was the only one with no updated expression. Specifically, in addition to confirming presence of expression of the unc-25(ot1372) reporter allele in CP9, EF1/2, EF3/4, we also confirmed its lack of expression in anti-GABA-positive neurons R2A, R6A, and R9B (Gendrel et al., 2016; Serrano-Saiz et al., 2017b; Figure 11A, Supplementary file 3).
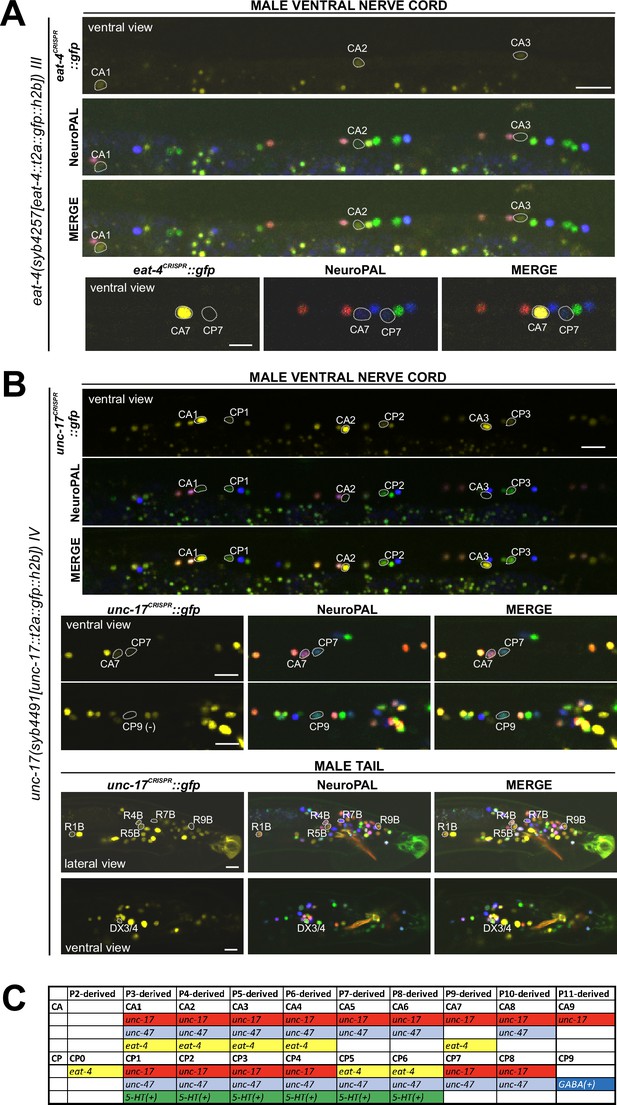
Expression of eat-4/VGLUT and unc-17/VAChT reporter alleles in the adult male.
Neuronal expression of eat-4(syb4257) and unc-17(syb4491) was characterized with landmark strain NeuroPAL (otIs696 and otIs669, respectively). Only selected neurons are shown to illustrate updates from previous studies. See Supplementary file 3 for a complete list of neurons. (A) eat-4(syb4257) expression. Top, long panels: CA1, CA2, and CA3 show visible, albeit very dim, novel expression of eat-4 (also expressed in CA4). Bottom panels: CA7 strongly expresses eat-4(syb4257), whereas CP7 does not. Neuron IDs for these two neurons were previously switched (Serrano-Saiz et al., 2017b). (B) unc-17(syb4491) expression. Top, long panels: ventral view of a male ventral nerve cord showing high levels of expression in CA1, CA2, and CA3 and previously unreported low levels of expression in CP1, CP2, and CP3. Middle panels: low levels of expression in CA7 and CP7. There is no visible expression in CP9. Bottom panels: lateral view of a male tail showing previously unreported dim expression in R1B, R4B, R5B, R7B, and R9B; ventral view of the preanal ganglion showing expression in DX3/4. Scale bars, 10 μm. (C) The updated neurotransmitter atlas underscores the molecular diversity of the male-specific ventral cord neuron class CA and CP. Based on their expression patterns for neurotransmitter genes, these neurons can be grouped into four CA and five CP subclasses.
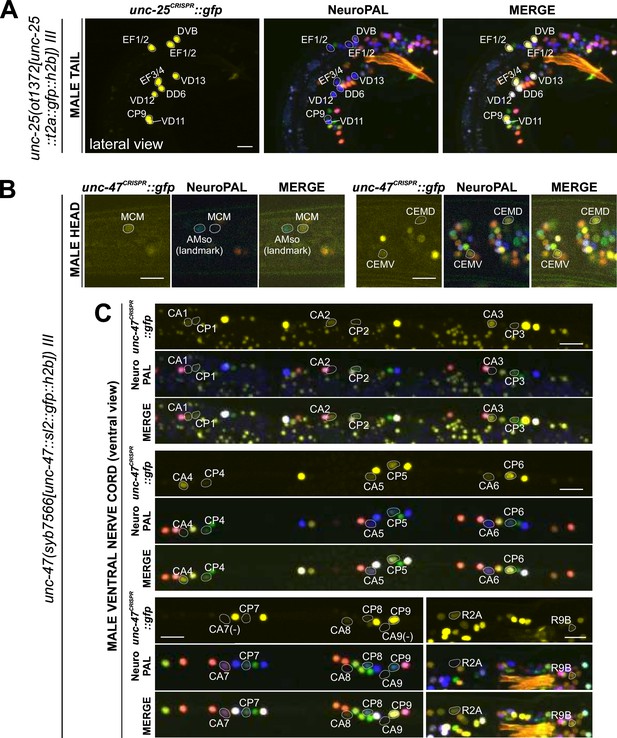
Expression of GABAergic reporter alleles in the adult male.
Neuronal expression of unc-25(ot1372) and unc-47(syb7566) reporter alleles was characterized with landmark strain NeuroPAL (otIs669). Only selected neurons are shown to illustrate updates from previous reports. See Supplementary file 3 for a complete list of neurons. (A) unc-25(ot1372) is expressed in male-specific CP9 and EF neurons as well as a few sex-shared neurons, all consistent with previous reports (Gendrel et al., 2016; Serrano-Saiz et al., 2017b). (B) unc-47(syb7566) shows expression in male head neuron classes MCM and CEM, the former previously undetected and the latter consistent with fosmid-based reporter otIs564. (C) unc-47(syb7566) shows expression in a number of ventral cord CA and CP neurons, largely consistent with reported otIs564 fosmid-based reporter expression except for no visible expression of syb7566 in CA7 (due to its initial confusion with CP7, described in Figure 10) and presence of very dim expression in CP7. The syb7566 reporter allele is also not visible in CA9. Scale bars, 10 μm.
In the preanal ganglion, we observed weak expression of unc-17(syb4491) in DX3/4 (Figure 10B, Supplementary file 3), hence assigning previously unknown neurotransmitter identity to these neurons. Related to DX3/4, we also confirmed expression of unc-17 in DX1/2 in the dorsorectal ganglion, consistent with fosmid-based reporter data (Supplementary file 3; Serrano-Saiz et al., 2017b). In the lumbar ganglion, we detected novel expression of unc-17(syb4491) in five pairs of type B ray neurons, namely R1B, R4B, R5B, R7B, and R9B (Figure 10B, Supplementary file 3). Expression in all these neurons is low, possibly explaining why it is not observed with an unc-17 fosmid-based reporter (Serrano-Saiz et al., 2017b).
In the ventral nerve cord, we found additional, very weak expression of eat-4(syb4257) in CA1 to CA4 (Figure 10A, Supplementary file 3), as well as weak expression of unc-17(syb4491) in CP1 to CP4 (Figure 10B, Supplementary file 3), all undetected by previous analysis of fosmid-based reporters (Serrano-Saiz et al., 2017b). Conversely, two neurons lack previously reported expression of fosmid-based reporters; CP9 does not show visible unc-17(syb4491) expression (Figure 10B) and neither does CA9 show visible expression of unc-47(syb7566) expression (Figure 11C). We also realized that the neuron identifications of CA7 and CP7 were previously switched (Serrano-Saiz et al., 2017b), due to lack of proper markers for those two neurons. With NeuroPAL, we are now able to clearly distinguish the two and update their classic neurotransmitter reporter expression: CA7 expresses high levels of eat-4(syb4257) (Figure 10A, Supplementary file 3), very low levels of unc-17(syb4491) (Figure 10B), and no unc-47(syb7566) (Figure 11C); CP7 expresses no eat-4(syb4257) (Figure 10A, Supplementary file 3), very low levels of unc-17(syb4491) (Figure 10B), and very low levels of unc-47(syb7566) as well (Figure 11C). Taken together, the analysis of reporter alleles reveals a remarkable diversity of CA and CP neurons, summarized in Figure 10C.
