The common Sting1 HAQ, AQ alleles rescue CD4 T cellpenia, restore T-regs, and prevent SAVI (N153S) inflammatory disease in mice
Figures

Splenocytes from HAQ, AQ, and Q293 mice are resistant to STING1-mediated cell death ex vivo.
(A) C57BL/6 N splenocytes were treated directly (no transfection) with diABZI (100 ng/ml), RpRpss-Cyclic di-AMP (5 μg/ml) or 2′3′-cGAMP (10 μg/ml), DMXAA (25 μg/ml) for 24 hr in culture. CD4, CD8 T cells and CD19 B cells death were determined by PI staining. (B). Splenocytes from C57BL/6 N mice were pre-treated with indicated small molecules, GSK2334470 (1.25 µM), GSK8612 (2.5 µM), Bx-795 (0.5 µM), QVD-OPH (25 µM) for 2 hrs. diABZI (100 ng/ml) was added in culture for another 24 hr. Dead cells were determined by PI staining. (C–D). Flowcytometry of HAQ, AQ, IFNAR1-/- or C57BL/6 N splenocytes treated with diABZI (100 ng/ml) for 24 hrs. Cell death was determined by PI staining. (E–F). Q293 or the WT littermates splenocytes were treated with diABZI (100 ng/ml), RpRpss-Cyclic di-AMP (5 μg/ml) or 2′3′-cGAMP 10 μg/ml for 24 hr. Cell death was determined by PI staining. (G). WT/HAQ, WT/AQ, or WT/WT littermates splenocytes were treated with DMXAA (10, 25 or 100 µg/ml) for 24 hr. Cell death was determined by PI staining. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test (A, E, G) or unpaired student T-test (B, D) * p<0.05. n.s: not significant.

Bx-795 inhibits diABZI-induced mouse splenocyte death.
(A) Splenocytes from C57BL/6N mice were treated with 100ng/ml diABZI in culture for the indicated time. Dead cells were determined by PI and Annexin V staining. (B) Splenocytes from C57BL/6N mice were treated with indicated dose of diABZi in culture for 24hrs. Dead cells were determined by PI staining. (C) Splenocytes from C57BL/6N mice were pre-treated with indicated small molecules, H151 (10µg/ml), C176 (1µM), MCC950 (1.25µM), 2-BP (20µM), GSK872 (312.5nM), Liproxstatin-1 (1µM), necrostatin-1 (10µM), VX-765 (1µM), Z-DEVD-FMK (25µM), Bx-795 (0.5µM) for 2 hr. diABZi (100ng/ml) was added in culture for another 24 hr. Dead cells were determined by PI staining. (D) IFNβ was determined in the cell supernatant from C by ELISA. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. n=3~5 mice/group. p values are determined by one-way ANOVA Tukey’s multiple comparison test (A, D) or unpaired student T-test (C). * p.
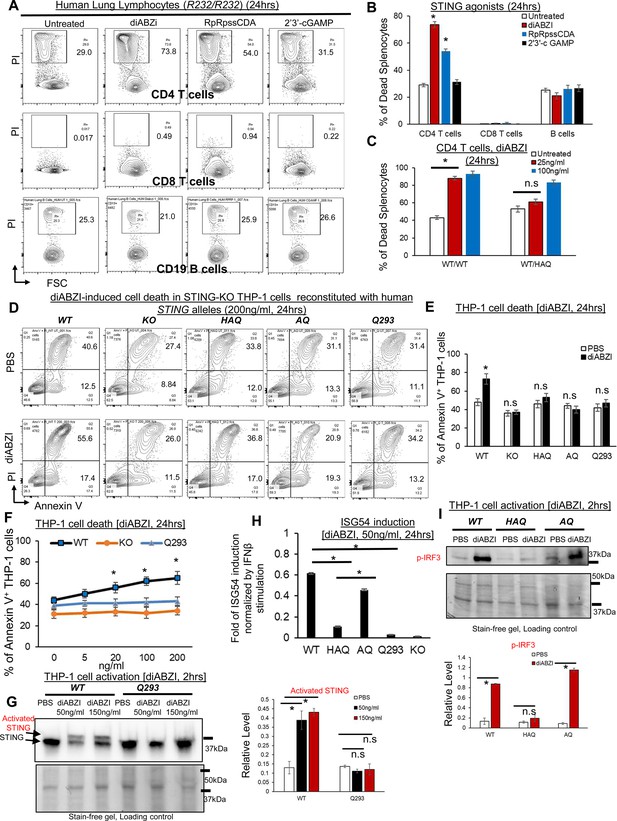
HAQ, AQ, and Q293 human cells are resistant to STING1 agonists-induced death.
(A–B) Total human Lung cells from WT/WT individuals were treated with diABZi (100 ng/ml) for 24 hr. Cell death in CD4, CD8 T cells and CD19 B cells were determined by PI staining. (C). Total lung cells from a WT/HAQ (2 individuals) and a WT/WT (3 individuals) were treated with diABZi (25, 100 ng/ml) for 24 hr. Cell death in CD4 T cells was determined by PI staining. (D–E). STING1-KO THP-1 cells (Invivogen,, cat no. thpd-kostg) were stably reconstituted with human WT (R232), HAQ, AQ, Q293. Cells were treated with diABZI (200 ng/ml) in culture for 24 hr. Dead cells were determined by Annexin V staining. (F). STING1-KO THP-1 cells stably reconstituted with human WT (R232), Q293 were treated with indicated dose of diABZI for 24 hr in culture. Dead cells were determined by Annexin V staining. (G). STING1-KO THP-1 cells stably reconstituted with human WT (R232), Q293 were treated with indicated dose of diABZI for 2hs in culture. STING1 activation was detected by anti-STING1 antibody (Proteintech, #19851–1-AP). (H). STING1-KO THP-1 cells stably reconstituted with human WT (R232), HAQ, AQ, Q293 were treated with 50 ng/ml diABZI in culture for 24 hr. ISG-54 reporter luciferase activity was determined in cell supernatant and normalized to 10 ng/ml IFNβ-stimulated ISG-54 luciferase activity. (I). STING1-KO THP-1 cells stably reconstituted with human WT (R232), HAQ, AQ were treated with 50 ng/ml diABZI in culture for 2 hr. STING1 and IRF3 activation were determined by anti-STING1 antibody and anti-p IRF3 antibody (CST, Ser396, clone 4D4G). Densitometry was determined by ImageLab 5. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values determined by one-way ANOVA Tukey’s multiple comparison test (B, C, F, H, G) or unpaired student T-test (E, I). * p<0.05, n.s: not significant.

