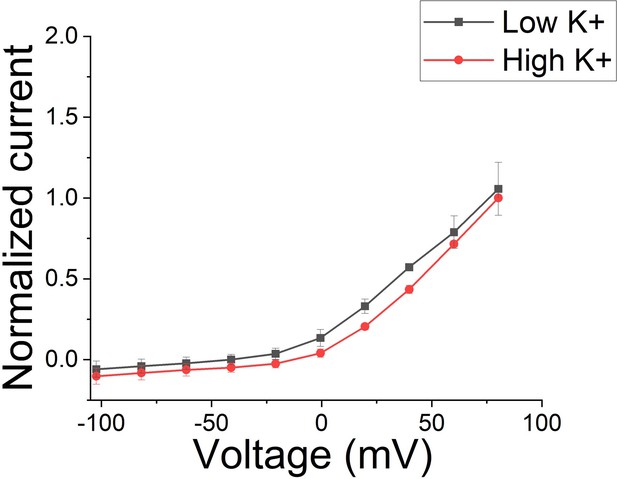
PUFA stabilizes a conductive state of the selectivity filter in IKs channels
Figures
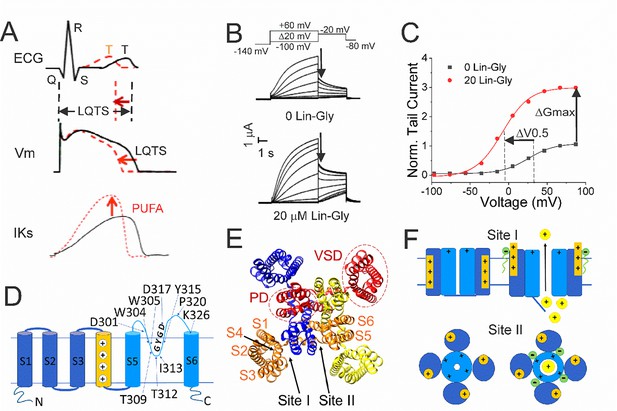
Polyunsaturated fatty acids (PUFAs) activate KCNQ1/KCNE1 channels.
(A) (Black) Prolonged QT interval in the ECG is due to, for example, loss-of-function mutations of KCNQ1/KCNE1 channels that generate the IKs current that normally contributes to the repolarizing phase of the ventricular action potential (AP). (Red) PUFAs are potent activators of KCNQ1/KCNE1 channels that can restore the normal functioning of the channel and restore the AP duration and the QT interval. (B) Representative current traces of KCNQ1/KCNE1 in 0 μM and 20 μM of Lin-Glycine. Voltage protocol on top. (C) Conductance versus voltage curves from tail currents (measured at arrows in B). Channel activation by PUFA results in two main effects: a shift of the voltage dependence of activation (ΔV0.5) and an increase in the channel maximum conductance (ΔGmax). (D) KCNQ1 transmembrane topology. Residues mutated in this study are labeled. (E) KCNQ1 top view (PDB: 6UZZ) with PUFA binding sites: Site I, at the voltage sensor domain (VSD); and Site II, at the pore domain. The four subunits are shown in four different colors. (F) Cartoon of PUFA mechanism of action. Site I, top panel. Electrostatic interactions between PUFA head groups and positively charged residues in S4 facilitate channel activation by stabilizing the outward state of S4. Site II, bottom panel. PUFA interaction with residues in the pore domain facilitates the increase in the maximum channel conductance.
