The sperm hook as a functional adaptation for migration and self-organized behavior
Figures
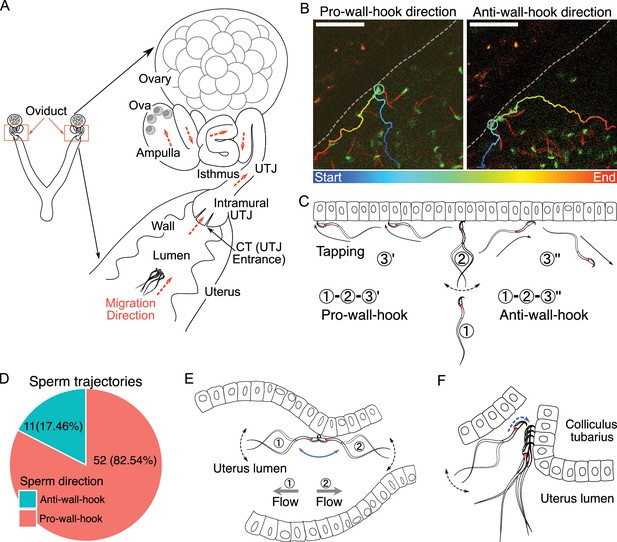
The structure of the female reproductive tract and various sperm behaviors and interactions with epithelia in the uterus.
(A) An illustration of the female reproductive tract and sperm migration direction (arrows in orange colour) inside the tract. Ova (egg cells) are in the ampulla. (B) Spermatozoa alter their travel direction based on their head orientation upon reaching the uterine wall (sperm hook functions as a pivot, Figure 1—video 2A). Left image shows a sperm trajectory exhibiting a pro-wall-hook direction, and the right image shows an anti-wall-hook direction. The trajectory is depicted in colors representing different time points. Dashed lines are guides to the eye to visualize the uterus wall. Scale bar: 50 µm. (C) Sperm moving direction changes over time when they reach the uterine epithelia. Numbers indicate sequences of sperm movement. (1) During sperm migration, they reach the uterine wall. (2) After reaching the uterine wall, they beat several times while facing their head towards the uterine wall. (3) Sperm beating results in a change in sperm orientation (1–3′: pro-wall-hook, 1–3″: anti-wall-hook). The pro-wall-hook orientation coincides with sperm travelling along the wall and the anti-wall-hook orientation coincides with sperm moving away from the wall. (D) When spermatozoa reach the uterine wall, their trajectories predominantly follow the pro-wall-hook direction, where the sperm hook is directed towards the uterine wall. (E) The sperm hook assists a spermatozoon in anchoring to the epithelia (hook as an anchor). This anchoring facilitates sperm attachment to the uterine and utero-tubal junction (UTJ) epithelium and prevents spermatozoa from being swept away by internal flow caused by peristaltic movement. (F) The sperm hook and thin sperm head aid spermatozoa in squeezing through the sperm-crowded UTJ entrance (colliculus tubarius, CT) and attaching to the epithelium by acting as an anchor (Figure 1—video 3). Note that in our experiments, we do not observe the principal and terminal pieces of spermatozoa due to a lack of fluorescence in our animal model. The observed acrosome and midpiece are shown coloured in the schematic figures.
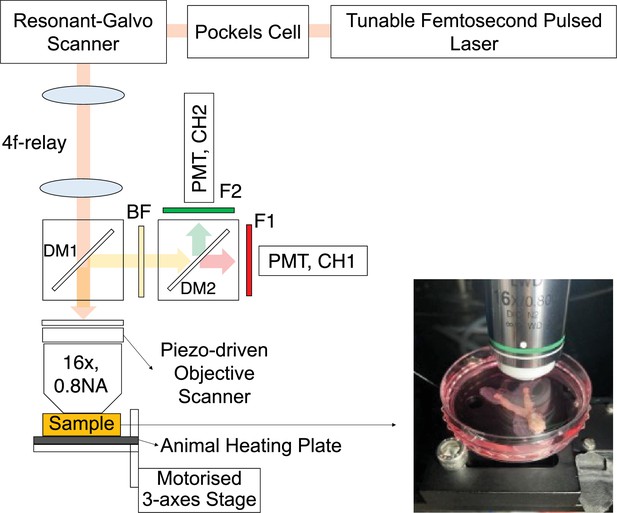
Schematic diagram of the custom-built 2PSLM.