In the head, we detected expression of unc-47(syb7566) in the male-specific neuron class MCM (Figure 11B, Supplementary file 3), previously not observed with fosmid-based reporters. Consistent with fosmid-based reporter data, the other male-specific head neuron class, CEM, shows expression of unc-17(syb4491) (Supplementary file 3) and unc-47(syb7566) (Figure 11B, Supplementary file 3) reporter alleles.
Expression of reporter alleles for monoaminergic neurotransmitter pathway genes in the male-specific nervous system
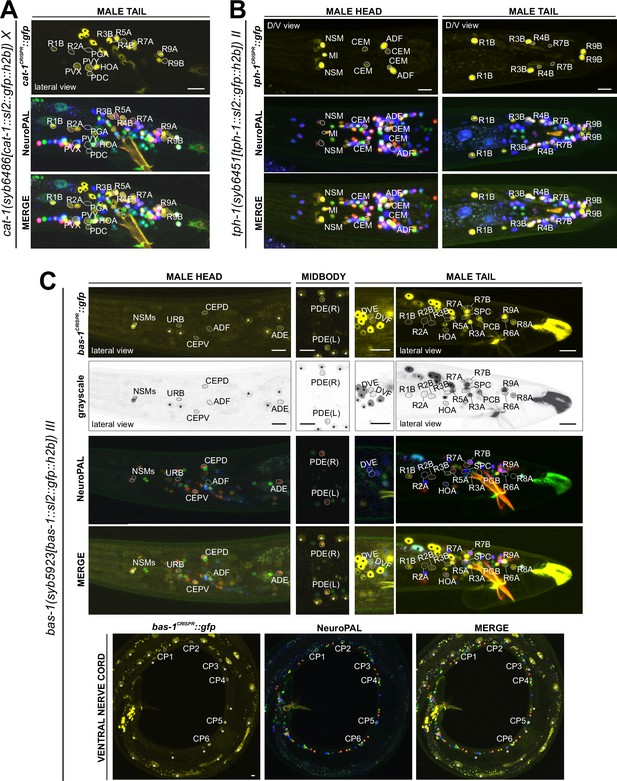
We analyzed the expression of reporter alleles for genes involved in monoamine biosynthesis and uptake in the male-specific nervous system: cat-1/VMAT (syb6486), tph-1/TPH (syb6451), cat-2/TH (syb8255), bas-1/AAAD (syb5923), tdc-1/TDC (syb7768), tbh-1/TBH (syb7786), mod-5/SERT (vlc47), oct-1/OCT (syb8870), and snf-3/BGT1 (syb7290). As in the hermaphrodite nervous system, we used the NeuroPAL reporter landmark (otIs669) for neuron ID (Tekieli et al., 2021). We found novel expression patterns in all male-specific ganglia (Figures 12 and 13, Supplementary file 3).
Expression of the cat-1/VMAT, tph-1/TPH, and bas-1/AAAD reporter alleles in the adult male.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). (A) Novel cat-1(syb6486) expression is seen in male-specific neurons PDC, PVY, PVX, R2A, and R4B. Consistent with previous reports, it is also expressed in HOA, PGA, R5A, R7A, R9A, R1B, and R8B. Its expression in ventral cord neurons CP1 to CP6 is consistent with earlier studies. (B) tph-1(syb6451) is expressed in male-specific head neuron class CEM and sex-shared neurons ADF, NSM, and MI. Similar to its expression in hermaphrodites, tph-1 in MI was previously undetected. In the tail, in addition to previously determined expression in R1B, R3B, and R9B, tph-1(syb6451) is also expressed at very low levels in R4B and R7B. Ventral cord expression of tph-1(syb6451) in CP1 to CP6 is consistent with previous reports and thus not shown here. (C) bas-1(syb5923) is expressed in previously identified NSM, ADE, PDE, and CEP neurons. In addition, we detected weak expression in URB as in the hermaphrodite. We also updated bas-1/AAAD expression in 39 male-specific neurons (see Supplementary file 3 for complete list). Neurons are also shown in grayscale for clearer visualization in some cases. Scale bars, 10 μm. Asterisks, non-neuronal expression, also see Figure 14 and Figure 14—figure supplement 1.
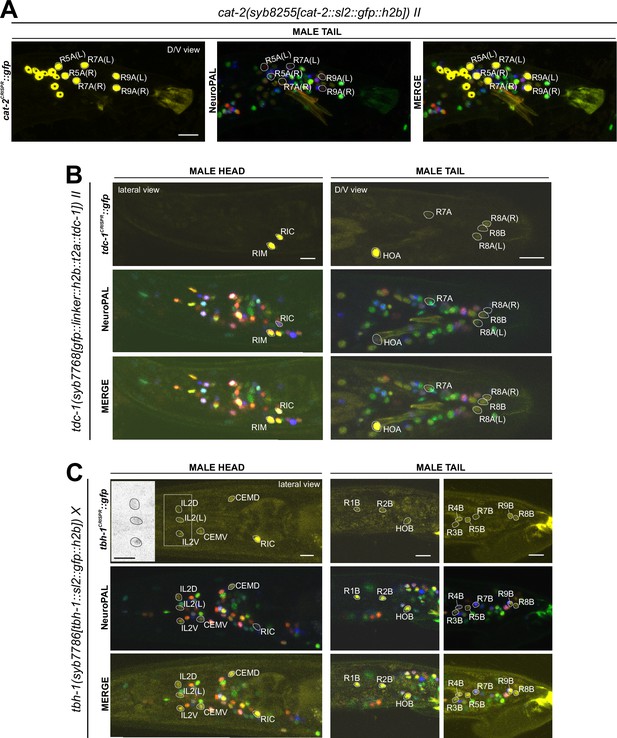
Expression of cat-2/TH, tdc-1/TDC, and tbh-1/TBH reporter alleles in the adult male.
Neuronal expression was characterized with landmark strain NeuroPAL (otIs669). (A) cat-2(syb8255) is expressed in male-specific neurons R4A, R7A, and R9B. This expression, as well as its expression in sex-shared neurons PDE, CEP, and ADE, is consistent with previous reports (Sulston et al., 1975; Sulston et al., 1980; Lints and Emmons, 1999). (B) tdc-1(syb7768) is expressed in sex-shared neurons RIM and RIC and male-specific neurons HOA, R8A, and R8B, all consistent with previous studies (Serrano-Saiz et al., 2017b). We also detected weak expression in R7A. (C) tbh-1(syb7786) is expressed in RIC, consistent with its previously reported expression in hermaphrodites. As in hermaphrodites, we also detected tbh-1(syb7786) in IL2 neurons of the male. In male-specific neurons, previously unreported expression is detected in CEM, HOB, and all type B ray neurons except for R6B. Intriguingly, this expression pattern resembles that of pkd-2 and lov-1, both genes essential for male mating functions (Barr and Sternberg, 1999; Barr et al., 2001). Inset, grayscale image showing dim expression for IL2 neurons. Scale bars, 10 μm. Asterisks, non-neuronal expression, also see Figure 14 and Figure 14—figure supplement 1.
Serotonin/5-HT synthesis
Serotonergic identity had been assigned to several male-specific neurons before (CP1 to CP6, R1B, R3B, R9B) (Loer and Kenyon, 1993), and we validated these assignments with our reporter alleles (Figure 12, Supplementary file 3). In addition, we detected previously unreported expression of tph-1 (Figure 12B) in the male-specific head neuron class CEM, as well as in a subset of B-type ray sensory neurons, R4B and R7B. However, not all of the neurons display additional, canonical serotonergic neuron features: While R4B and R7B express bas-1(syb5923) (with R4B expressing it variably) to generate serotonin, neither neuron was detected by anti-serotonin staining in the past. On the other hand, R9B and CEM stain positive for 5-HT (Serrano-Saiz et al., 2017b), but they do not express bas-1(syb5923), indicating that they may be producing 5-HTP rather than 5-HT (sertonin)(see more below on serotonin uptake). In addition, R4B and R9B, but not R7B or CEM, express cat-1(syb6486) for vesicular release of serotonin.
In the ventral nerve cord, consistent with previous fosmid-based reporter data (Serrano-Saiz et al., 2017b), we observed the expression of cat-1(syb6486) and tph-1(syb6451) in CP1 to CP6 (Supplementary file 3). Additionally, we also detected novel expression of bas-1(syb5923) in CP1 to CP4 and strongly in CP5 and CP6 (Figure 12C, Supplementary file 3). This updated expression supports the serotonergic identities of these neurons, which had been determined previously based only on their expression of cat-1/VMAT reporters and positive staining for serotonin (Loer and Kenyon, 1993; Serrano-Saiz et al., 2017b).