STING1 activation in primary human cells and THP-1 cells reconstituted with WT human STING1.
(A). Total human Lung cells from WT/WT individuals were treated with indicated dose of diABZi for 24 hr. Cell death in CD4, CD8 T cells and CD19 B cells were determined by PI staining. (B). Sequencing results of human STING1 gene in WT/WT and WT/HAQ individuals. (C–D). STING1-KO THP-1 cells stably reconstituted with human WT (R232) were treated with indicated dose of diABZI for indicated time in culture. ISG-54 reporter luciferase activity was determined in cell supernatant and normalized to 10 ng/ml IFNβ-stimulated ISG-54 luciferase activity. Data are representative of at least two independent experiments. Graphs represent the mean with error bars indication s.e.m. p values determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05, n.s: not significant.

HAQ and AQ rescue the lymphopenia and suppress myeloid cell expansion in SAVI (N153S) mice.
(A) The size and weight of spleens from WT/HAQ, HAQ/SAVI, AQ/SAVI, WT/AQ, WT/SAVI. (B–D). Spleen CD19+ B cells, CD4, CD8 T cells were determined in the indicated mice by Flow. (E–H). Spleen Ly6G+ neutrophils, Ly6Chi monocytes and F4/80+ macrophage was determined in the indicated mice by Flow. Data are representative of three independent experiments. n=3–5 mice/group. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05, n.s: not significant.

Splenocytes from HAQ/SAVI, AQ/SAVI partially resist to STING1-activation-induced cell death ex vivo.
(A–B). Flow cytometry of HAQ/SAVI, AQ/SAVI, WT/WT or WT/HAQ splenocytes treated with diABZI (100 ng/ml) or DMXAA (20 µg/ml) for 24 hr. Cell death was determined by PI staining. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05. n.s: not significant.

HAQ and AQ restore bone marrow monocytes in SAVI (N153S) mice.
(A–D) Flow analysis of Ly6G+CD11B+ neutrophils and Ly6G-Ly6C+CD11B+ bone marrow monocytes from HAQ/SAVI, AQ/SAVI, WT/SAVI and their littermates. n=3–5 mice/group. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05, n.s: not significant.

HAQ and AQ alleles prevent SAVI(N153S) disease in mice.
(A, B, G) The size and body weight of HAQ/SAVI, AQ/SAVI, WT/SAVI and their littermates WT/HAQ, WT/AQ mice. (D, E, H, I). Airway resistance, and pulmonary artery pressure were determined as described in Materials and methods. (C, F). HAQ/SAVI, AQ/SAVI, WT/SAVI (10 mice/group), were monitored for survival by Kaplan-Meier. (J, K). Representative hematoxylin and eosin (H&E) staining of lung, liver sections from indicated mice. n=3–5 mice/group. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05, **p<0.01. n.s.: not significant. (WBC): white blood cells; H: hepatocytes; K: Kupper cells; PV: portal vein; CV: central vein.