-
Figure 1—source data 1
Tail current data used to generate Figure 1C.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig1-data1-v1.docx
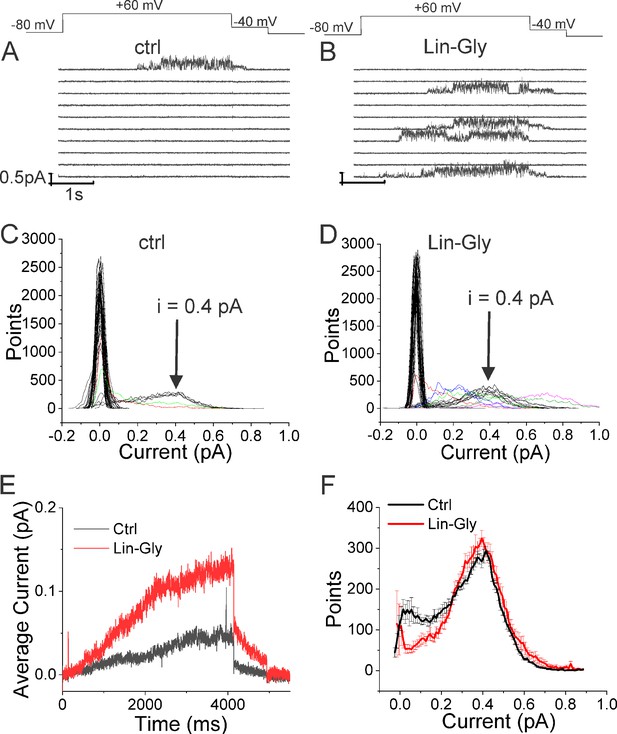
Lin-Glycine induces an increase in the Po of KCNQ1/KCNE1.
(A, B) Ten consecutive traces of KCNQ1/KCNE1 in (A) control and (B) in the presence of Lin-Glycine (20 μM). (Top) Protocol used for the recordings. (C, D). All-point amplitude histogram of 50 consecutive traces in (C) control and (D) Lin-Glycine. Note no change in the single-channel current amplitude; however, an increase in the number of sweeps with channel opening is observed. Note that there were at least two channels in this patch. Different sweeps were assigned different colors to better visualize different types of channel behaviors. Note panels (A–D) are all from the same patch. (E) Average currents of 100 sweeps in control and Lin-Glycine. (F) All-point histogram of the last second of non-empty sweeps in control and in Lin-Glycine. We estimated the open probability from the all-point amplitude histogram by Po = Sum (iN/(iestimateNtotal)), where N is the number of points for a specific current i in the histogram, iestimate = 0.4 pA from the peak of the histogram, and Ntotal = 10,000 is the total number of points in the last second of the trace. p=0.78 ± 0.02 (n = 8 sweeps) and p=0.87 ± 0.04 (n = 8 sweeps) for Control and Lin-Glycine, respectively, from the same patch.
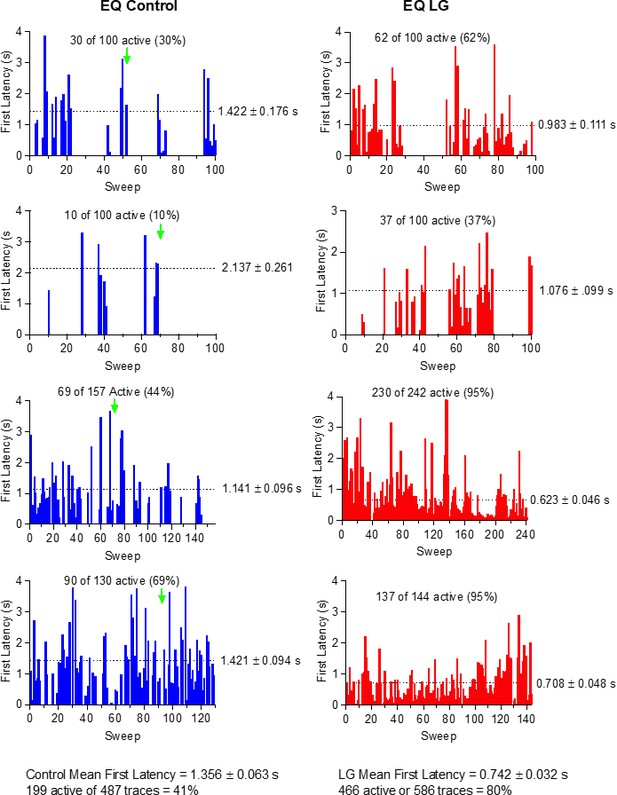
First latency to opening is shortened by Lin-Glycine in wt KCNQ1/KCNE1 channels.