Dopamine synthesis
We found that the expression of the dopamine-synthesizing cat-2(syb8255) reporter allele precisely matched previous assignments of dopaminergic identity (Sulston et al., 1975; Sulston et al., 1980; Lints and Emmons, 1999), i.e., expression was detected exclusively in R5A, R7A, and R9A (Figure 13A, Supplementary file 3), in addition to all sex-shared dopaminergic neurons. All these neurons show matching expression of bas-1/AAAD, the other essential enzyme for dopamine synthesis, and cat-1/VMAT, the vesicular transporter for dopamine (Figure 12A and C; Supplementary file 3).
Tyramine and octopamine synthesis
Reporter alleles for the two diagnostic enzymes, tdc-1/TDC and tbh-1/TBH, confirm the previously reported assignment of HOA as tyraminergic (Serrano-Saiz et al., 2017b), based on the presence of tdc-1(syb7768) but absence of tbh-1(syb7786) expression (Figure 13 B and C). The tdc-1 reporter allele reveals a novel site of expression in R7A. Due to lack of tbh-1 expression, R7A therefore classifies as another tyraminergic neuron. Both HOA and R7A also co-express cat-1/VMAT for vesicular release of tyramine (Figure 12A).
We detected no neurons in addition to the sex-shared RIC neuron class that shares all features of a functional octopaminergic neuron, i.e., co-expression of tbh-1/TBH, tdc-1/TDC, and cat-1/VMAT. While one male-specific neuron, R8B, shows an overlap of expression of tdc-1(syb7768) and tbh-1(syb7786) (Figure 13B and C), indicating that these neurons can synthesize octopamine, R8B does not express cat-1(syb6486), indicating that it cannot engage in vesicular release of octopamine.
Curiously, while there are no other male-specific neurons that co-express tdc-1 and tbh-1, several male-specific neurons express tbh-1, but not tdc-1 (Figure 13B and C; Table 2, Supplementary file 3). The absence of the TDC-1/AAAD protein, which produces tyramine, the canonical substrate of the TBH-1 enzyme (Figure 1A), indicates that TBH-1 must be involved in the synthesis of a compound other than octopamine. Moreover, bas-1/AAAD is expressed in several of the tbh-1(+); tdc-1(-) neurons (R1B, R2B, R3B, R4B, and R7B) (Figure 12C, Table 2, Supplementary file 3). Rather than using L-Dopa or 5-HTP as substrate, BAS-1/AAAD may decarboxylate other aromatic amino acids, which then may serve as a substrate for TBH-1. We consider the trace amine phenylethanolamine (PEOH) as a candidate end product (see Discussion).
Other monoaminergic neurons
In the preanal ganglion, we detected novel expression of the cat-1(syb6486) reporter allele in the cholinergic PDC, PVX, and PVY neurons (Figure 12A). Intriguingly, just as the sex-shared neuron AVL (Figure 6C), these neurons express no other serotonergic, dopaminergic, tyraminergic, or octopaminergic pathway genes. However, we did find PDC (but not PVX or PVY) to express the betaine uptake transporter reporter allele snf-3(syb7290) (Figure 9; more below). PVX and PVY may synthesize or uptake another aminergic transmitter. Such presumptive transmitter is not likely to be synthesized by hdl-1/AAAD since we detected no expression of the hdl-1 reporter allele syb4208 in the male nervous system.
The expression pattern of the bas-1/AAAD, which had not been previously analyzed in the male-specific nervous system, reveals additional novelties. In addition to the ‘canonical’ serotonergic and dopaminergic neurons described above, we detected bas-1(syb5923) reporter allele expression in a substantial number of additional neurons, including the tyraminergic HOA and R7A neurons, but also the DVE, DVF, R2A, R3A, R6A, R8A, R2B, R6B, R7B, PCB, and SPC neurons (Figure 12C, Supplementary file 3). As described above, a subset of the neurons co-express tbh-1(syb7786) (most B-type ray neurons), a few co-express tdc-1(syb7768) (HOA and several A-type ray neurons), and several co-express neither of these two genes. Only a subset of these neurons express cat-1(syb6486). Taken together, this expression pattern analysis argues for the existence of additional monoaminergic signaling system(s) (Table 2).
Serotonin/5-HT uptake
In the male-specific nervous system, we detected mod-5/SERT reporter allele expression in CEM, PGA, R3B, R9B, and ventral cord neurons CP1 to CP6 (Figure 8D). We found that anti-serotonin staining in CP1 to CP6, R1B, and R3B is unaffected in mod-5(n3314) mutant animals, consistent with these neurons expressing the complete serotonin synthesis machinery (i.e. tph-1 and bas-1) (Table 2, Figure 8B, D, and G; Supplementary file 3). Hence, like several other monoaminergic neurons, these serotonergic neurons both synthesize, synaptically release, and reuptake serotonin. In contrast, anti-serotonin staining is lost from the R9B and PGA neurons of mod-5(n3314) mutant animals, indicating that the presence of serotonin in these neurons depends on serotonin uptake, consistent with them not expressing the complete serotonin synthesis pathway (Table 2; Supplementary file 3). Since R9B and PGA express cat-1/VMAT (Figure 12A), these neurons have the option to utilize serotonin for vesicular release after mod-5-dependent uptake.
Tyramine and betaine uptake
We did not observe oct-1(syb8870) reporter allele expression in male-specific neurons. As in the hermaphrodite nervous system, we detected snf-3(syb7290) in a number of neurons that do not express CAT-1/VMAT (Supplementary file 1), including in male-specific neurons PHD, and variably, PVV (Figure 9C). As mentioned earlier, the male-specific neuron PDC expresses both cat-1(syb6486) and snf-3(syb7290), making it a likely betaine-signaling neuron.
Sexually dimorphic neurotransmitter deployment in sex-shared neurons
eat-4/VGLUT
We had previously noted that a fosmid-based eat-4/VGLUT reporter is upregulated in the sex-shared neuron PVN, specifically in males (Serrano-Saiz et al., 2017b). Since PVN is also cholinergic (Figure 4D; Pereira et al., 2015), this observation indicates a sexually dimorphic co-transmission configuration. As described above (Figure 4B, Supplementary file 2), our eat-4 reporter allele revealed low levels of eat-4/VGLUT expression in hermaphrodites PVN, but in males the eat-4 reporter allele showed strongly increased expression, compared to hermaphrodites. Hence, rather than being an ‘on’ vs. ‘off’ dimorphism, dimorphic eat-4/VGLUT expression in male PVN resembles the ‘scaling’ phenomenon we had described previously for eat-4/VGLUT in male PHC neurons, compared to hermaphrodite PHC neurons (Serrano-Saiz et al., 2017a). Both PHC and PVN display a substantial increase in the amount of synaptic output of these neurons in males compared to hermaphrodites (Cook et al., 2019), providing a likely explanation for such scaling of gene expression. The scaling of eat-4/VGLUT expression in PVN is not accompanied by scaling of unc-17/VAChT expression, which remains comparable in both sexes (Figure 4D).
We also examined AIM, another neuron class that was previously reported to be sexually dimorphic in that AIM expresses eat-4/VGLUT fosmid-based reporters in juvenile stages in both sexes, whereas upon sexual maturation its neurotransmitter identity is switched from being glutamatergic to cholinergic only in adult males and not hermaphrodites (Pereira et al., 2015; Pereira et al., 2019). With the eat-4(syb4257) reporter allele, we also detected a downregulation of eat-4 expression to low levels in young adult males and almost complete elimination in 2-day-old adult males, while expression in hermaphrodites stays high.
unc-17/VAChT
The unc-17/VAChT reporter allele syb4491 confirms that cholinergic identity is indeed male-specifically turned on in the AIM neurons (Figure 4C), thereby confirming the previously reported neurotransmitter switch (Pereira et al., 2015). The fosmid-based unc-17 reporter also showed sexually dimorphic expression in the AVG neurons (Serrano-Saiz et al., 2017b). This is also confirmed with the unc-17 reporter allele, which shows dim and variable expression in hermaphrodites and slightly stronger, albeit still dim, AVG expression in males (Figure 4C, showing a hermaphrodite representing animals with no visible expression and a male with representative dim expression).
unc-47/VGAT
unc-47(syb7566) confirms previously reported sexually dimorphic expression of unc-47/VGAT in several sex-shared neurons, including ADF, PDB, PVN, PHC, AS10, and AS11 (Figure 5B, right side panels) (Serrano-Saiz et al., 2017b). The assignment of AS10 was not definitive in our last report (we had considered either DA7 or AS10), but with the help of NeuroPAL the AS10 assignment could be clarified. In all these cases expression was only detected in males and not hermaphrodites. It is worth mentioning that expression of the mCherry-based unc-47/VGAT fosmid-based reporter (otIs564) in some of these neurons was so dim that it could only be detected through immunostaining against the mCherry fluorophore and not readily visible with the fosmid-based reporter by itself (Serrano-Saiz et al., 2017b; Supplementary file 1). In contrast, the unc-47/VGAT reporter allele is detected in all cases except the PQR neuron class.
mod-5/SERT
Expression of the mod-5(vlc47) reporter allele is sexually dimorphic in the pheromone-sensing ADF neurons, with higher levels in hermaphrodites compared to males (Figure 8F). Notably, the serotonin-synthesizing enzyme (tph-1) and vesicular acetylcholine transporter (unc-17) do not exhibit this dimorphism in ADF (Figure 8F). This suggests that the sex difference specifically involves serotonin signaling mechanisms, particularly serotonin uptake rather than synthesis.