DsRed and eGFP were excited nonlinearly using a tunable high peak power femtosecond laser (Chameleon discovery/Coherent) and a water-immersion objective lens (CFI75 LWD 16 X W/Nikon). The fluorescence emitted was collected by the same objective lens and detected by a pair of GaAsP photomultiplier tubes (PMT, H10770PA-40/Hamamatsu). Dichroic mirrors and filters; DM1(T735lpxrxt-UF3/Chroma), DM2(T565lxr/Chroma), BF(ET720SP-2P8/Chroma), F1(ET605/70 m/Chroma), and F2(ET525/70 m/Chroma) were used to split the excitation beam and emission light. A resonant-galvo scanner (RESCAN-GEN/Sutter instrument) enabled real-time fluorescence imaging of sperm behavior at a speed of 30 frames per second for 512 pixels per line acquisition. A Pockels cell (M350-80-LA-02 KDP/Conoptics) allowed for rapid control of the laser beam intensity, homogenising the illumination across the field of view, and applying varying laser power per tissue depth. The sample position in all three dimensions was controlled by a Piezo-driven objective scanner (P-725.4CA/PI) and a motorized 3-axes stage (3DMS/Sutter instrument). A small animal heating plate (HP-4M/Physitemp) maintained the warmth of the mouse female reproductive tract at body temperatures.
Sperm migration in the uterus.
Imaging was conducted around the area labelled as ‘Lumen’ in Figure 1A. (A) Most spermatozoa within the uterine volume are moving back and forth following the flow in the uterus. (B) Spermatozoa near the uterine wall (uterine epithelium) are more active and swim faster than those in the centre of the lumen. Sperm trajectories for the analysis in Figure 2 were also present later in the video. Trajectories with different colours correspond to the curvilinear velocity (VCL) of each trajectory (Blue: slow, Red: Fast). (C) Vertical scan imaging in different depths shows that most spermatozoa at the uterine wall (depth: 0 µm) are active and fast-moving. The further away from the uterine wall, e.g., depth: 30 µm, there are increasing number of inactive or slow-moving spermatozoa.
The sperm hook helps sperm to determine migration directions in the uterus.
Imaging was conducted around the area labeled as ‘Wall’ in Figure 1A. (A) When sperm reach the uterine wall, sperm change their migration direction. Most sperm change their heading direction in a way that their apical hook faces the uterine wall (pro-wall-hook direction) which allows sperm to migrate straighter along the wall. In contrast, a few sperm exhibit an opposite heading direction such that their hook faces the uterine lumen (anti-wall-hook direction). This heading direction usually results in a departure of the sperm from the wall. (B) Sperm are tapping along the epithelium with their hook while migrating along the uterine wall.
Sperm use their hook as an anchor to be attached to the uterine epithelium.
Imaging was conducted around the area labeled as ‘colliculus tubarius (CT) (utero-tubal junction, UTJ Entrance)’ in Figure 1A. (A) Sperm use their hook to be attached to the uterine epithelium (sperm hooking behavior for anchoring). The apical sperm hook also helps sperm squeeze through other sperm in a confined space. (B) Unattached (unanchored) or loosely attached sperm may be more easily squeezed out by uterine muscle contraction or fluid flow.
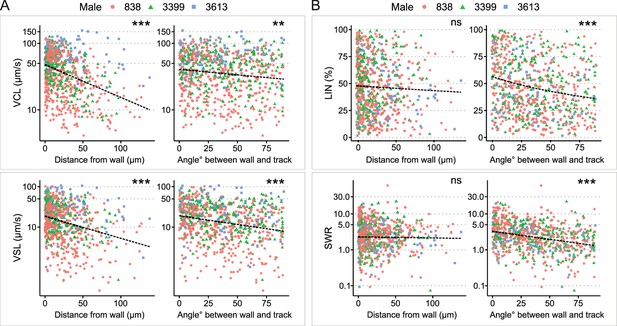
Analysis of sperm kinetic parameters relative to the distance and angle between the sperm trajectory and uterine wall.