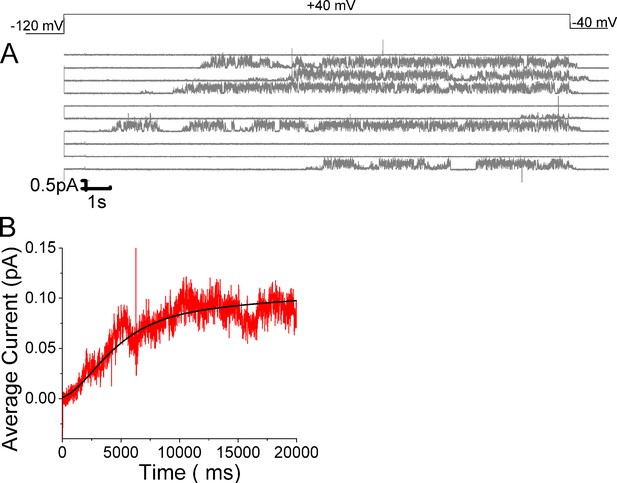
Open probability stays high in KCNQ1/KCNE1 channels once opened.
(A) Ten consecutive traces of KCNQ1/KCNE1in response to 20-s-long voltage steps in control solutions. (Top) Protocol used for the recordings. (B) Average currents of 57 sweeps in control solution.
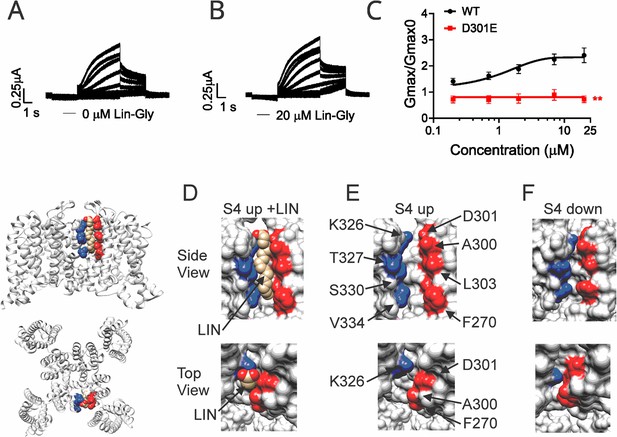
Polyunsaturated fatty acid (PUFA) binds to a state-dependent small crevice between K326 and D301.
(A, B) KCNQ1_D301E/KCNE1 representative current traces (A) in 0 μM of Lin-Glycine. (B) After perfusion of 20 μM of Lin-Glycine. (C) Gmax/Gmax0 for KCNQ1/KCNE1 channels and KCNQ1_D301E /KCNE1 channels. Gmax/Gmax0 was significantly reduced for the D301E mutation compared to WT channels (p=0.0018, n = 4 oocytes). Student’s t-test was used to conduct statistical analysis. (D–F) Structures of crevice between S5 and S6 in KCNQ1 with S4 activated (S4 up) (D, E) and S4 resting (S4 down) (F). Residues that surround the crevice from S6 shown in blue (K326, T327, S330, V334) and from the pore helix and S5 in red (D301, A300, L303, F270). Remaining KCNQ1 residues shown in light gray. On the left is shown the location of the crevice in KCNQ1 (top) side view and (bottom) top view. (D) In MD simulations (Yazdi et al., 2021), linoleic acid (LIN: gold color) fits in a narrow crevice present in the cryoEM structure of activated state KCNQ1. (E) Same view as in (D) but without LIN. (F) In the cryoEM structure with S4 in the resting state, the crevice between K326 and D301 is too narrow to fit LIN.
