Impact of the clinically approved BTK inhibitors on the conformation of full-length BTK and analysis of the development of BTK resistance mutations in chronic lymphocytic leukemia
Figures

Inhibition of Bruton’s tyrosine kinase (BTK) is an effective way to treat chronic lymphocytic leukemia (CLL).
(a) Domain organization of full-length (FL) BTK and the BTK linker-kinase domain (LKD) fragment used in this study: PHTH, Pleckstrin homology-Tec homology domain; PRR, proline-rich region; SH3, Src homology 3 domain; SH2, Src homology 2 domain, SH2-kinase linker (L) and the catalytic kinase domain. Key residues are indicated above each domain. (b) Autoinhibited conformation of FL BTK based on the crystal structure of FL BTK (Lin et al., 2024). The PHTH domain (purple) is dynamic, in transient contact with several regions on the core SH3-SH2-kinase domain, and is not visible in the crystal structure of full-length BTK (Lin et al., 2024). Dynamics of the PHTH domain is represented by the multiple poses of the PHTH domain and the double-headed arrow. (c) Co-crystal structure of BTK LKD (light cyan cartoon) bound to Ibrutinib (PDB ID: 5P9J) showing the location of C481 (yellow spheres), T474, and L528 (red spheres) within the kinase active site (broken oval). (c) Pie charts showing the prevalence of the BTK resistance mutations in CLL patients treated with various BTK inhibitors. The total number of patients with mutations in BTK are indicated below each chart. See Supplementary file 1 for additional details.

The Bruton’s tyrosine kinase (BTK) kinase domain can interconvert between active and inactive conformations.
(a) Superposition of the structure of BTK linker-kinase domain (LKD) bound to Dasatinib (PDB ID: 3K54) in the active kinase conformation (gray cartoon) with the Ibrutinib bound structure (PDB ID: 5P9J) in the inactive conformation (red cartoon). The expanded inset shows the inward movement of the αC-helix, the change in W395 rotamer conformation, and the K430/E445 salt bridge formation that accompanies kinase activation. (b–f) Co-crystal structures of BTK LKD (light cyan cartoon) bound to Ibrutinib (PDB ID: 5P9J), Acalabrutinib (PDB ID: 8FD9), Zanubrutinib (PDB ID: 6J6M), Tirabrutinib (PDB ID: 5P9M) and Pirtobrutinib (PDB ID: 8FLL) in the inactive kinase conformation. The inhibitors are shown as dark blue sticks, the kinase activation loop is purple and C481, Y551, and W395 residues are shown as sticks with transparent spheres. Electron density for part of the activation loop is missing in the Ibrutinib co-crystal structure and is indicated as dotted lines (b). In the Acalabrutinib structure, the activation loop has several mutations (Lin and Andreotti, 2023) and the SH2-kinase linker (including W395) is absent (c). Electron density for the W395 sidechain is missing in the BTK:Pirtobrutinib co-crystal structure (f). (g) Overlay of the BTK:Ibrutinib, Acalabrutinib, Zanubrutinib, Tirabrutinib, and Pirtobrutinib co-crystal structures. With the exception of the Tirabrutinib co-crystal structure (e), no major structural variation is observed in the kinase domains. The activation loop in the Tirabrutinib bound structure adopts a different conformation compared to the other co-crystal structures.