(A) Both curvilinear velocity (VCL) (top) and straight-line velocity (VSL) (bottom) decreased with an increase in the distance between the sperm trajectory and the uterine wall. Similarly, VCL and VSL decreased as the angle between the sperm trajectory and uterine wall increased. (B) The distance between the sperm trajectory and uterine wall did not significantly affect linearity of forward progression (LIN) (top left) and SWR (bottom left). However, both LIN and straight line-to-sideward movement ratio (SWR) decreased when the angle between the sperm trajectory and uterine wall increased. The total number of sperm trajectories is 694. Data from different males are represented in different colors and shapes. The dotted lines indicate regression lines from simple regressions to aid visual interpretation. Check model estimates for more details and precise interpretation of the models (Supplementary file 1). The y-axis of each figure is displayed in the log-scale except for LIN. Images for sperm tracking were acquired around the area labeled as ‘Wall’ in the uterus in Figure 1A. Sperm trajectories used in this analysis are presented in Figure 1—video 1B. The effect of the two random variables (males and females) are visualized in Figure 2—figure supplement 2. ***: p < 0.001, **: p < 0.01, *: p < 0.05, ns: not significant.
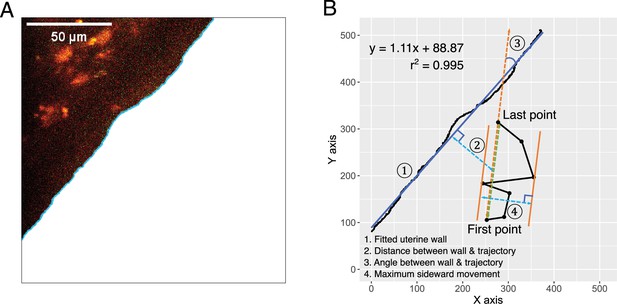
Uterus wall and parameters that were used to measure sperm migration speed and linearity.
(A) Areas along the uterine wall that were relatively straight were selected. The boundary of these areas was identified using the object selection tool in Adobe Photoshop CC (23.1.0 version). (B) The uterine wall was approximated as a linear line using linear regression (1). The distance between a spermatozoon and the uterine wall was defined as the minimum distance between the midpoint of the track displacement and a sperm trajectory (2). The angle between sperm trajectories and the uterine wall was calculated as the angle (in radians) between the uterine wall (approximated line) and the straight line that connected the first and last points of a sperm trajectory–track displacement line (3). The maximum sideward movement was determined as the greatest distance between the parallel lines that aligned with the track displacement line at the positions of the sperm trajectory (4). Straight line-to-sideward movement ratio (SWR) was then computed by dividing the track displacement by the maximum sideward movement.
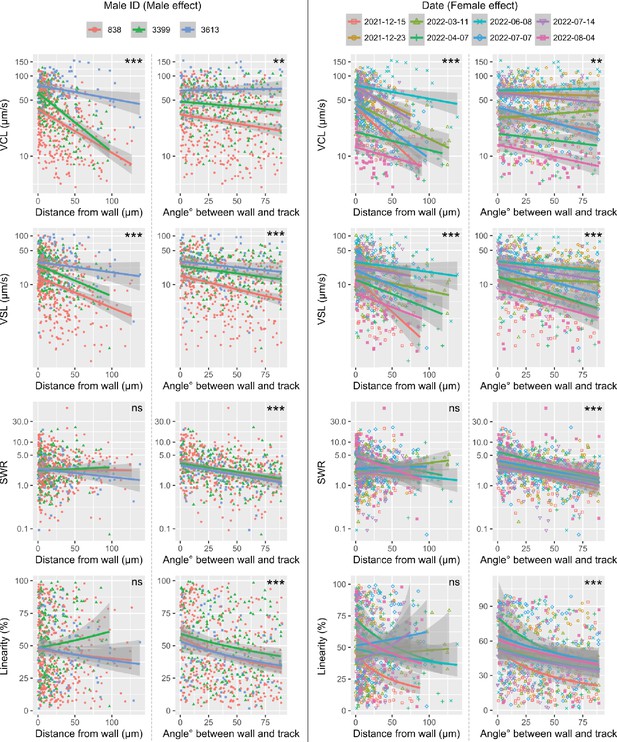
Visualization of male and female effects (two random effects) on sperm migration kinematic parameters.