-
Figure 3—source data 1
Gmax data used to generate Figure 3C.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig3-data1-v1.docx
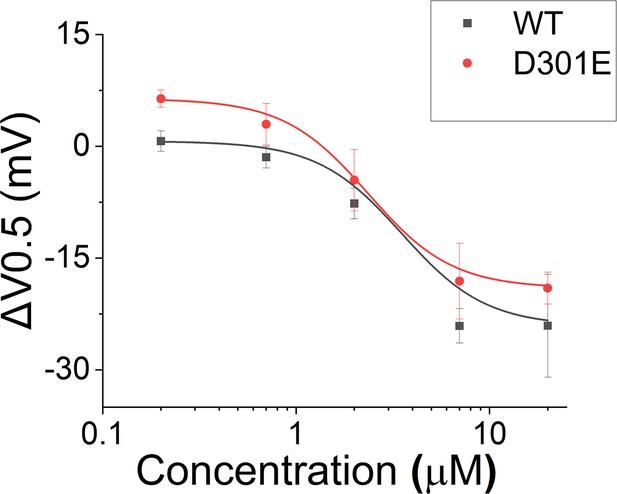
Effect of Lin-Glycine in shifting the voltage dependence of activation (ΔV0.5).
A similar shift in the voltage dependence of activation was found for KCNQ1_D301E/KCNE1 and KCNQ1/KCNE1 after perfusion of several concentrations of Lin-Glycine. Comparisons at 20 μM of Lin-Glycine revealed no significant difference between the effect seen in KCNQ1/KCNE1 and KCNQ1_D301E (p=0.52; n = 3 oocytes).
-
Figure 3—figure supplement 1—source data 1
ΔV0.5 data used to generate the figure.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig3-figsupp1-data1-v1.docx
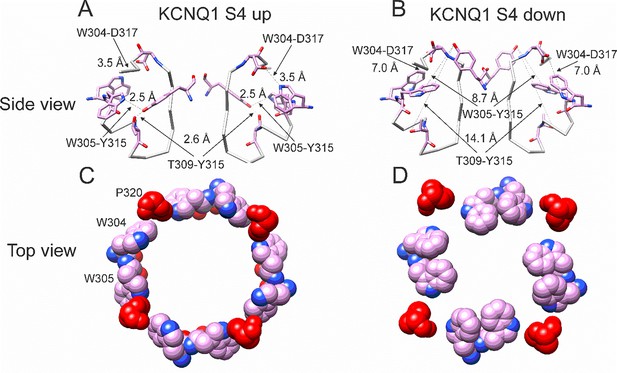
Different conformations of selectivity filter in cryoEM structures with S4 activated or resting.
(A) Selectivity filter of KCNQ1 with S4 activated (S4 up; PDB: 8SIK). Distances between D317 and W304 and between T309 and Y315 are short enough to form hydrogen bonds (dashed lines). (B) Selectivity filter of KCNQ1 with S4 in resting state (S4 down; PDB: 8SIN). Distances between D317 and W304 and between T309 and Y315 are too long for hydrogen bonds (dashed lines). Only two subunits are shown for clarity. (C, D). Aromatic cuff in KCNQ1 with (C) S4 activated and (D) resting S4. Note how P320 (red) moves away from its position in between W304 and W305 from two different subunits in the S4 down conformation.
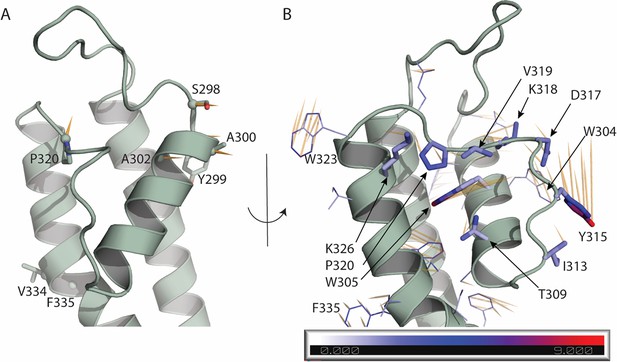
Atomic displacements at the selectivity filter and nearby regions in the transition from up to down state.