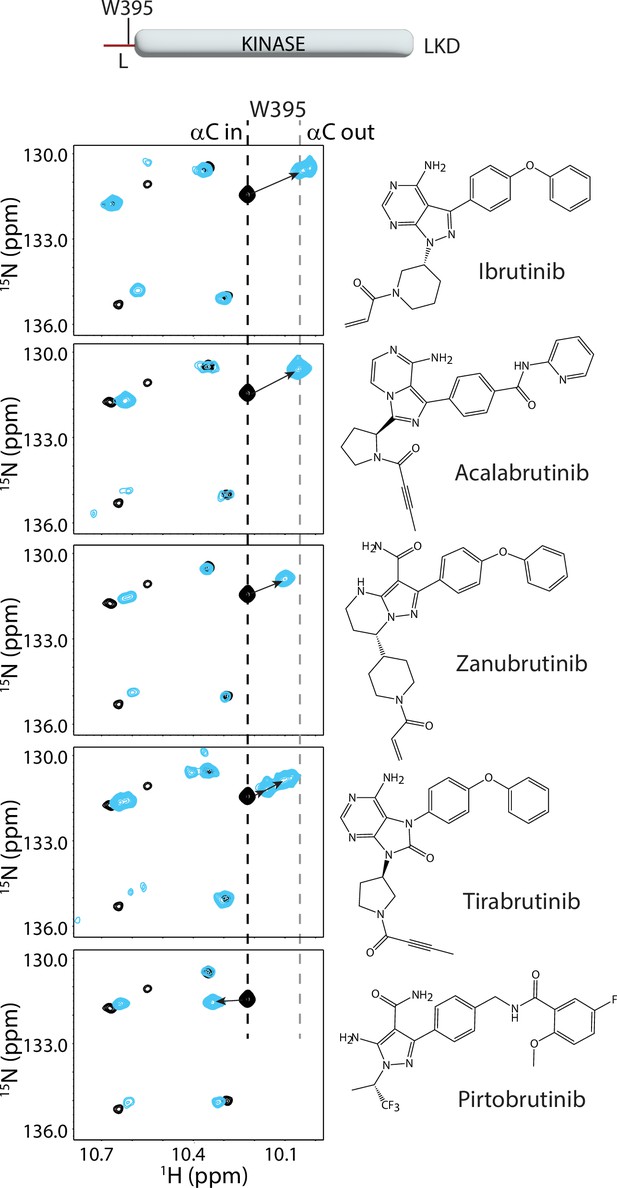
Bruton’s tyrosine kinase (BTK) inhibitors stabilize the inactive kinase conformation in solution.
The tryptophan side chain region of the 1H-15N TROSY HSQC spectra of 15N-labeled apo BTK linker-kinase domain (black spectrum) overlaid with that of the inhibitor bound spectrum (cyan spectrum). Here and in subsequent figures, the broken black and gray lines indicate the position of the BTK W395 resonance in the active (αC-in) and inactive (αC-out) states, respectively as shown in Figure 2a. The shift in the BTK W395 resonance upon inhibitor binding is indicated by an arrow in each spectrum. The structures of each inhibitor are shown on the right. The BTK W395 indole NH resonance is in the inactive (αC-out) position in the Ibrutinib (published earlier Joseph et al., 2020), Acalabrutinib, Zanubrutinib and Tirabrutinib bound BTK linker-kinase domain (LKD) samples. Multiple peaks corresponding to W395 are seen in the Tirabrutinib bound spectrum suggesting that the kinase adopts multiple conformations in solution. The downfield shift observed in W395 in the Pirtobrutinib bound structure is likely due to local changes in the chemical environment due to the distinct chemical structure of Pirtobrutinib. W395 assignments in the inhibitor-bound spectra were confirmed by acquiring inhibitor-bound spectra with the BTK LKD W395A mutant (see Figure 3—figure supplement 1).

Nuclear magnetic resonance (NMR) analysis of inhibitor binding.
(a) Cartoon of the Bruton’s tyrosine kinase (BTK) linker-kinase domain (LKD) fragment used for NMR analysis. (b) Assignment of W395 in inhibitor-bound spectra of BTK LKD. The tryptophan side chain region of the 1H-15N TROSY HSQC spectra of 15N-labeled inhibitor-bound BTK LKD wild-type (WT) (black spectrum) overlaid with that of the inhibitor-bound BTK LKD W395A spectrum (cyan spectrum). The boxed peak indicates the W395 resonance in each of the WT inhibitor-bound spectrums.