We had previously reported that the PVW neuron stains with anti-serotonin antibodies exclusively in males but we did not detect expression of a fosmid-based reporter for the serotonin-synthesizing enzyme TPH-1 (Serrano-Saiz et al., 2017b). We confirmed the lack of tph-1 expression with our new tph-1 reporter allele in both males and hermaphrodites, and also found that hermaphrodite and male PVW does not express the reporter allele for the other enzyme in the serotonin synthesis pathway, bas-1. Because of very dim cat-1::mCherry fosmid-based reporter expression that was only detected upon anti-mCherry antibody staining, we had assigned PVW as a serotonin-releasing neuron (Serrano-Saiz et al., 2017b). However, we failed to detect expression of our new cat-1/VMAT reporter allele in PVW. Neither did we detect expression of the mod-5(vlc47) reporter allele. Taken together, PVW either synthesizes or uptakes serotonin by unconventional means, akin to the pharyngeal I5 neuron.
In conclusion, although there are some updates in the levels of dimorphic gene expression (PVN and ADF neuron classes), our analysis with reporter alleles does not reveal pervasive novel sexual dimorphism in sex-shared neurons compared to those that we previously identified in Serrano-Saiz et al., 2017b. These sexual dimorphisms are summarized in Supplementary file 4.
Neurotransmitter pathway genes in glia
In vertebrates, glia can produce various signaling molecules, including neurotransmitters (Araque et al., 2014; Savtchouk and Volterra, 2018). There is some limited evidence for neurotransmitter synthesis in C. elegans glia. In males, it had been reported that the socket glia of spicule neurons synthesize and utilize dopamine, based on their expression of cat-2/TH and bas-1/AAAD (Lints and Emmons, 1999; Hare and Loer, 2004; LeBoeuf et al., 2014). We confirmed this notion with cat-2/TH and bas-1/AAAD reporter alleles (Figure 14A). Additionally, we detected expression of the cat-1/VMAT reporter allele in these cells (Figure 14A), indicating that these glia secrete dopamine by canonical vesicular transport. We did not detect cat-1/VMAT in other glial cell types. In addition to the spicule socket glia, we also observed bas-1(syb5923) reporter allele expression in cells that are likely to be the spicule sheath glia (Figure 14A), as well as in additional glial cell types in the head and tail (Figure 14B). We detected no glial expression of other monoaminergic synthesis machinery.
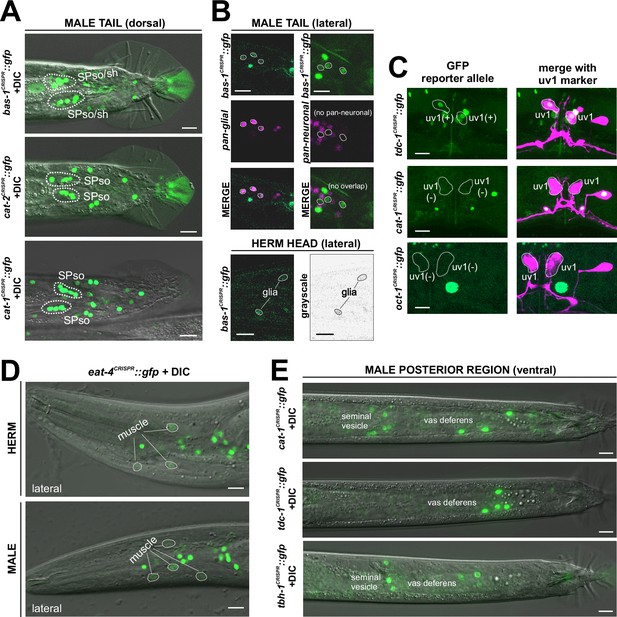
Expression of neurotransmitter pathway genes in non-neuronal cell types.
Multiple neurotransmitter pathway genes show expression in glial cells (A, B) and other non-neuronal cell types (C–E). Also see Figure 14—figure supplement 1 for whole-worm views that capture more non-neuronal expression. (A) bas-1(syb5923), cat-2(syb8255), and cat-1(syb6486) reporter alleles exhibit expression in the male spicule glial cell types, largely consistent with previous reports (Lints and Emmons, 1999; Hare and Loer, 2004; LeBoeuf et al., 2014). (B) Top 6 panels: bas-1(syb5923) is expressed in additional, multiple glial cell types in the male tail. Left 3 panels: bas-1(syb5923) crossed into a pan-glial reporter otIs870[mir-228p::3xnls::TagRFP], confirming its expression in glial cells; right 3 panels: bas-1(syb5923) shows no overlap with the pan-neuronal marker component in NeuroPAL (otIs669). Bottom 2 panels: bas-1(syb5923) also shows expression in at least two glial cells in the head. A hermaphrodite head is shown here. Expression is similar in the male. (C) In the hermaphrodite vulval region, tdc-1(syb7768) is expressed in uv1, consistent with previous reports (Alkema et al., 2005). This expression in uv1 is not observed for either cat-1(syb6486) or oct-1(syb8870). An ida-1p::mCherry integrant vsls269[ida-1::mCherry] was used for identifying uv1. (D) Detection of eat-4(syb4257) expression in muscle cells in both sexes, most prominently in the head. (E) cat-1(syb6486), tdc-1(syb7768), and tbh-1(syb7786) are expressed in the male somatic gonad. All three have expression in the vas deferens; additionally, cat-1 and tbh-1 are also expressed in the seminal vesicle.
We detected no expression of vesicular transporters or biosynthetic synthesis machinery for non-aminergic transmitters in glia of either sex. This observation contrasts previous reports on GABA synthesis and release from the AMsh glial cell type (Duan et al., 2020; Fernandez-Abascal et al., 2022). We were not able to detect signals in AMsh with anti-GABA staining, nor with an SL2- or T2A-based GFP-based reporter allele for any unc-25 isoform (Gendrel et al., 2016) (M Gendrel, pers. comm.; this paper).
There is, however, abundant evidence for neurotransmitter uptake by C. elegans glial cells, mirroring this specific function of vertebrate glia (Henn and Hamberger, 1971). We had previously shown that one specific glia-like cell type in C. elegans, the GLR glia, take up GABA via the GABA uptake transporter SNF-11 (Gendrel et al., 2016). We did not detect unc-47/VGAT fosmid-based reporter expression in the GLRs (Gendrel et al., 2016) and also detected no expression with our unc-47/VGAT reporter allele. Hence, these glia are unlikely to release GABA via classic vesicular machinery. Other release mechanisms for GABA can of course not be excluded. Aside from the snf-11 expression in GLR glia (Gendrel et al., 2016), we detected expression of the putative tyramine uptake transporter oct-1/OCT in a number of head glial cells (Figure 8K), as well as broad glial expression of the betaine uptake transporter snf-3/BGT1 in the head, midbody, and tail (Figure 9E and F). These results indicate tyramine and betaine clearance roles for glia.
Neurotransmitter pathway gene expression outside the nervous system
We detected expression of a few neurotransmitter pathway genes in cells outside the nervous system. The most prominent sites of reporter allele expression are located within the gonad. We detected expression of tdc-1(syb7768) and tbh-1(syb7786) reporter alleles in the gonad of hermaphrodite as well as tdc-1(syb7768) expression in the neuroendocrine uv1 cells (Figure 14C; Figure 14—figure supplement 1), as previously reported (Alkema et al., 2005). Intriguingly, while cat-1(syb6486) is expressed in a midbody gonadal cell posterior to the vulva, likely the distal valve (Figure 6C, Figure 14—figure supplement 1), we observed no expression of cat-1(syb6486) in the gonad or the uv1 cells (Figure 14C). This suggests alternative release mechanisms for tyramine and octopamine. A vertebrate homolog of the putative tyramine uptake transporter, oct-1, has been found to be located presynaptically and to co-purify with synaptosomes (Berry et al., 2016; Matsui et al., 2016), therefore indicating that this transporter may have the potential to also act in tyramine release, at least in vertebrate cells. However, we observed no expression of our oct-1 reporter allele in uv1 or gonadal cells.