Each vector (shown in orange) starts from the position of a given atom in the up state (PDB ID 8SIK) and points to the position of the same atom in the down state (PDB ID 8SIN). (A) Displacement of the Ca atoms of residues 298–341 is shown in orange if greater than 2 Å. The side chain of such residues is shown as sticks. (B) Displacement of the side chains of residues 298–341. The side chain of residues W304, W305, T309, I313, Y315, D317, K318, V319, P320, K326 are highlighted as sticks and atoms are colored based on the atomic displacement values. Scale bar is in Å. In (A, B), for clarity, only one monomer of the up state of KCNQ1 is shown.
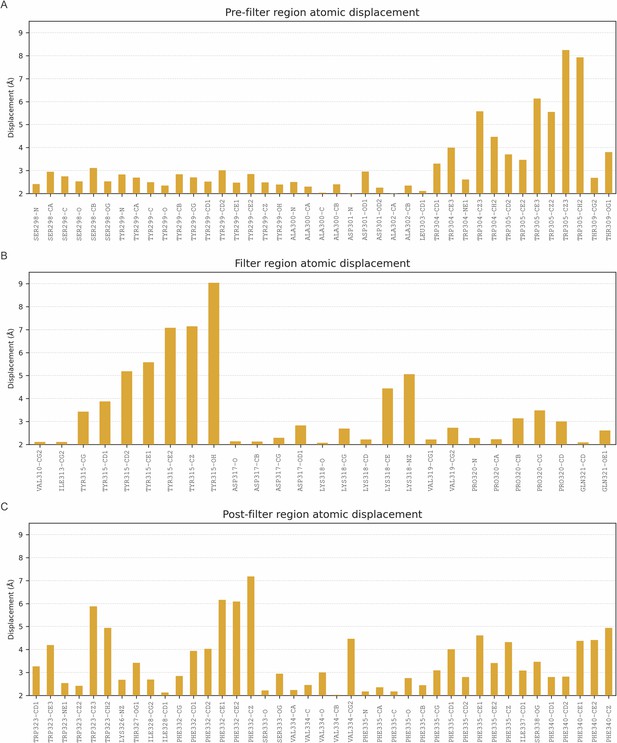
Graphic indicating the atomic displacement of residues at the selectivity filter and nearby regions in the transition from up to down state.
Displacement of the Ca atoms of residues 298–341 is shown if greater than 2 Å. (A) Displacement of atoms (Å) before the selectivity filter; (B) residues of the selectivity filter; and (C) residues located just outside the selectivity filter region.
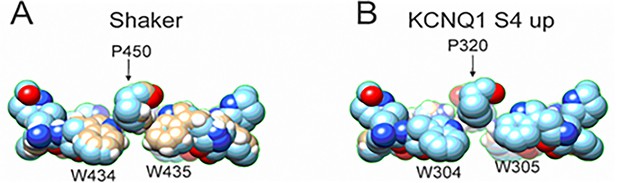
Comparison between the aromatic ring cuff configuration of Shaker and KCNQ1 channels.
(A) In Shaker, P450 sits in between the two tryptophan and stabilizes the aromatic ring cuff. (B) In contrast, in KCNQ1 the P320 is positioned further outward and away from the two tryptophan, generating a looser arrangement of the aromatic ring cuff.
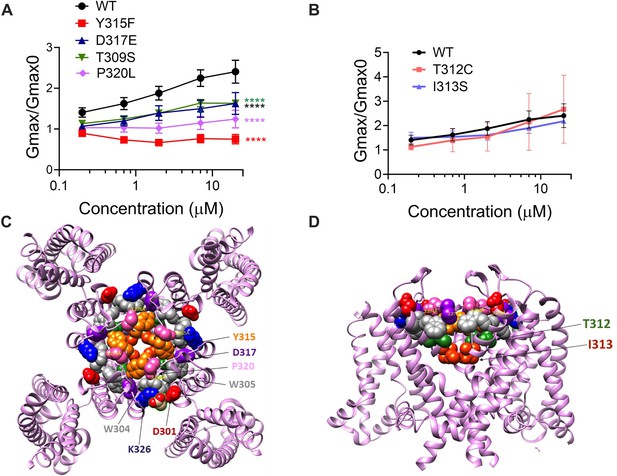
The ability of Lin-Glycine to increase the channel conductance is reduced when channel pore residues are mutated.