HAQ, AQ suppress lung myeloid cells infiltration in SAVI(N153S) mice.
(A–C) Flow analysis of lung Ly6G+CD11B+ neutrophils and Ly6G-Ly6C+CD11B+ monocytes from HAQ/SAVI, AQ/SAVI, WT/SAVI and their littermates WT/HAQ, WT/AQ mice. n=3–5 mice/group. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05.
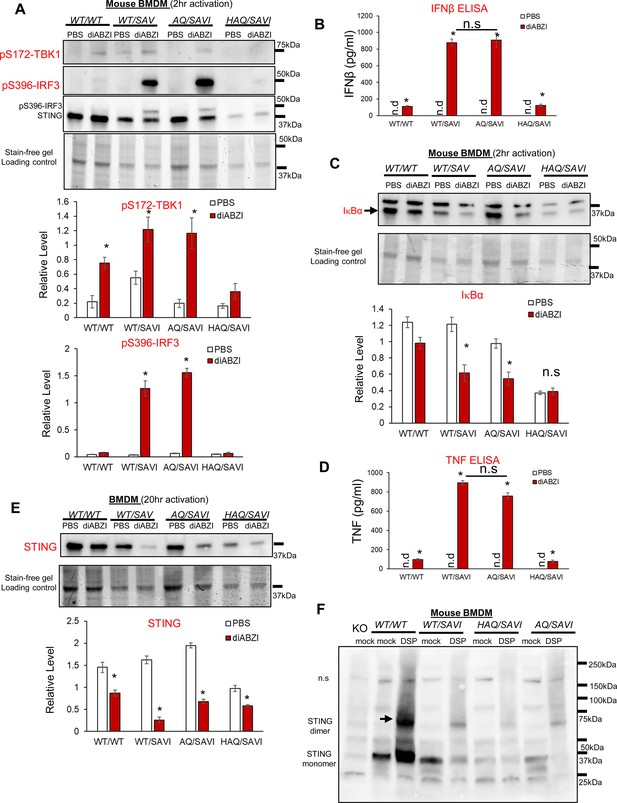
AQ/SAVI(N153S) cells had similar TBK1-IRF3, NFκB activation and STING1 degradation as the WT/SAVI(N153S) cells.
(A, C). BMDM from WT/WT, WT/SAVI, HAQ/SAVI and AQ/SAVI were treated with 100 ng/ml diABZi in culture for 2 hr. Cells were lysed and run on a 4~20% Mini-PROTEAN TGX Stain-Free Precast Gel. The blot was probed for phospho-Thr172-TBK1 antibody (CST, clone D52C2), phosphor-Ser 396-IRF3 (CST, clone 4D4G), STING1 (Proteintech, #19851–1-AP) and IκBα (CST, clone 44D4) antibody. (E). BMDM from WT/WT, WT/SAVI, HAQ/SAVI and AQ/SAVI were treated with 100 ng/ml diABZi in culture for 24 hr. Cells were lysed and run on a 4~20% Mini-PROTEAN TGX Stain-Free Precast Gel. The blot was probed for STING1 antibody (Proteintech, 19851–1-AP). (B, D). IFNβ and TNF were determined by ELISA in the cell supernatant from E. (F). BMDM from WT/WT, WT/SAVI, HAQ/SAVI and AQ/SAVI were treated with 400 µM cleavable chemical crosslinker DSP (Pierce, cat no: PG82081) in PBS for 1 hr at 4 °C. Cells were washed with PBS and lysed in RIPA buffer. Whole cell lysate was mixed with 4 x Laemmli Sample Buffer (BioRad, cat no 1610747) containing 5% 2-mercaptoethanol, heated at 95 °C for 10 min and, run on a 4~20% Mini-PROTEAN TGX Stain-Free Precast Gel. The blot was probed for STING1 antibody (Proteintech, 19851–1-AP). Densitometry was determined by ImageLab 5. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by unpaired student T-test (A–E) or one-way ANOVA Tukey’s multiple comparison test (D, B). * p<0.05, **p<0.01, ***p<0.001. n.s.: not significant; n.d: not detected.

HAQ/SAVI(N153S) and AQ/SAVI(N153S) cells had 10-fold and 20-fold increased spleen T-regs compared to WT/SAVI mice.
(A) Flow cytometry analysis of IFNγ producing spleen CD4+ T cells from WT/HAQ, WT/AQ, WT/SAVI, HAQ/SAVI and AQ/SAVI mice. (B) Flow cytometry analysis of CD4+ FoxP3+ spleen T-regs. n=3–5 mice/group. Data are representative of three independent experiments. Graphs represent the mean with error bars indication s.e.m. p values are determined by one-way ANOVA Tukey’s multiple comparison test. * p<0.05, **p<0.01, ***p<0.001. n.s.: not significant.