In the male, tdc-1(syb7768), tbh-1(syb7786), cat-1(syb6486), and oct-1(syb8870) animals also show reporter expression in the somatic gonad: while all four genes are expressed in the vas deferens, cat-1 and tbh-1, but not tdc-1 or oct-1, are expressed in the seminal vesicle (Figure 14C, Figure 8K). A similar pattern of cat-1(+); tbh-1(+); tdc-1(-); oct-1(-) is detected in several male-specific neurons and may indicate the usage of a novel transmitter (e.g. PEOH, see Discussion) by these cells. snf-3/BGT1 is also expressed in male somatic gonad cells, indicating that these cells could also use betaine for signaling (Figure 9E).
The AAADs tdc-1 and bas-1 are also prominently expressed in the intestine, where bas-1 has been shown to be involved in generating serotonin-derived glucosides (Yu et al., 2023). bas-1, but not tdc-1, is also expressed in the hypodermis and seam cells, as is the betaine uptake transporter snf-3 (Figure 9, Figure 14—figure supplement 1). The tph-1 reporter allele expresses in a subset of pharyngeal non-neuronal cells during the L1 to L4 larval stages of development (Figure 6—figure supplement 1), which is consistent with low levels of tph-1 transcripts detected in pharyngeal muscles in the CeNGEN scRNA dataset (Taylor et al., 2021). Additionally, we observed previously uncharacterized eat-4/VGLUT expression in muscle cells in both sexes (Figure 14D).
Discussion
Using CRISPR/Cas9-engineered reporter alleles we have refined and extended neurotransmitter assignments throughout all cells of the C. elegans male and hermaphrodite. We conclude that in both hermaphrodites and males, about one quarter of neurons are glutamatergic (eat-4/VGLUT-positive), a little more than half are cholinergic (unc-17/VAChT-positive), around 10% are GABAergic (unc-25/GAD-positive), and about another 10% are monoaminergic (cat-1/VMAT-positive). We compiled comprehensive lists for gene expression and neuron identities, which are provided in Supplementary file 2 for hermaphrodites and Supplementary file 3 for males. Figure 3 presents a summary of neurotransmitter usage and atlases showing neuron positions in worm schematics. Additionally, we summarize our rationale for assigning neurotransmitter usage and updates to previously reported data in Tables 1 and 2, and Supplementary file 5. Given the complexity and nuances in determining neurotransmitter usage, we refer the reader to all the individual tables for a comprehensive description of the subject matter, rather than encouraging sole reliance on the summary in Figure 3.
Neurotransmitter synthesis versus uptake
Direct detection of neurotransmitters through antibody staining has shown that at least two neurotransmitters, GABA and serotonin, are present in some neurons that do not express the synthesis machinery for these transmitters (Tables 1 and 2). Instead, these neurons acquire GABA and serotonin through uptaking them via defined uptake transporters, SNF-11/BGT1 for GABA (Mullen et al., 2006) and MOD-5/SERT for serotonin (Ranganathan et al., 2001; Jafari et al., 2011). A combination of CeNGEN scRNA transcriptome and our reporter allele data corroborates the absence of synthesis machinery in these presumptive uptake neurons (Tables 1 and 2). One interesting question that relates to these uptake neurons is whether they serve as ‘sinks’ for clearance of a neurotransmitter or whether the taken-up neurotransmitter is subsequently ‘recycled’ for synaptic release via a vesicular transporter. Previous data, as well as our updated expression profiles, provide evidence for both scenarios: ALA and AVF do not synthesize GABA via UNC-25/GAD, but they stain with anti-GABA antibodies in a manner that is dependent on the uptake transporter SNF-11 (Gendrel et al., 2016). ALA expresses unc-47, hence it is likely to synaptically release GABA, but AVF does not, and it is therefore apparently involved only in GABA clearance. Similarly, RIH, AIM, and PGA express the serotonin uptake transporter mod-5/SERT and stain for serotonin in a MOD-5-dependent manner (Jafari et al., 2011) (this study), but only RIH and PGA, not AIM, expresses the vesicular transporter cat-1/VMAT, suggesting RIH and PGA are likely serotonergic signaling neurons whereas AIM is a clearance neuron.
Some neurons do not obviously fall into the synthesis or uptake category, most notably, the anti-GABA-antibody-positive AVA and AVB neurons (both of which conventional cholinergic neurons). None of these neurons express unc-25/GAD, nor the snf-11/BGT1 uptake transporter, yet unc-25/GAD is required for their anti-GABA-positive staining (Gendrel et al., 2016). This suggests that GABA may be acquired by these neurons through non-canonical uptake or synthesis mechanisms. Also, the AVA and AVB neurons do not express UNC-47 (Gendrel et al., 2016; Taylor et al., 2021) (this study); hence, it is not clear if or how GABA is released from them. A member of the bestrophin family of ion channels has been shown to mediate GABA release from astrocyte glia in vertebrates (Lee et al., 2010) and, more recently, from C. elegans glia (Cheng et al., 2024; Graziano et al., 2024). However, while there are more than 20 bestrophin channels encoded in the C. elegans genome (Hobert, 2013), they do not appear to be expressed in the AVA or AVB neurons, based on CeNGEN scRNA data (Taylor et al., 2021).
The co-expression of a specific uptake transporter and a vesicular transporter corroborates the potential usage of betaine as a neurotransmitter. Betaine is known to be synthesized in C. elegans but is also taken up via its diet (Peden et al., 2013; Hardege et al., 2022). Betaine has documented effects on C. elegans behavior and acts via activation of several betaine-gated ion channels (Peden et al., 2013; Hardege et al., 2022). Expression of biosynthetic enzymes suggests betaine production in at least the RIM neuron class, which also expresses the vesicular transporter cat-1/VMAT, capable of transporting betaine (Hardege et al., 2022). The expression of the betaine uptake transporter snf-3/BGT1 in CAN, AUA, RIR, ASI, and male-specific neuron PDC, coupled with their co-expression of cat-1/VMAT, suggests that several distinct neuron classes in different parts of the nervous system may uptake betaine and engage in vesicular betaine release via CAT-1/VMAT to gate betaine-activated ion channels, such as ACR-23 (Peden et al., 2013) or LGC-41 (Hardege et al., 2022). Additionally, we detected the snf-3/BGT1 reporter allele in several other neuron classes that do not co-express cat-1/VMAT. This indicates that these neurons could function as betaine clearance neurons.
Lastly, based on sequence similarity and expression pattern, we predict that the ortholog of the OCT subclass of SLC22 family, oct-1, could serve as a tyramine uptake transporter in C. elegans. We identified RIM as the only neuron expressing an oct-1 reporter allele, suggesting that like several other monoaminergic neuron classes, RIM both synthesizes its monoaminergic transmitter and reuptakes it after release.
Evidence for usage of currently unknown neurotransmitters
Novel amino acid transmitters?
unc-47/VGAT is expressed in a substantial number of non-GABAergic neurons (95 out of 302 total neurons in hermaphrodites, plus 61 out of 93 male-specific neurons). However, expression in many of these non-GABAergic neurons is low and variable and such expression may not lead to sufficient amounts of a functional gene product. Yet, in some neurons (e.g. the SIA neurons) expression of unc-47 is easily detectable and robust (based on fosmid-based reporter, reporter allele, and scRNA data), indicating that VGAT may transport another presently unknown neurotransmitter (Gendrel et al., 2016). In vertebrates, VGAT transports both GABA and glycine, and the same is observed for UNC-47 in vitro (Aubrey et al., 2007). While the C. elegans genome encodes no easily recognizable ortholog of known ionotropic glycine receptors, it does encode anion channels that are closely related by primary sequence (Hobert, 2013). Moreover, a recently identified metabotropic glycine receptor, GPR158 (Laboute et al., 2023), has a clear sequence ortholog in C. elegans, F39B2.8. Therefore, glycine may also act as a neurotransmitter in C. elegans. VGAT has also been shown to transport β-alanine (Juge et al., 2013), another potential, but as yet unexplored, neurotransmitter in C. elegans. However, it needs to be pointed out that most of the additional unc-47-positive neurons do not co-express the LAMP-type UNC-46 protein, which is important for sorting UNC-47/VGAT to synaptic vesicles in conventional GABAergic neurons (Schuske et al., 2007). In vertebrates, the functional UNC-46 ortholog LAMP5 is only expressed and required for VGAT transport in a subset of VGAT-positive, GABAergic neurons (Tiveron et al., 2016; Koebis et al., 2019), indicating that alternative vesicular sorting mechanisms exist for UNC-47/VGAT.