The model-fitted lines, separated by male ID and date (female), in the non-significant models, indicated as ‘ns’, show non-significant effects of the distance (straight line-to-sideward movement ratio, SWR) or an inconsistent effect of the distance (Linearity) on the kinetic parameters. ***: p < 0.001, **: p < 0.01, *: p < 0.05, ns: not significant.
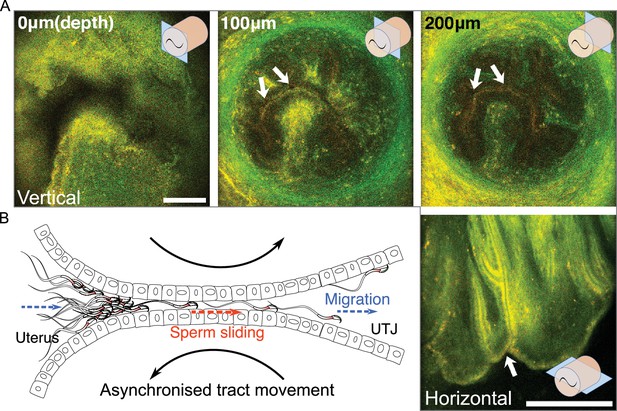
The structure of the entrance of intramural utero-tubal junction (UTJ) and sperm passage.
(A) The upper three images are the vertical view of the colliculus tubarius (CT) (entrance to the UTJ) of the intramural UTJ from an unmated female. The bottom right image is a horizontal view of the intramural UTJ. There are only a few small gaps, indicated by arrows, between mucosal folds, which control sperm migration into the UTJ from the uterus (Figure 3—video 1). Illustrations at upper and lower right conners indicate imaging planes (blue rectangles) and the intramural UTJ (orange cylindrical structure) with the CT (black tilde). Scale bar: 100 µm, Arrow line thickness: 10 µm for top three images, 5 µm right bottom image. (B) Asynchronized movement of mucosal folds (sliding against each other) at the CT due to uterine and UTJ contractions enables spermatozoa to slide into the intramural UTJ from the uterus (Figure 3—video 2). Two dashed arrows (in blue) indicate the direction of sperm migration from the uterus to the UTJ, and the dashed arrow in the centre (in orange) indicates the direction of sperm sliding in the intramural UTJ. The two curved black arrows indicate a asynchronized (opposite) movement of confronting mucosal folds in the intramural UTJ.
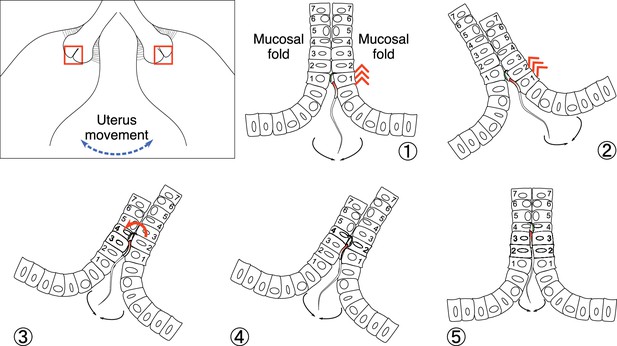
A hypothetical model for sperm migration from the uterus to utero-tubal junction (UTJ).