(A) Gmax/Gmax0 values obtained for KCNQ1_WT/KCNE1 channel (black) and mutant channels. KCNQ1_Y315F/KCNE1 (red), KCNQ1_D317D/KCNE1 (blue), KCNQ1_T309S/KCNE1 (green), and KCNQ1_P320L/KCNE1 (purple), the Gmax/Gmax0 is significantly reduced (p<0.0001 n = 4 oocytes). One-way ANOVA with Dunnett’s post hoc multiple-comparisons test was used for statistical analysis. (B) Gmax/Gmax0 values for KCNQ1_T312C/KCNE1 and KCNQ1_I313S/KCNE1 (p=0.14 and p=0.10, respectively) (n = 3 oocytes). One-way ANOVA with Dunnett’s post hoc multiple-comparisons test was used for statistical analysis. (C, D) Top view and side view of KCNQ1 channel with mutated residues highlighted.
-
Figure 5—source data 1
Gmax data used to generate Figure 5A and B.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig5-data1-v1.docx
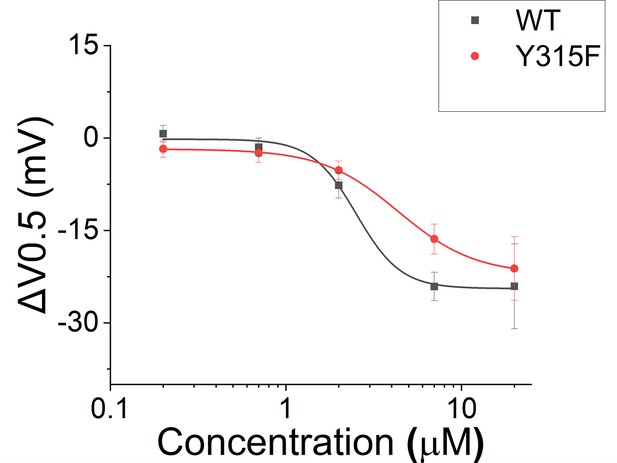
Effect of Lin-Glycine in shifting the voltage dependence of activation (ΔV0.5).
A similar effect in shifting the voltage dependence of activation was found for KCNQ1_Y315F/KCNE1 and KCNQ1/KCNE1 after perfusion of several concentrations of Lin-Glycine. Comparisons at 20 μM of Lin-Glycine revealed no significant difference between the effect seen in KCNQ1/KCNE1 and KCNQ1_Y315F (p=0.7435; n = 4 oocytes).