Novel monoaminergic transmitters?
Three neuron classes (AVL, PVX, and PVY) express cat-1/VMAT but do not express the canonical synthesis machinery for serotonin, tyramine, octopamine, or dopamine. Neither do they show evidence for uptake of known monoamines. There are also several cat-1/VMAT-positive male-specific neurons that express only a subset of the biosynthetic machinery involved in the biosynthesis of known aminergic transmitters in the worm. That is, some neurons express cat-1/VMAT and bas-1/AAAD, but none of the previously known enzymes that produce the substrate for BAS-1, i.e., CAT-2 or TPH-1 (Figure 1A). In these neurons, BAS-1/AAAD may decarboxylate an unmodified (i.e. non-hydroxylated) aromatic amino acid as substrate to produce, for example, the trace amine PEA from phenylalanine (Table 2, Figure 1—figure supplement 1A). A subset of these neurons (all being B-type ray sensory neurons) co-express tbh-1, which may use PEA as a substrate to produce the trace amine, PEOH. PEOH is a purported neurotransmitter in Aplysia (Saavedra et al., 1977) and the vertebrate brain (Saavedra and Axelrod, 1973) and can indeed be detected in C. elegans extracts (F Schroeder, pers. comm.).
bas-1/AAAD may also be responsible for the synthesis of histamine, an aminergic neurotransmitter that can be found in extracts of C. elegans (Pertel and Wilson, 1974). The only other AAAD that displays reasonable sequence similarity to neurotransmitter-producing AAADs is the hdl-1 gene (Hare and Loer, 2004; Hobert, 2013; Figure 1—figure supplement 1B), for which we, however, did not detect any expression in the C. elegans nervous system (Figure 1—figure supplement 1C and D). Since there are neurons that only express bas-1/AAAD, but no enzyme that produces canonical substrates for bas-1/AAAD (tph-1/TPH, cat-2/TH; Figure 1A), and since at least a subset of these neurons express the monoamine transporter cat-1/VMAT (Table 2), bas-1/AAAD may be involved in synthesizing another currently unknown bioactive monoamine.
Conversely, based on the expression of tph-1, but concurrent absence of bas-1/AAAD, the pharyngeal MI neuron, hermaphrodite VC4 and VC5, and male neurons CEM and R9B may produce 5-HTP (Table 2). 5-HTP may either be used directly as a signaling molecule or it may be metabolized into some other serotonin derivative, an interesting possibility in light of serotonin derivatives produced elsewhere in the body (Yu et al., 2023).
Additionally, three neuron classes (IL2, HOB, and R5B) express tbh-1 but lack expression of any other genes in canonical monoaminergic pathways, including bas-1 (Table 2). Taken together, canonical monoaminergic pathway genes are expressed in unconventional combinations in several neuron classes, pointing toward the existence of yet undiscovered amino acid-derived neuronal signaling systems.
Neurons devoid of canonical neurotransmitter pathway genes may define neuropeptide-only neurons
We identified neurons that do not express any conventional, well-characterized vesicular neurotransmitter transporter families, namely UNC-17/VAChT, CAT-1/VMAT (the only SLC18 family members), UNC-47/VGAT (only SLC32 family member), or EAT-4/VGLUT (an SLC17 family member). Six sex-shared neurons (AVH, BDU, PVM, PVQ, PVW, RMG) and one male-specific neuron (SPD) fall into this category. Most of these neurons exhibit features that are consistent with them being entirely neuropeptidergic. First, electron microscopy has revealed a relative paucity of clear synaptic vesicles in most of these neurons (White et al., 1986; Cook et al., 2019; Witvliet et al., 2021). Second, not only do these neurons express a multitude of neuropeptide-encoding genes (Taylor et al., 2021), but they also display a dense interconnectivity in the ‘wireless’ neuropeptidergic connectome (Ripoll-Sánchez et al., 2023).
That said, electron microscopy shows that some of the neurons devoid of conventional neurotransmitter pathway genes generate synapses with small, clear synaptic vesicles, indicative of the use of non-peptidergic transmitters (e.g. the sex-shared RMG and PVM neurons or the male-specific SPD neurons) (White et al., 1986; Cook et al., 2019; Witvliet et al., 2021). It is therefore conceivable that either conventional neurotransmitters utilize non-conventional neurotransmitter synthesis and/or release pathways, or that completely novel neurotransmitter systems remain to be discovered. Although the C. elegans genome does not encode additional members of the SLC18A2/3 (cat-1/VMAT, unc-17/VAChT) or SLC32A1 (unc-47/VGAT) family of vesicular neurotransmitter transporters, it does contain a number of additional members of the SLC17A6/7/8 (VGLUT) family (Hobert, 2013). These may serve as non-canonical vesicular transporters of more uncommon neurotransmitters or, alternatively, may be involved in modulating release of glutamate (Serrano-Saiz et al., 2020; Choi et al., 2021). Uncharacterized paralogs of bona fide neurotransmitter uptake transporters (SLC6 superfamily) may also have functions in neurotransmitter release rather than uptake. However, based on CeNGEN scRNA data, no robust or selective expression of these SLC17 or SLC6 family members is observed in these ‘orphan neurons’.
Co-transmission of multiple neurotransmitters
Our analysis expands the repertoire of neurons that co-transmit multiple neurotransmitters (Figure 3). Neurotransmitter co-transmission has been observed in multiple combinations in the vertebrate brain (Wallace and Sabatini, 2023). In C. elegans, the most frequent co-transmission configurations are a classic, fast transmitter (acetylcholine or glutamate) with a monoamine. Co-transmission of two distinct monoaminergic systems also exists. In several cases, however, it is not clear whether the second neurotransmitter is indeed used for communication or whether its presence is merely a reflection of this neuron being solely a clearance neuron. For example, the glutamatergic AIM neuron stains positive for serotonin, which it uptakes via the uptake transporter MOD-5, but it does not express the vesicular monoamine transporter cat-1/VMAT (Figures 3, 6, and 8, Tables 1 and 2).
Co-transmission of small, fast-acting neurotransmitters (glutamate, GABA, acetylcholine) does exist, but it is rare (Figure 3). The most prominent co-transmission configuration is acetylcholine with glutamate, but acetylcholine can also be co-transmitted with GABA. There are no examples of co-transmission of glutamate and GABA, as observed in several regions of the vertebrate brain (Wallace and Sabatini, 2023). There are also examples of possible co-transmission of three transmitters (Figure 3).
Interestingly, co-transmission appears to be much more prevalent in the male-specific nervous system, compared to the sex-shared nervous system (Figure 3, Supplementary file 3). This may relate to male-specific neurons displaying a greater degree of anatomical complexity compared to the hermaphrodite nervous system, both in terms of branching patterns and extent of synaptic connectivity (Jarrell et al., 2012; Cook et al., 2019). Given that all co-transmitting neurons display multiple synaptic outputs (Cook et al., 2019), it appears possible that each individual neurotransmitter secretory system is distributed to distinct synapses. Based on vertebrate precedent (Wallace and Sabatini, 2023), co-release from the same vesicles is also possible.
Sexual dimorphisms in neurotransmitter usage
The observation of sexual dimorphisms in neurotransmitter abundance in specific regions of the mammalian brain has been one of the earliest molecular descriptors of neuronal sex differences in mammals (McCarthy et al., 1997). However, it has remained unclear whether such differences are the result of the presence of sex-specific neurons or are indications of distinctive neurotransmitter usage in sex-shared neurons. Using C. elegans as a model, we have been able to precisely investigate (a) whether sex-specific neurons display a bias in neurotransmitter usage and (b) whether there are neurotransmitter dimorphisms in sex-shared neurons (Pereira et al., 2015; Gendrel et al., 2016; Serrano-Saiz et al., 2017b) (this paper). We found that male-specific neurons display a roughly similar proportional usage of individual neurotransmitter systems and note that male-specific neurons display substantially more evidence of co-transmission, a possible reflection of their more elaborate morphology and connectivity. We also confirmed evidence for sexual dimorphisms in neurotransmitter usage in sex-shared neurons (Supplementary file 4), which are usually correlated with sexual dimorphisms in synaptic connectivity of these sex-shared neurons (Cook et al., 2019).
Neurotransmitter pathway genes in glia and gonad
Neurotransmitter uptake is a classic function of glial cells across animal phylogeny (Henn and Hamberger, 1971), and such uptake mechanisms are observed in C. elegans as well. Previous reports demonstrated glutamate uptake by CEPsh (Katz et al., 2019) and GABA uptake by GLR glia (Gendrel et al., 2016). We now add to this list betaine uptake by most glia, as inferred from the expression pattern of SNF-3/BGT1 (Figure 9, Supplementary file 1).