The upper left inset represents a uterine horn that moves to the left or right due to muscle contraction (exaggerated for visualization). The five subfigures with numbering represent the zoom-in of the two red square frames in the inset. When the uterine horn moves from the centre to the right (1 to 2), two facing surfaces between the two mucosal folds slide against each other. This sliding results in an opening space where sperm can ascend – note that the sperm moves from cell 1 to cell 2 of the right mucosal fold (2). When the uterine horn moves from right to left (3), the two surfaces between the mucosal folds slide in opposite directions where the sperm can now reach cell 4 of the left mucosal fold. If sperm can turn over, its head can be attached to cell 4 of the left mucosal fold (4 to 5). Repetition of these procedures will make the sperm finally pass the colliculus tubarius (CT) and migrate through narrow gaps between mucosal folds in intramural UTJ. This process appears to be as if sperm may slide through the space between mucosal folds when the space is too small for normal beating (Figure 3—video 2C).
The entrance of intra-mural utero-tubal junction (UTJ) (or colliculus tubarius, CT) in the uterus has small spacing (almost closed inter-fold gaps) for mouse sperm to pass through.
Imaging was conducted after excising and clearing the ‘Intramural UTJ’ part in Figure 1A. (A) These closed inter-fold gaps continue for about 100 µm from the entrance of UTJ. (B) The existence of a copulatory plug or mating history does not influence the opening of the inter-fold gap at the UTJ entrance (UTJ entrance seen from an orthogonal perspective with respect to A). (C) Even after mating with a male with working sperm, the width of inter-fold gaps does not considerably change. Only a few sperm can pass through the inter-fold gap at a time due to its narrow width. Scanning direction of the intra-mural UTJ to get images is indicated with an arrow at the right upper corner in each Video.
Sperm behaviors and the movement of mucosal folds at the entrance of utero-tubal junction (UTJ) (colliculus tubarius, CT).
Imaging was conducted around the area labeled as ‘CT (UTJ Entrance)’ in Figure 1A. (A) There is no upsuck-like passive sperm transfer from the uterus to UTJ. Some unanchored sperm are sometimes pulled off by muscle contraction (peristaltic movement) in the intramural UTJ (indicated by an arrow). (B) Two facing mucosal folds sometimes move in an opposite direction which causes the two mucosal folds to slide against each other. Sperm may use this moment to enter UTJ from the uterus. (C) As the sperm head is round enough despite its apical sperm hook, sperm can move forward (head direction) by sliding through a narrow lumen between mucosal folds. However, due to the sperm hook shape (anchor), it will not be easier for sperm to move backwards (tail direction).
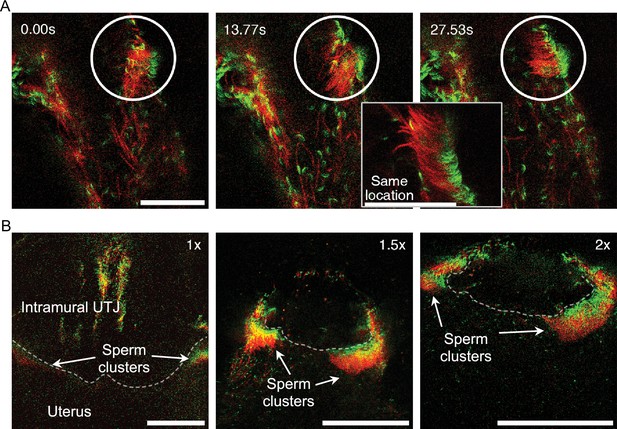
Self-organized sperm behavior at colliculus tubarius (CT) inside the uterus.
(A) The apical shape of the sperm head, due to the sperm hook, results in head asymmetry. This asymmetrical falciform head shape may facilitate sperm re-arrangement and clustering at a uterine wall (Figure 4—video 1). The circle highlights spermatozoa undergoing unidirectional re-arrangement over time. The elapsed time after the first frame is shown in the upper left of the images. The right lower zoom-in inset shows an instant of synchronized motion and unidirectional re-arrangement. Scale bar: 50 µm. (B) Unidirectional sperm clustering at the entrance of the intramural utero-tubal junction (UTJ) (CT, indicated by a dashed line) is marked with arrows. Such large sperm clustering resulted in synchronized sperm beating at the CT (Figure 4—video 2). Scale bar: 200 µm. Note that due to a lack of fluorescence, the principal and terminal pieces of sperm tails are not seen in the images.