-
Figure 5—figure supplement 1—source data 1
ΔV0.5 data used to generate Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig5-figsupp1-data1-v1.docx
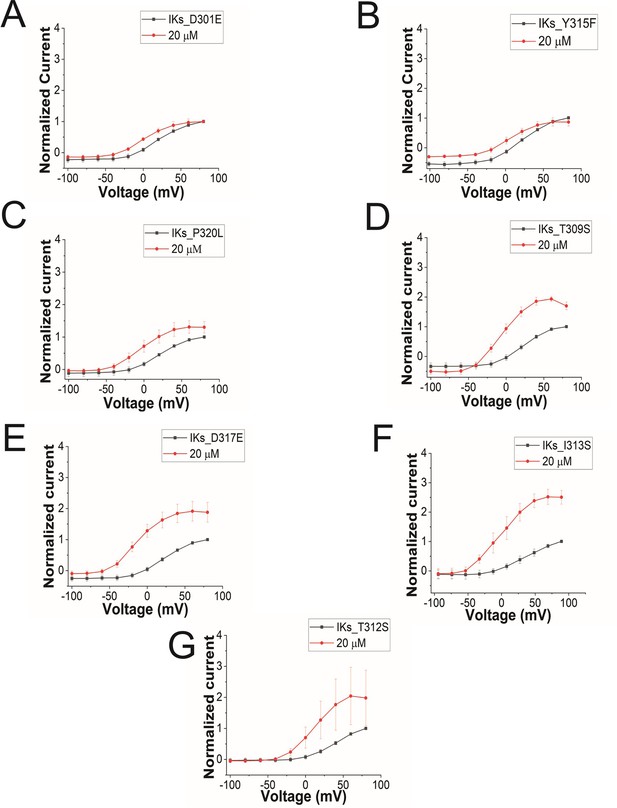
GV curves for KCNQ1 mutants.
GV curves in control (black) and after perfusion of 20 μM of Lin-Gly (red) for (A) KCNQ1_D301E/KCNE1, (B) KCNQ1_Y315F/KCNE1, (C) KCNQ1_P320L/KCNE1, (D) KCNQ1_T309S/KCNE1, (E) KCNQ1_D317E/KCNE1, (F) KCNQ1_I313S/KCNE1, and (G) KCNQ1_T312S/KCNE1.
-
Figure 5—figure supplement 2—source data 1
Gmax data used to generate Figure 5—figure supplement 2A–G.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig5-figsupp2-data1-v1.docx
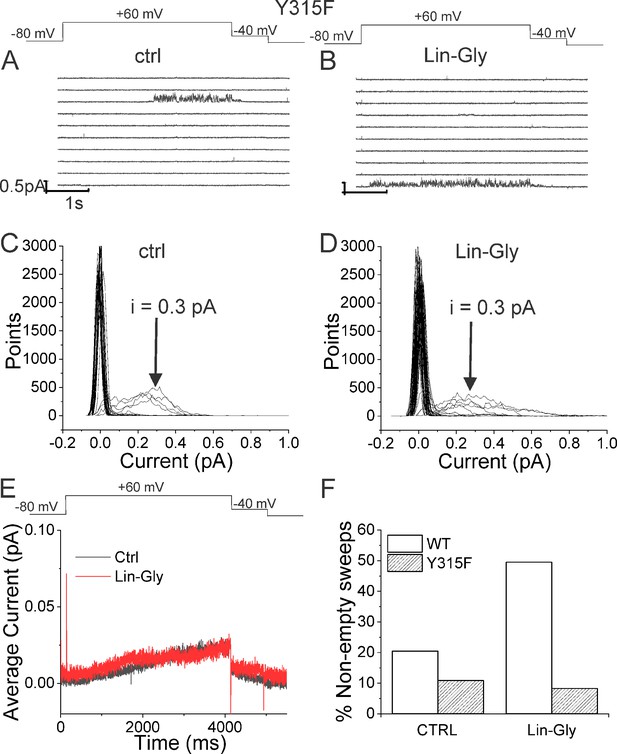
Lin-Glycine does not increase the Po of KCNQ1_Y315F/KCNE1.
(A, B) Ten consecutive traces of KCNQ1_Y315F/KCNE1 in (A) control and (B) in the presence of 20 μM Lin-Glycine. (Top) Protocol used for the recordings. (C, D) All-point amplitude histogram of 50 consecutive traces in (C) control and (D) Lin-Glycine. The single-channel current amplitude was reduced to 0.3 pA compared to 0.4 pA for WT KCNQ1/KCNE1 (Figure 2C and D). Note that there were at least two channels in this patch. (E) Average currents of 478 sweeps in control and 533 sweeps in Lin-Glycine. Note that panels (A–D) were all from the same patch.
-
Figure 6—source data 1
Percentage of non-empty sweeps used to generate Figure 6F.