Studies in vertebrates have also suggested that specific glial cell types synthesize and release several neurotransmitters (Araque et al., 2014; Savtchouk and Volterra, 2018). For example, astrocytes were recently shown to express VGLUT1 to release glutamate (de Ceglia et al., 2023). Evidence of neurotransmitter synthesis and release also exists in C. elegans glia. Previous work indicated that glia associated with male-specific spicule neurons synthesize (through cat-2/TH and bas-1/AAAD) the monoaminergic transmitter dopamine to control sperm ejaculation (LeBoeuf et al., 2014). Our identification of cat-1/VMAT expression in these glia indicate that dopamine is released via the canonical vesicular monoamine transporter. We also detected expression of bas-1/AAAD in additional male and hermaphrodite glia, indicating the production of other signaling substances released by these glia. bas-1 has indeed recently been shown to be involved in the synthesis of a class of unconventional serotonin derivatives (Yu et al., 2023).
There have been previous reports on GABA synthesis and release from the AMsh glial cell type (Duan et al., 2020; Fernandez-Abascal et al., 2022). We were not able to detect AMsh with anti-GABA staining, nor with reporter alleles of unc-25/GAD. However, since very low levels of unc-25 are observed in the AMsh scRNA datasets (Taylor et al., 2021; Purice et al., 2023), the abundance of GABA in AMsh may lie below conventional detection levels.
Outside the nervous system, the most prominent and functionally best characterized usage of neurotransmitters lies in the hermaphrodite somatic gonad, which has been shown to synthesize octopamine and use it to control oocyte quiescence (Alkema et al., 2005; Kim et al., 2021). Intriguingly, we also detected tbh-1, tdc-1, and cat-1 expression in the somatic gonad of the male, specifically the vas deferens, which is known to contain secretory granules that are positive for secretory molecular markers (Nonet et al., 1993). The presence of octopamine is unexpected because, unlike oocytes, sperm are not presently known to require monoaminergic signals for any aspect of their maturation. It will be interesting to assess sperm differentiation and function of tbh-1 or tdc-1 mutant animals. The usage of monoaminergic signaling systems in the gonad is not restricted to C. elegans and has been discussed in the context of sperm functionality and oocyte maturation in vertebrates (Mayerhofer et al., 1999; Ramírez-Reveco et al., 2017; Alhajeri et al., 2022).
Comparing approaches and caveats of expression pattern analysis
Our analysis also provides an unprecedented and systematic comparison of antibody staining, scRNA transcript data, reporter transgene expression, and knock-in reporter allele expression. The bottom-line conclusions of these comparisons are: (1) Reporter alleles reveal more sites of expression than fosmid-based reporters. It is unclear whether this is due to the lack of cis-regulatory elements in fosmid-based reporters or issues associated with the multicopy nature of these reporters (e.g. RNAi-based gene silencing of multicopy arrays or squelching of regulatory factors). Another factor to consider is that neuron identification for most fosmid-based reporters was carried out prior to the introduction of NeuroPAL. Consequently, errors occasionally occurred, as exemplified by the misidentification of neuron IDs for CA7 and CP7 in previous instances (Serrano-Saiz et al., 2017b). (2) The best possible reporter approaches (i.e. reporter alleles) show very good overlap with scRNA data, thereby validating each approach. However, our comparisons also show that no single approach is perfect. CeNGEN scRNA data can miss transcripts and can also show transcripts in cells in which there is no independent evidence for gene or protein expression. Conversely, antibody staining displays vagaries related to staining protocols and protein localization, which can be overcome with reporter approaches, but the price to pay with reporter alleles is that if they are based on SL2 or T2A strategies, they may fail to detect additional levels of posttranslational regulation, which may result in protein absence even in the presence of transcripts. The existence of such mechanisms may be a possible explanation for cases where the expression of synthesis and/or transport machinery expression does not match up (e.g. tdc-1(-); tbh-1(+) neurons).
Our detailed analysis of reporter allele expression has uncovered several cases where expression of a neurotransmitter pathway gene in a given neuron class appears very low and variable from animal to animal. Such variability only exists when expression is dim, thus one possible explanation for it is that expression levels merely hover around an arbitrary microscopical detection limit. However, we cannot rule out the other possibility that this may also reflect true on/off variability of gene expression. Taking this notion a step further, we cannot exclude the possibility that expression observed with reporter alleles misses sites of expression. This possibility is raised by our inability to detect unc-25/GAD reporter allele expression in AMsh glia (Duan et al., 2020; Fernandez-Abascal et al., 2022) or eat-4 reporter allele expression in AVL and DVB neurons, in which some (but not other) multicopy reporter transgenes revealed expression of the respective genes (Li et al., 2023). Functions of these genes in the respective cell types were corroborated by cell-type-specific RNAi experiments and/or rescue experiments; whether there is indeed very low expression of these genes in those respective cells or whether drivers used in these studies for knock-down and/or rescue produce very low expression in other functionally relevant cells remains to be resolved.
Conclusions
In conclusion, we have presented here the most complete neurotransmitter map that currently exists for any animal nervous system. Efforts to map neurotransmitter usage on a system-wide level are underway in other organisms, most notably, Drosophila melanogaster (Deng et al., 2019; Eckstein et al., 2024). The C. elegans neurotransmitter map presented here comprises a critical step toward deciphering information flow in the nervous system and provides valuable tools for studying the genetic mechanisms underlying cell identity specification. Moreover, this neurotransmitter map opens new opportunities for investigating sex-specific neuronal differentiation processes, particularly in the male-specific nervous system, where a scarcity of molecular markers has limited the analysis of neuronal identity control. Lastly, our analysis strongly suggests that additional neurotransmitter systems remain to be identified.
While the gene expression patterns delineated here enable informed predictions about novel neuronal functions and neurotransmitter identities, further investigations involving genetic perturbations, high-resolution imaging, complementary functional assays, and analyses across developmental stages are needed to shed further light on neurotransmitter usage. Nonetheless, this comprehensive neurotransmitter map provides a robust foundation for deciphering neural information flow, elucidating developmental mechanisms governing neuronal specification, exploring sexual dimorphisms in neuronal differentiation, and potentially uncovering novel neurotransmitter systems awaiting characterization.
Materials and methods
Transgenic reporter strains
Request a detailed protocolKnock-in reporter alleles were generated either by SunyBiotech (syb alleles) or in-house (ot alleles) using CRISPR/Cas9 genome engineering. Most genes were tagged with a nuclear-targeted gfp sequence (gfp fused to his-44, a histone h2b gene) at the 3' end of the locus to capture all isoforms, except tdc-1 which was tagged at the 5' end. For unc-25, both isoforms were individually tagged since a single tag would not capture both. Transgene schematics are shown in Figure 2.
Reporter alleles generated in this study:
unc-25(ot1372[unc-25a.1c.1::t2a:gfp::h2b]) III
unc-25(ot1536[unc-25b.1::t2a::gfp::h2b]) III
unc-46(syb7278[unc-46::sl2::gfp:h2b]) V
unc-47(syb7566[unc-47::sl2::gfp::h2b]) III
cat-1(syb6486[cat-1::sl2::gfp::h2b]) X
tph-1(syb6451[tph-1::sl2::gfp::h2b]) II
tbh-1(syb7786[tbh-1::sl2::gfp::h2b]) X
tdc-1(syb7768[gfp::linker::h2b::t2a::tdc-1]) II
cat-2(syb8255[cat-2::sl2::gfp::h2b]) II
snf-3(syb7290[snf-3::TagRFP::sl2::gfp::h2b]) II
oct-1(syb8870[oct-1::sl2::gfp::h2b]) I
hdl-1(syb1048[hdl-1::gfp]) IV
hdl-1(syb4208[hdl-1::t2a::3xnls::cre]) IV
Since we did not detect fluorophore signals in the hdl-1(syb1048[hdl-1::gfp]) strain, we attempted to amplify low-level signals, by inserting Cre recombinase at the C-terminus of the hdl-1 locus (hdl-1(syb4208[hdl-1::t2a::3xnls::cre])). We crossed this strain to the recently published ‘Flexon’ strain (arTi361[rps-27p::gfp"flexon"-h2b::unc-54–3'UTR]) (Shaffer and Greenwald, 2022). Even low expression of hdl-1 should have led to Cre-mediated excision of the flexon stop cassette, which is designed to abrogate gene expression by a translational stop and frameshift mutation, and subsequently can result in strong and sustained gfp expression under the control of the rps-27 promoter and thereby providing information about cell-specific hdl-1 expression. However, no robust, consistent reporter expression was seen in hdl-1(syb4208[hdl-1::t2a::3xnls::cre]); arTi361[rps-27p::gfp"flexon"-h2b::unc-54–3'UTR] animals.