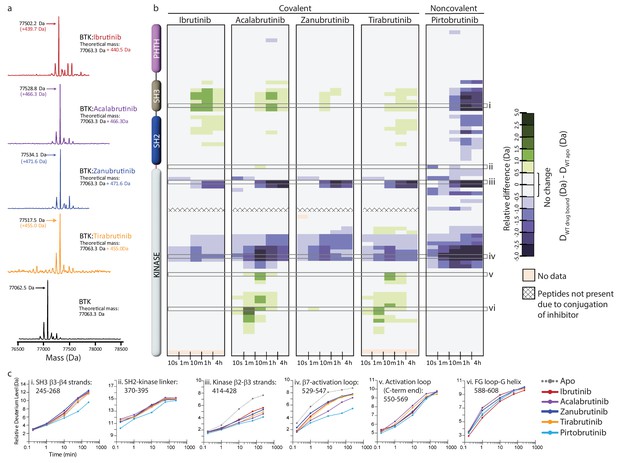
Assessing the impact of Bruton’s tyrosine kinase (BTK) inhibitors on full-length BTK by hydrogen deuterium exchange mass spectrometry (HDX-MS).
(a) Intact mass analysis of wild-type full-length (FL) BTK before (bottom spectrum, black) and after 1 hr incubation with a twofold molar excess of covalent BTK inhibitors: Ibrutinib (red), Acalabrutinib (purple), Zanubrutinib (blue), and Tirabrutinib (orange) show a mass increase of one inhibitor molecule. (b) Clinically approved BTK inhibitors induce allosteric changes in full-length BTK. Relative deuterium level of peptides in apo BTK was subtracted from the deuterium level of the corresponding peptide from each drug-bound form of BTK (DWT drug-bound-DWT apo) and the differences were colored according to the scale shown. In this and subsequent figures, peptic peptides are shown from N- to C-terminus, top to bottom, and the amount of time in deuterium is shown from left to right. The relative difference data shown here represents a curated set of peptides that are coincident across all 6 states (apo and five drug-bound BTK forms). The identification of these chosen peptides, the relative difference values, and the complete data set for each state can be found in the Supplementary file 2. The approximate position of the domains of BTK, as described in Figure 1a, is shown on the left. Deuterium incorporation curves of selected peptides (indicated with a gray box in panel b and labeled i-vi) from various regions of the protein are shown below. Data for Ibrutinib has been previously published (Joseph et al., 2020).
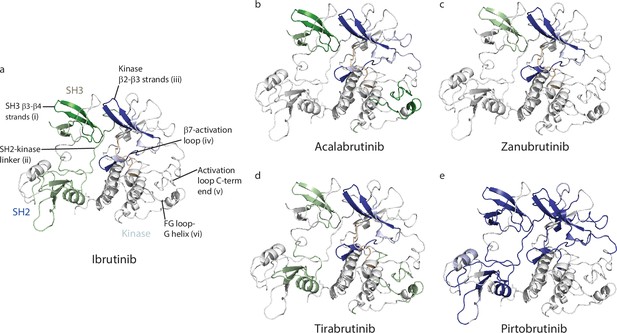
Inhibitor binding causes changes in Bruton’s tyrosine kinase (BTK).
(a–e) Mapping the hydrogen deuterium exchange mass spectrometry (HDX-MS) changes induced by each BTK inhibitor on the structure of the BTK SH3-SH2-kinase fragment (PDB ID: 4XI2).Changes mapped onto the structure represent the maximal HDX-MS change that occurred at any time point. Major differences greater than 1.0 Da are shown as dark blue (decrease) or dark green (increase); modest differences between 0.5 Da and 1.0 Da are shown as light blue (decrease) and light green (increase). Localization of the changes in deuterium incorporation was accomplished using overlapping peptides included in the complete peptide data set provided in the Supplementary file 2. The location of peptides i – vi from Figure 4 are indicated in panel a. Data corresponding to Ibrutinib has been previously published (Joseph et al., 2020).
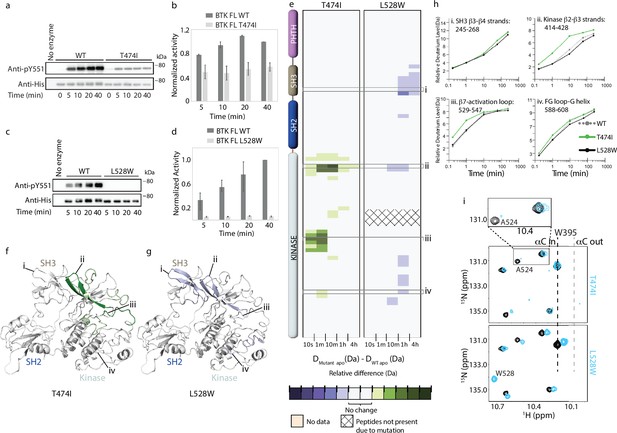
Probing the impact of the Bruton’s tyrosine kinase (BTK) resistance mutations T474I and L528W on BTK.