Sperm unidirectional re-arrangement in a sperm cluster at a uterine crypt.
Imaging was conducted around the area labelled as ‘colliculus tubarius (CT) (utero-tubal junction, (UTJ) Entrance)’ in Figure 1A. (A) Asymmetrical sperm head shape may facilitate sperm unidirectional re-arrangement in sperm clusters at uterine crypts and CT. The unidirectional sperm clustering then results in synchronized sperm beating that pushes out other sperm by generating fluid flow or by beating other sperm directly. (B) Re-arranged sperm in a cluster sometimes move together in the same direction.
An enormous unidirectional sperm cluster at colliculus tubarius (CT) in the uterus exhibits synchronized sperm beating.
Imaging was conducted around the areas labeled as ‘CT (utero-tubal junction, UTJ Entrance)’ in Figure 1A. The synchronized sperm beating may prevent or hinder other sperm from approaching the UTJ entrance (CT) by pushing out approaching sperm.
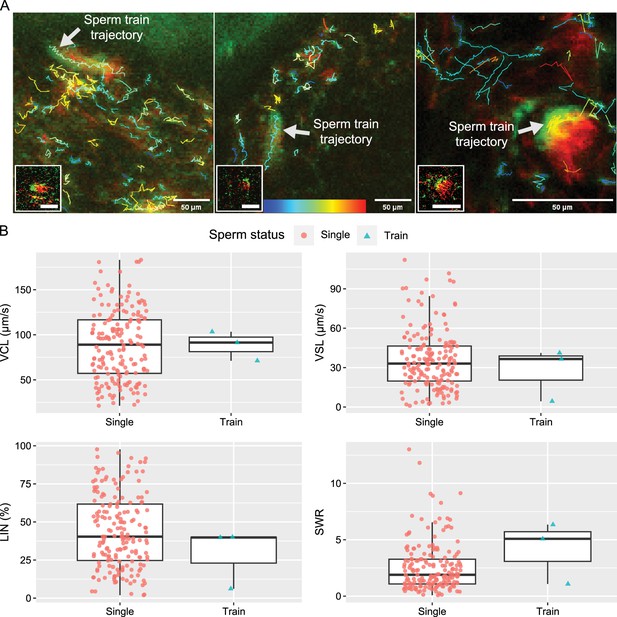
Comparative trajectories and kinetics of 3 linked spermatozoa (sperm trains) and 181 unlinked single spermatozoa.
(A) The projected images, comprising 60 frames, depict the trajectories of sperm trains and unlinked single spermatozoa. Each of the images in the lower left corner shows a sperm train that was traced. Figure 5—video 1 shows how the three traced sperm trains are moving. The colour bar located at the bottom centre represents the VCL of each sperm trajectory, with blue indicating slower speeds and red indicating faster speeds. (B) The boxplots, which include individual data points, represent the kinetic parameters of the sperm trains and unlinked single spermatozoa. The parameters, including curvilinear velocity (VCL), straight-line velocity (VSL), linearity of forward progression (LIN), and straight line-to-sideward movement ratio (SWR), were computed using images of 100x100 pixels that contained a sperm train. The three sperm trains that were traced did not exhibit a faster VCL or VSL, nor a higher LIN. However, it is still possible that the SWR is higher in the sperm train. The lines within the boxes represent the medians, the whiskers represent 1.5 times the interquartile ranges, and the symbols show the individual data points.
Accumulated sperm (sperm trains) are not found to swim faster than unlinked individual sperm in the uterus.
Moreover, a large sperm accumulation cannot pass the narrow luminal space near the utero-tubal junction (UTJ) entrance (colliculus tubarius, CT). These results suggest a disadvantage of sperm trains in sperm migration from the uterus to UTJ. Imaging was conducted around the area labeled as ‘Wall’ and ‘Lumen’ in Figure 1A.