- https://cdn.elifesciences.org/articles/95852/elife-95852-fig6-data1-v1.docx
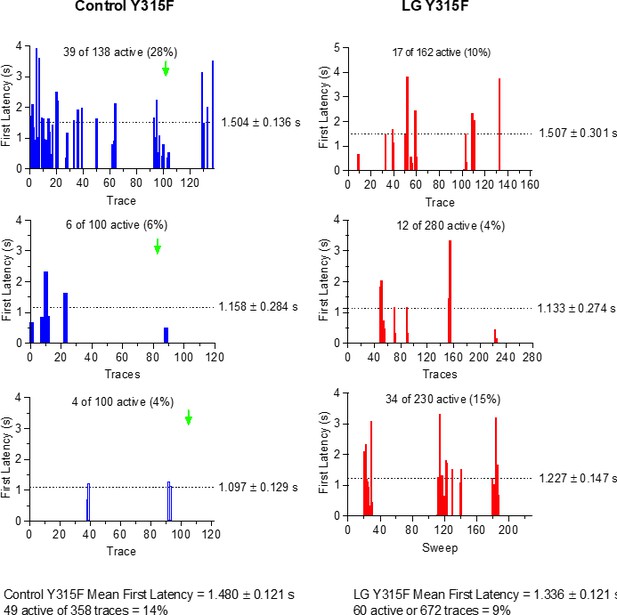
First latency to opening is not shortened by Lin-Glycine in Y315Y channels.
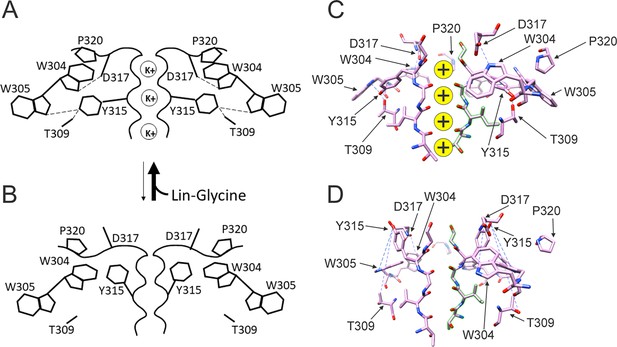
Conformational changes occurring at the pore during the transitions between non-conductive and conductive states.
(A) The binding of polyunsaturated fatty acid (PUFA) to Site II between K326 and D301 induces a series of interactions between residues near the external part of the selectivity filter. W304-D317 and W305-Y315 form hydrogen bonds (dash line, black), Y315 interacts also with T309 (dash line, black), and P320 is reoriented to sit on top of W304 and W305 to favor a more stable configuration of the aromatic ring cuff. The result of those new interactions is a more stable and conductive pore. (B) In the non-conductive state, those interactions are likely to be absent and this results in a more unstable selectivity filter. Also, P320 is now flipped from its position on top of the two tryptophans of the aromatic ring cuff. (C) CryoEM selectivity filter with S4 in the activated-state representative of a conductive selectivity filter. (D) CryoEM selectivity filter with S4 in the resting state, representative of a non-conductive selectivity filter.
Videos
Large conformational changes in selectivity filter between Kv7.1 structures with S4 up and S4 down.
Interpolation between the cryoEM structures of Kv7.1 with S4 in the activated conformation (PDB 8SIK) and S4 in the resting conformation (PDB SIN) showing the position of residues W304, W305, T309, Y315, and D317 that are important for the stability of the selectivity filter in related Kv channels. Dashed lines between residues proposed to make hydrogen bonds in Kv channels.
Large conformational changes in aromatic cuff between Kv7.1 structures with S4 up and S4 down.
Interpolation between the cryoEM structures of Kv7.1 with S4 in the activated conformation (PDB 8SIK) and S4 in the resting conformation (PDB SIN) showing the position of the aromatic cuff residues W304, W305, and P320 (red) that are important for the stability of the selectivity filter in related Kv channels.