(a–d) Western blot comparing the kinase activity of full-length (FL) BTK wild-type (WT), T474I and L528W mutants. BTK autophosphorylation was monitored using the BTK pY551 antibody and the total protein levels were monitored using the Anti-His antibody. (b, d) Histogram quantifying the western blots shown in (a, c). The blots were quantified and normalized as described in the Materials and Methods. Data shown are the average of three independent experiments with standard deviations. (e) HDX difference data for the BTK T474I and L528W mutants (DMutant apo -DWT apo). Color scale and peptide/time course arrangement are the same as in Figure 4. See the Supplementary file 2 for additional information, including all peptide identifications and deuterium values. (f, g) Mapping the mutational induced hydrogen deuterium exchange mass spectrometry (HDX-MS) changes on the structure of the BTK SH3-SH2-kinase fragment. Changes mapped onto the structure represent the maximal HDX-MS change that occurred at any time point. (h) Deuterium incorporation curves of selected peptides (indicated with a gray box in panel e and labeled i-iv) from various regions of the protein are shown. (i) The tryptophan side chain region of the 1H-15N TROSY HSQC spectra of 15N-labeled apo WT BTK linker-kinase domain (black spectrum) overlaid with that of the apo mutant kinase spectrum (cyan spectrum). The boxed region in the T474I spectral overlay is expanded above.
-
Figure 6—source data 1
Original unedited files for western blot analysis displayed in Figure 6a.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig6-data1-v1.zip
-
Figure 6—source data 2
Figure 6a along with original files for western blots displayed in Figure 6a with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig6-data2-v1.zip
-
Figure 6—source data 3
Original unedited files for western blot analysis displayed in Figure 6c.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig6-data3-v1.zip
-
Figure 6—source data 4
Figure 6c along with original files for western blots displayed in Figure 6c with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig6-data4-v1.zip
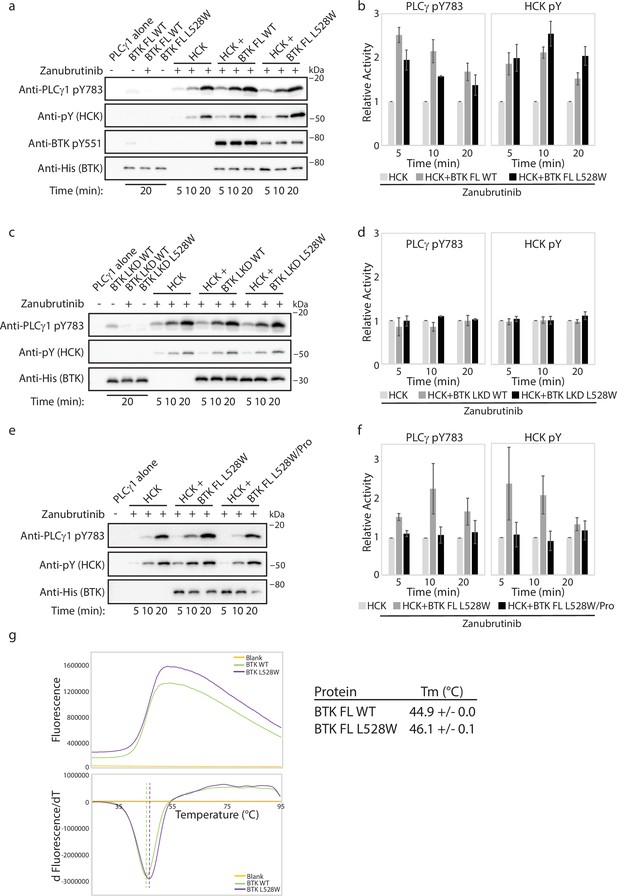
The Bruton’s tyrosine kinase (BTK) L528W mutant can activate HCK.