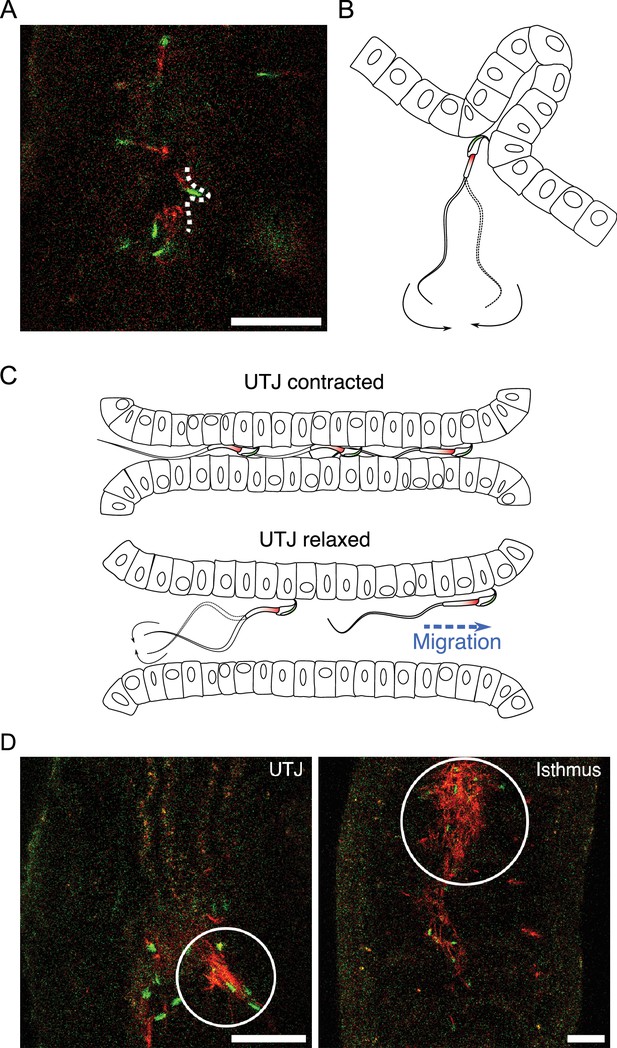
Sperm migration through narrow luminal space in utero-tubal junction (UTJ) and various sizes of accumulated sperm in the oviduct.
(A, B) Spermatozoa can penetrate narrow spaces with their thin head and anchor their hook when they pass through the narrow gaps between mucosal folds during migration in the UTJ (Figure 6—video 1A). Scale bar: 50 µm. (C) Spermatozoa can only migrate through the UTJ when the luminal space is extended due to oviductal contraction and relaxation in the UTJ. (D) Entangled spermatozoa in the oviduct, including the UTJ and isthmus, were predominantly made up of inactive acrosome-reacted spermatozoa. These entangled spermatozoa obstruct the migration of other active spermatozoa and can cause damage to live spermatozoa. Scale bar: 50 µm.
Various sperm behaviors in utero-tubal junction (UTJ).
Imaging was conducted around the area labelled as ‘UTJ’ in Figure 1A. (A) Sperm interact with the UTJ epithelium using their hook in various ways. They put their hook into a gap (crypt) and exhibit tapping- and stroking-like behavior while they migrate through UTJ. (B) Sperm can more easily migrate when UTJ lumens get wider. Attached (anchored) sperm also beat faster when UTJ lumens get wider. (C) Dead or inactive sperm are accumulated in UTJ and may damage live sperm through collisions or hinder sperm migration in UTJ.
The beating rate of the attached (anchored) sperm in utero-tubal junction (UTJ) changes over time.
The speed of fluid flow and the luminal width of UTJ may be related to the beating rate. Imaging was conducted around the area labelled as ‘UTJ’ in Figure 1A.
Additional files
-
Supplementary file 1
Tables with experiment animal information and results of the generalized linear mixed models (GLMM) analysis.
(a) Basic information of 4 males that were used for the mating experiment. (b) Mating records and the information of the females for sperm tracking. (c) Summary results of the GLMM.
- https://cdn.elifesciences.org/articles/96582/elife-96582-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/96582/elife-96582-mdarchecklist1-v1.pdf