Three of the reporter alleles that we generated were already previously examined in specific cellular contexts:
unc-17(syb4491[unc-17::t2a::gfp:h2b]) IV (Vidal et al., 2022)
eat-4(syb4257[eat-4::t2a::gfp::h2b]) III (Vidal et al., 2022)
bas-1(syb5923[bas-1::sl2::gfp::h2b]) III (Yu et al., 2023)
One of the reporter alleles was obtained from the Caenorhabditis Genetics Center (CGC):
mod-5(vlc47[mod-5::t2a::mNeonGreen]) I (Maicas et al., 2021)
Microscopy and image processing
Request a detailed protocolFor adult animal imaging, 15–25 (exact number depending on the difficulty of neuron ID) same-sex L4 worms were grouped on NGM plates 6–9 hr prior to imaging to control for accurate staging and avoid mating. Young adult worms were then anesthetized using 50–100 mM sodium azide and mounted on 5% agarose pads on glass slides. Z-stack images were acquired with ZEN software using Zeiss confocal microscopes LSM880 and LSM980 or a Zeiss Axio Imager Z2 and processed with ZEN software or FIJI (Schindelin et al., 2012) to create orthogonal projections. Brightness and contrast, and in some cases gamma values, were adjusted to illustrate dim expression and facilitate neuron identification.
Neuron class and cell-type identification
Request a detailed protocolNeuron classes were identified by crossing the gfp reporter alleles with the landmark strain ‘NeuroPAL’ (allele otIs669 or otIs696, for bright reporters and dim reporters, respectively) and following published protocols (Tekieli et al., 2021; Yemini et al., 2021) (also see ‘lab resources’ at hobertlab.org). For neuron identification of the eat-4(syb4257), unc-46(syb7278), and unc-47(syb7566) reporter alleles in hermaphrodites, the reporter alleles were also crossed into the fosmid-based reporter transgenes of the same gene [eat-4(otIs518), unc-46(otIs568), and unc-47(otIs564)] as a ‘first-pass’ to identify potential non-overlapping expression of the two alleles. For tph-1(syb6451) analysis, an eat-4 fosmid-based reporter (otIs518) was also used. For identification of VC4, VC5, HSN, and uv1, an ida-1p::mCherry integrant (LX2478, lin-15(n765ts) X; vsls269[ida-1::mCherry]) was also used in some cases (Fernandez et al., 2020). For phasmid neurons, dye-filling with DiD (Thermo Fisher Scientific) was sometimes used to confirm neuron ID. For glial expression, a panglial reporter otIs870[mir-228p::3xnls::TagRFP] was used. For hypodermal cells identification, a dpy-7p::mCherry reporter stIs10166 [dpy-7p::his-24::mCherry+unc-119(+)] was used (Liu et al., 2009).
Resource availability
Lead contact
Request a detailed protocolOliver Hobert (or38@columbia.edu) is the Lead Contact.
Materials availability
Request a detailed protocolAll newly generated strains are available at the Caenorhabditis Genetics Center (CGC).
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
-
Alternative splicing leads to two cholinergic proteins in Caenorhabditis elegansJournal of Molecular Biology 241:627–630.https://doi.org/10.1006/jmbi.1994.1538
-
Neurotransmitters, neuropeptides and calcium in oocyte maturation and early developmentFrontiers in Cell and Developmental Biology 10:980219.https://doi.org/10.3389/fcell.2022.980219
-
The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotypeThe Journal of Neuroscience 27:6273–6281.https://doi.org/10.1523/JNEUROSCI.1024-07.2007
-
Catecholamine transport by the organic cation transporter type 1 (OCT1)British Journal of Pharmacology 125:218–224.https://doi.org/10.1038/sj.bjp.0702065
-
Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoaminesAmerican Journal of Physiology. Cell Physiology 280:C1616–C1622.https://doi.org/10.1152/ajpcell.2001.280.6.C1616
-
Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegansThe Journal of Comparative Neurology 506:398–408.https://doi.org/10.1002/cne.21551
-
Cellular expression and functional roles of all 26 neurotransmitter GPCRs in the C. elegans egg-laying circuitThe Journal of Neuroscience 40:7475–7488.https://doi.org/10.1523/JNEUROSCI.1357-20.2020
-
BookThe neuronal genome of Caenorhabditis elegansIn: Hobert O, editors. WormBook: The Online Review of C. elegans Biology. WormBook. pp. 1–106.
-
Vesicular GABA transporter (VGAT) transports β-alanineJournal of Neurochemistry 127:482–486.https://doi.org/10.1111/jnc.12393
-
Maintenance of quiescent oocytes by noradrenergic signalsNature Communications 12:6925.https://doi.org/10.1038/s41467-021-26945-x
-
Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegansThe Journal of Neuroscience 13:5407–5417.https://doi.org/10.1523/JNEUROSCI.13-12-05407.1993
-
Sources and function of neuronal signalling molecules in the gonadsMedicina 59:542–545.
-
Excitatory neurotransmission and sexual differentiation of the brainBrain Research Bulletin 44:487–495.https://doi.org/10.1016/s0361-9230(97)00230-x
-
The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABAMolecular Biology of the Cell 17:3021–3030.https://doi.org/10.1091/mbc.e06-02-0155
-
The SLC22 transporter family: A paradigm for the impact of drug transporters on metabolic pathways, signaling, and diseaseAnnual Review of Pharmacology and Toxicology 58:663–687.https://doi.org/10.1146/annurev-pharmtox-010617-052713
-
Betaine acts on a ligand-gated ion channel in the nervous system of the nematode C. elegansNature Neuroscience 16:1794–1801.https://doi.org/10.1038/nn.3575
-
Histamine content of the nematode, Caenorhabditis elegansComparative and General Pharmacology 5:83–85.https://doi.org/10.1016/s0306-3623(74)80011-x
-
Neuronal signaling repertoire in the mammalian sperm functionalityBiology of Reproduction 96:505–524.https://doi.org/10.1095/biolreprod.116.144154
-
BookNeurotransmitter assignments for specific neuronsIn: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C.elegans II. Cold Spring Harbor Laboratory Press. pp. 1049–1052.
-
Gliotransmission: Beyond black-and-whiteThe Journal of Neuroscience 38:14–25.https://doi.org/10.1523/JNEUROSCI.0017-17.2017
-
Fiji: an open-source platform for biological-image analysisNature Methods 9:676–682.https://doi.org/10.1038/nmeth.2019
-
UNC-46 is required for trafficking of the vesicular GABA transporterNature Neuroscience 10:846–853.https://doi.org/10.1038/nn1920
-
Dopaminergic neurons in the nematode Caenorhabditis elegansThe Journal of Comparative Neurology 163:215–226.https://doi.org/10.1002/cne.901630207
-
The Caenorhabditis elegans male: postembryonic development of nongonadal structuresDevelopmental Biology 78:542–576.https://doi.org/10.1016/0012-1606(80)90352-8
-
The structure of the nervous system of the nematode Caenorhabditis elegansPhilosophical Transactions of the Royal Society of London. Series B, Biological Sciences 314:1–340.https://doi.org/10.1098/rstb.1986.0056
-
Parallel pathways for serotonin biosynthesis and metabolism in C. elegansNature Chemical Biology 19:141–150.https://doi.org/10.1038/s41589-022-01148-7
Article and author information
Author details
Funding
National Institutes of Health (NS039996)
- Oliver Hobert
Howard Hughes Medical Institute
- Oliver Hobert
National Institutes of Health (Office of Research Infrastructure Programs P40 OD010440)
- Oliver Hobert
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Chi Chen for generating nematode strains. We thank Emily Bayer, James Rand, Piali Sengupta, and Esther Serrano-Saiz for comments on the manuscript, Frank Schroeder and Marie Gendrel for discussion and communicating unpublished results, Aakanksha Singhvi for discussing glia scRNA data and Michael Koelle for an ida-1 reporter strain. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was funded by the Howard Hughes Medical Institute and by NIH R01 NS039996.
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.95402. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Wang et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 5,123
- views
-
- 500
- downloads
-
- 30
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 10
- citations for umbrella DOI https://doi.org/10.7554/eLife.95402
-
- 3
- citations for Reviewed Preprint v1 https://doi.org/10.7554/eLife.95402.1
-
- 2
- citations for Reviewed Preprint v2 https://doi.org/10.7554/eLife.95402.2
-
- 15
- citations for Version of Record https://doi.org/10.7554/eLife.95402.3