(a–f) Kinase activity of HCK in the presence or absence of full-length BTK L528W mutant was compared in a western blot assay by monitoring PLCγ1 phosphorylation (pY783 antibody) and HCK autophosphorylation (pY antibody). BTK phosphorylation on Y551 was monitored using the Anti-pY551 antibody. Total protein levels were monitored using the Anti-His antibody. Full-length wild-type (WT) BTK preincubated with Zanubrutinib was used as a control. (b, d, f) Histogram quantifying the western blots shown in (a, c, e). The blots were quantified and normalized as described in the Materials and methods. Data shown are the average of three independent experiments with standard deviations. (b, c) Kinase activity of HCK in the presence or absence of the isolated linker-kinase domain (LKD) fragment of the BTK L528W mutant (b) or the full-length proline mutant of BTK L528W (BTK full-length (FL) L528W/Pro: BTK L528W/ P189A/P192A/P203A/P206A, (c)) was compared as in (a). (g) Thermal stability analysis of BTK FL WT and BTK FL L528W. Data shown are the average of three independent experiments with standard deviations.
-
Figure 7—source data 1
Original unedited files for western blot analysis displayed in Figure 7a.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data1-v1.zip
-
Figure 7—source data 2
Figure 7a with original files for western blots displayed in Figure 7a with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data2-v1.zip
-
Figure 7—source data 3
Original unedited files for western blot analysis displayed in Figure 7c.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data3-v1.zip
-
Figure 7—source data 4
Figure 7c with original files for western blots displayed in Figure 7c with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data4-v1.zip
-
Figure 7—source data 5
Original unedited files for western blot analysis displayed in Figure 7e.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data5-v1.zip
-
Figure 7—source data 6
Figure 7e with original files for western blots displayed in Figure 7e with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-data6-v1.zip
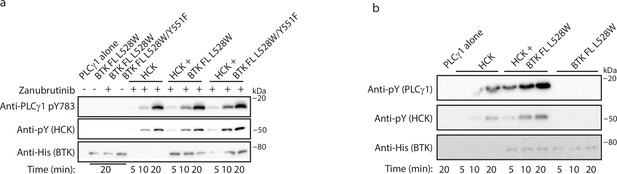
Bruton’s tyrosine kinase (BTK) full-length (FL) L528W is able to activate HCK.
(a) Phosphorylation on BTK Y551 is not required for HCK activation by BTK FL L528W. Kinase activity of HCK in the presence or absence of full-length BTK L528W single or L528W/Y551F double mutant was compared in a western blot assay by monitoring PLCγ1 phosphorylation (pY783 antibody) and HCK autophosphorylation (pY antibody). Total protein levels were monitored using the Anti-His antibody. (b) Zanubrutinib does not require activation of HCK by BTK FL L528W. Kinase activity of HCK in the presence or absence of full-length BTK L528W mutant, without Zanubrutinib was compared in a western blot assay by monitoring PLCγ1 phosphorylation and HCK autophosphorylation (pY antibody). Total protein levels were monitored using the Anti-His antibody.
-
Figure 7—figure supplement 1—source data 1
Original unedited files for western blot analysis displayed in Figure 7—figure supplement 1a.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-figsupp1-data1-v1.zip
-
Figure 7—figure supplement 1—source data 2
Figure 7—figure supplement 1a with original files for western blots displayed in Figure 7—figure supplement 1a with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-figsupp1-data2-v1.zip
-
Figure 7—figure supplement 1—source data 3
Original unedited files for western blot analysis displayed in Figure 7—figure supplement 1b.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-figsupp1-data3-v1.zip
-
Figure 7—figure supplement 1—source data 4
Figure 7—figure supplement 1b with original files for western blots displayed in Figure 7—figure supplement 1b with sample labels.
- https://cdn.elifesciences.org/articles/95488/elife-95488-fig7-figsupp1-data4-v1.zip
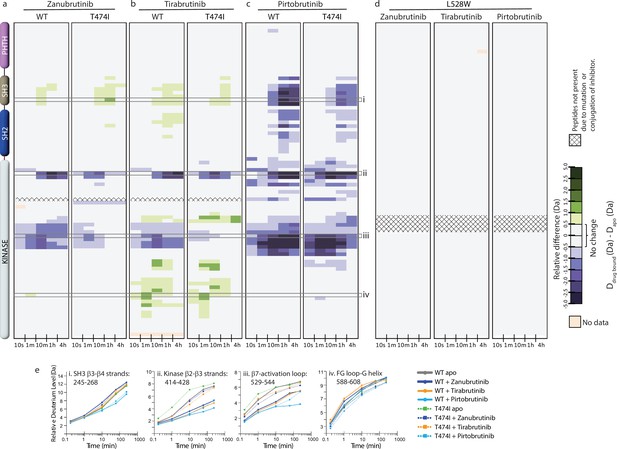
Hydrogen deuterium exchange mass spectrometry (HDX-MS) analysis of Bruton’s tyrosine kinase (BTK) inhibitor binding to BTK T474I and L528W mutants.
(a–d) Relative deuterium level of peptides in apo mutant BTK was subtracted from the deuterium level of the corresponding peptide from each inhibitor-bound form of BTK (Ddrug-bound-Dapo) and compared to the changes in the wild-type (WT) protein. The differences are colored according to the scale shown. (e) Deuterium incorporation curves of selected peptides (indicated with a gray box in panels a-c, and labeled i-iv) are shown.
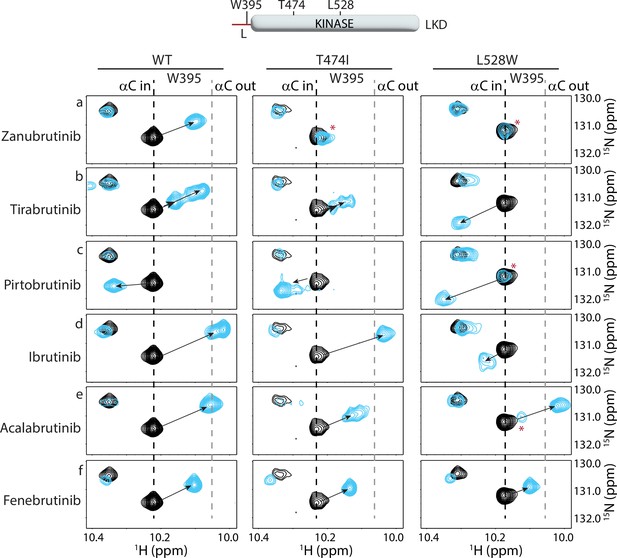
Nuclear magnetic resonance (NMR) analysis of Bruton’s tyrosine kinase (BTK) inhibitor binding to the BTK T474I and L528W mutants.
The tryptophan side chain region of the 1H-15N TROSY HSQC spectra of 15N-labeled apo BTK linker-kinase domain (black spectrum) overlaid with that of the inhibitor bound spectrum (cyan spectrum). The broken black and gray lines indicate the position of the BTK W395 resonance and have been described earlier in Figure 3. The shift in the BTK W395 indole NH resonance upon inhibitor binding is indicated by an arrow in each spectrum. The red asterisks indicate the presence of an unbound kinase domain in the inhibitor-bound NMR sample.
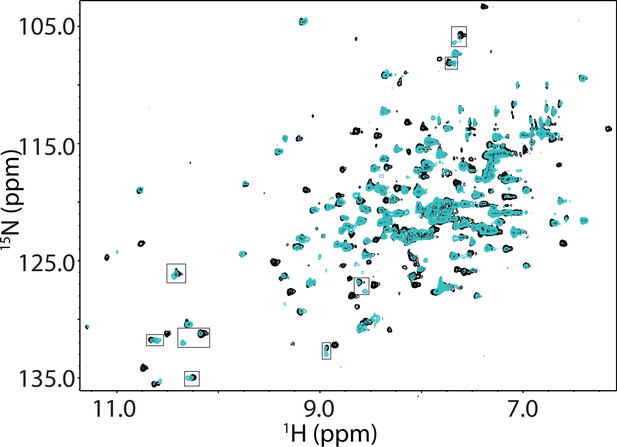
Overlay of the 1H-15N TROSY HSQC spectra of 15N-labeled apo Bruton’s tyrosine kinase (BTK) linker-kinase domain (LKD) L528W (black spectrum) with that of the Pirtobrutinib bound BTK LKD L528W spectrum (cyan spectrum).
The boxed resonances show resonance doubling in the Pirtobrutinib bound spectrum, one of which overlaps with the apo BTK LKD L528W spectrum, indicating a mixture of bound and unbound protein.
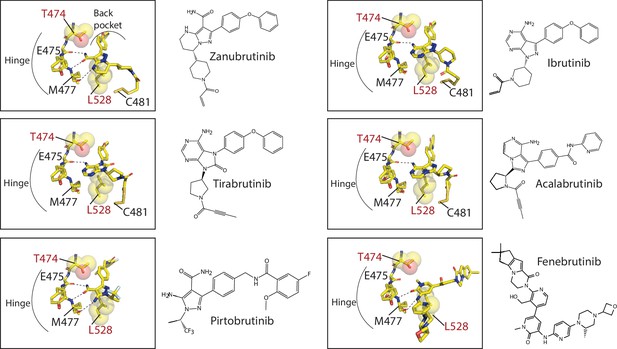
Close-up of Bruton’s tyrosine kinase (BTK) inhibitors bound to the BTK kinase active site.
The covalent BTK inhibitors (Zanubrutinib, Tirabrutinib, Acalabrutinib, and Ibrutinib, represented as sticks) are bound to BTK C481. All BTK inhibitors, covalent and non-covalent (Pirtobrutinib and Fenebrutinib) interact with the hinge region of the kinase and with the exception of Fenebrutinib, extend into the back pocket of the kinase. Hydrogen bonds between the inhibitor and hinge region are shown as dotted lines. The binding of these BTK inhibitors are incompatible with nucleotide binding. The location of BTK T474 and L528, residues that are frequently mutated in chronic lymphocytic leukemia (CLL) patients are shown as sticks and spheres. BTK T474 is adjacent to the hinge region and BTK L528 is at the ‘base’ of the kinase active site.
Tables
Half-life of clinically approved Bruton's tyrosine kinase (BTK) inhibitors: Ibrutinib (Advani et al., 2013), Acalabrutinib (Podoll et al., 2019), Zanubrutinib (Tam et al., 2019), Tirabrutinib (Walter et al., 2016), and Pirtobrutinib (Mato et al., 2021).
| Inhibitor | Half-life (h) |
|---|---|
| Ibrutinib | 2–3 |
| Acalabrutinib | 0.6–2.8 |
| Zanubrutinib | 4 |
| Tirabrutinib | 6.5–8 |
| Pirtobrutinib | 20 |
Additional files
-
Supplementary file 1
Bruton's tyrosine kinase (BTK) mutations detected in chronic lymphocytic leukemia (CLL) patients treated with BTK inhibitors.
In addition to mutations in BTK C481, T474, and L528, other BTK mutations that have been detected include: R28S, E108K, G164D, V416L, A428D, M437R, R490H, Q516K, V537I, and T316A (Maddocks et al., 2015; Ahn et al., 2017; Woyach et al., 2014; Blombery et al., 2022; Wang et al., 2022; Jackson et al., 2023; Handunnetti et al., 2019; Woyach et al., 2019; Woyach et al., 2017; Kanagal-Shamanna et al., 2019; Gángó et al., 2020; Sharma et al., 2016).
- https://cdn.elifesciences.org/articles/95488/elife-95488-supp1-v1.xlsx
-
Supplementary file 2
Excel file providing enhanced experimental details for hydrogen deuterium exchange mass spectrometry (HDX-MS) including minimum criteria specified by Masson et al., 2019, lists of all peptides by residue number, sequence, as well as deuterium levels measured for each Figure.
The value of each deuterium difference for every colored box in each Figure as well as the complete dataset for each state are also found in this file.
- https://cdn.elifesciences.org/articles/95488/elife-95488-supp2-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/95488/elife-95488-mdarchecklist1-v1.docx





